Abstract
Mononuclear metal–dioxygen species are key intermediates that are frequently observed in the catalytic cycles of dioxygen activation by metal-loenzymes and their biomimetic compounds. In this work, a side-on cobalt-(III)–peroxo complex bearing a macro-cyclic N-tetramethylated cyclam (TMC) ligand, [CoIII(15-TMC)(O2)]+, was synthesized and characterized with various spectroscopic methods. Upon protonation, this cobalt(III)–peroxo complex was cleanly converted into an end-on cobalt(III)–hydroperoxo complex, [CoIII(15-TMC)(OOH)]2+. The cobalt(III)–hydroperoxo complex was further converted to [CoIII(15-TMC-CH2-O)]2+ by hydroxylation of a methyl group of the 15-TMC ligand. Kinetic studies and 18O-labeling experiments proposed that the aliphatic hydroxylation occurred via a CoIV–oxo (or CoIII–oxyl) species, which was formed by O–O bond homolysis of the cobalt(III)–hydroperoxo complex. In conclusion, we have shown the synthesis, structural and spectroscopic characterization, and reactivities of mono-nuclear cobalt complexes with peroxo, hydroperoxo, and oxo ligands.
Keywords: aliphatic hydroxylation, bioinorganic chemistry, cobalt, macrocyclic ligands, oxygen
Introduction
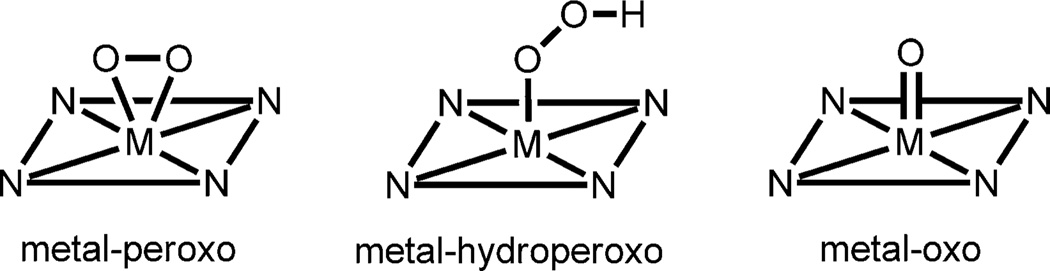
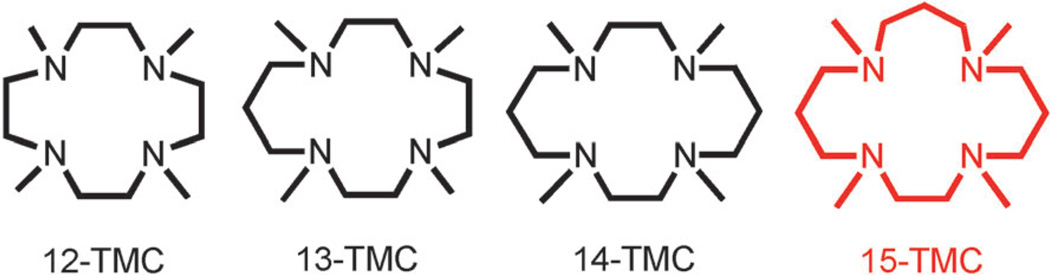
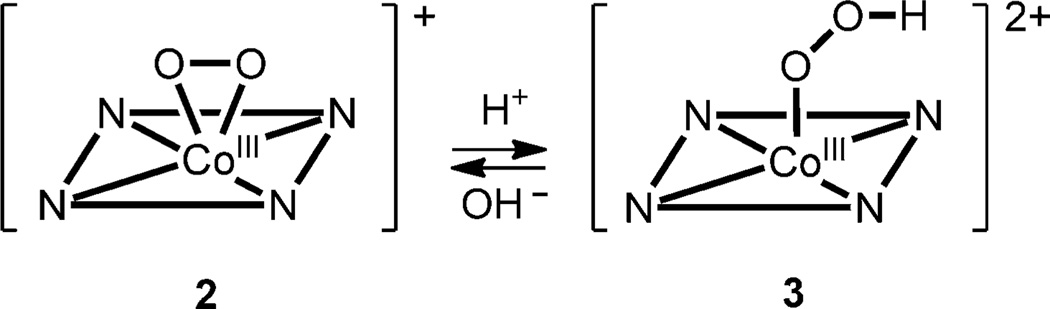
Oxygen-coordinating metal species, such as metal–peroxo,–hydroperoxo, and –oxo complexes (Scheme 1), have been frequently invoked as reactive intermediates in the catalytic cycles of dioxygen activation by metalloenzymes, such as heme and nonheme iron monooxygenases and copper-containing enzymes.[1] In biomimetic studies, synthetic analogues of these active oxygen intermediates have been intensively investigated to understand their structures, mechanisms of interconversion, and the factors that control their chemical reactivities in oxidation reactions.[2] In those studies, it has been shown that supporting ligands play an important role in tuning the geometric and electronic structures, stabilities, and reactivities of the oxygen-coordinating metal complexes. One notable example of the supporting ligands is N-tetramethylated cyclam (TMC) and its derivatives,[3] which have proved to be versatile ligands in the biomimetic chemistry of dioxygen activation by metal complexes (see Scheme 2 for the ligand structures of n-TMC).[4] A variety of metal–TMC complexes with oxo, superoxo, peroxo, and hydroperoxo ligands have been synthesized and spectroscopically and/or structurally characterized.[2h, 5–10] In particular, the first high-resolution crystal structures of mononuclear nonheme iron(IV)–oxo and iron(III)–peroxo complexes, such as [FeIV(14-TMC)(O)]2+ and [FeIII(14-TMC)(O2)]+, were successfully obtained by using the 14-TMC macrocyclic ligand.[7a, 10a] It was also shown that the iron(III)–peroxo complex [FeIII(14-TMC)(O2)]+ was cleanly converted to the corresponding iron(III)–hydroperoxo complex, [FeIII(14-TMC)(OOH)]2+, upon protonation; this was followed by clean conversion of the iron(III)–hydroperoxo complex to an iron(IV)–oxo complex, [FeIV(14-TMC)(O)]2+, through homolytic O–O bond cleavage.[7a] As part of our ongoing efforts to elucidate the chemistry of metal–O2 complexes, we synthesized a novel cobalt(II) complex bearing a 15-TMC ligand, [CoII(15-TMC)]2+ (1; see the structure of the 15-TMC ligand in Scheme 2) and used it for the synthesis of cobalt(III)–peroxo and –hydroperoxo complexes ([CoIII(15-TMC)(O2)]+ (2) and [CoIII(15-TMC)(OOH)]2+ (3)).[11] We also show that the cobalt(III)–hydroperoxo complex (3) is cleanly converted to [CoIII(15-TMC-CH2-O)]2+ (4) by hydroxylation of an N-CH3 group of the 15-TMC ligand. In the latter reaction, we propose that the hydroperoxide group in 3 is homolytically cleaved to generate a high-valent cobalt(IV)–oxo (or cobalt(III)–oxyl) intermediate as an active oxidant that effects the C–H bond activation of the 15-TMC ligand.
scheme 1.
A schematic drawing of metal–peroxo, metal–hydroperoxo, and metal–oxo complexes.
scheme 2.
A schematic drawing of n-TMC ligands.[4]
Results and Discussion
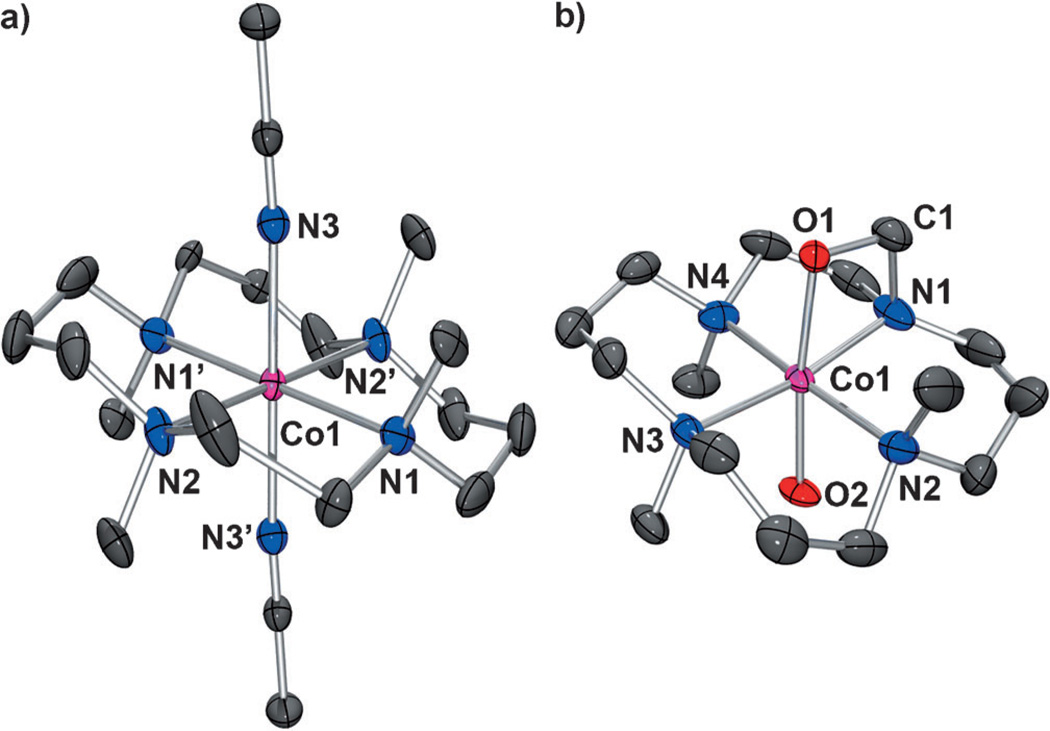
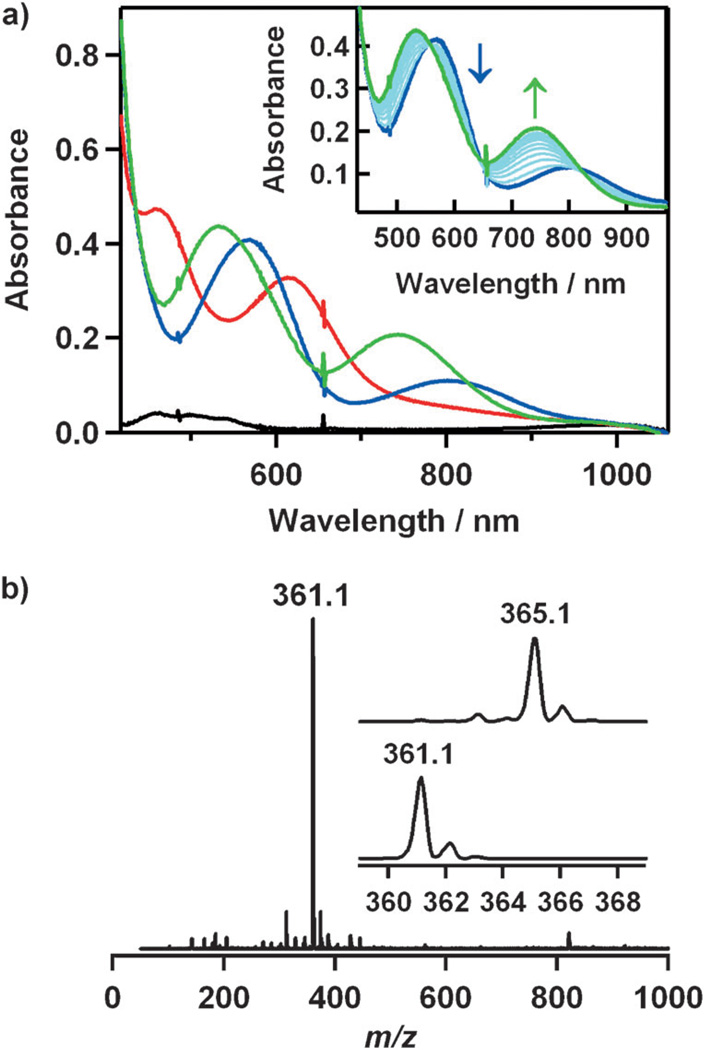
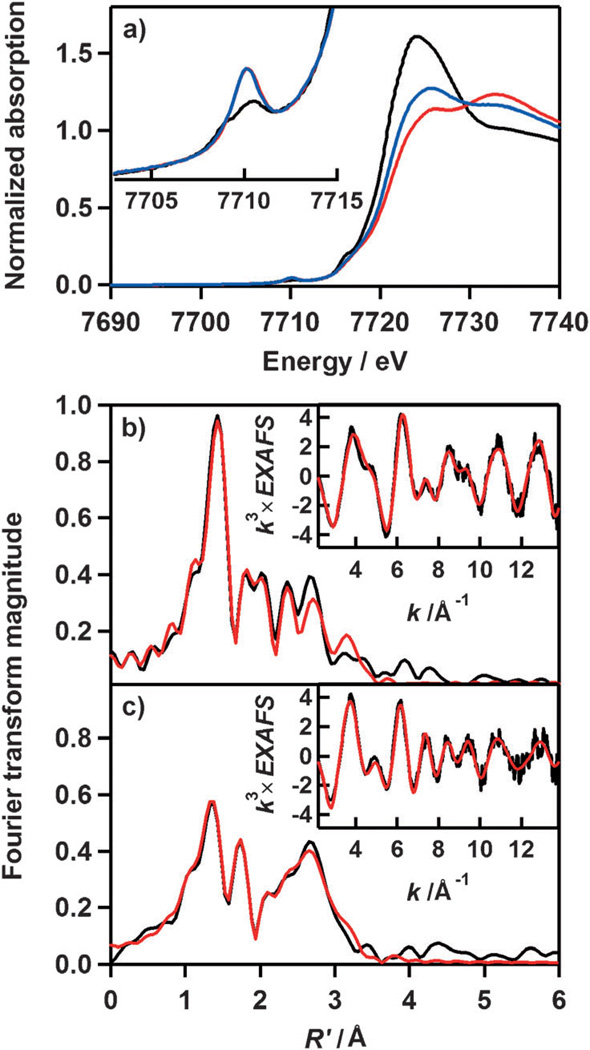
The starting cobalt complex, [CoII(15-TMC)(CH3CN)2]2+ ([1•(CH3CN)2]), was synthesized by the reaction of equimolar amounts of Co(ClO4)2•6H2O and 15-TMC in CH3CN (Figure 1 a for X-ray crystal structure; Tables S1 and S2 and Figures S1 and S2 in the Supporting Information for structural and spectral data, respectively). Addition of aqueous H2O2 (10 equiv relative to 1) to a reaction solution containing 1 and triethylamine (TEA; 5 equiv relative to 1) in CH3CN at 0 °C afforded the green intermediate 2 with absorption bands at 464 (ε = 120m−1cm−1) and 615 nm (ε = 90m−1cm−1; Figure 2 a). Complex 2 was metastable at 0 °C (t1/2≈ 2 h), which allowed us to characterize it with various spectroscopic techniques. The ESI mass spectrum of 2 exhibits a prominent signal at m/z 361.1 (Figure 2 b), and the mass and isotope distribution pattern correspond to [Co(15-TMC)(O2)]+ (calcd: m/z 361.2). When the reaction was carried out with isotopically labeled H2 18O2, a mass peak corresponding to [Co(15-TMC)(18O2)]+ appeared at m/z 365.1 (calcd: m/z 365.2; Figure 2b, inset). The observation of a four-mass-unit upshift upon substitution of 16O atoms with 18O atoms indicates that 2 contains an O2 unit. The X-band EPR spectrum of 2 is silent at 4.3 K, and the determination of the spin state of 2 by using the 1H NMR method of Evans[12] clearly indicates that 2 is a low-spin state (S = 0) cobalt(III) d6 species.[8] The oxidation of 1 (Co2+) to 2 (Co3+) was confirmed by using Co K-edge XAS data, which show a clear shift in the pre-edge and rising edge to higher energy upon going from 1 to 2 (Figure 3 a; Table S3 in the Supporting Information). Figure 3b shows the Fourier transform and corresponding non-phase-shift-corrected EXAFS data for 2. The EXAFS data are consistent with a six-coordinate site in 2 with two short Co–O bond lengths of 1.89 Å and four Co–N bond lengths of 2.07 Å. Based on the spectroscopic and structural characterization, complex 2 is assigned as a cobalt(III) complex with a ‘side-on’ peroxo group, [CoIII(15-TMC)(O2)]+.
Figure 1.
X-ray crystal structures of a) [CoII(15-TMC)(CH3CN)2]2+ [1•-(CH3CN)2] and b) [CoIII(15-TMC-CH2-O)(OH)]+ [4•(OH)] with thermal ellipsoids drawn at the 30% probability. Hydrogen atoms are omitted for clarity. Selected bond lengths [Å] and angles [°] for [4•(OH)]: Co–O1 1.896(3), Co–O2 1.878(4), Co–N1 1.988(5), Co–N2 2.125(5), Co–N3 2.089(4), Co–N4 2.082(5); O1–Co–O2 173.36(17), Co–O1–C1 92.7(3), O1–C1–N1 104.9(4).
Figure 2.
a) UV/Vis spectra of 1 (black), 2 (red), 3 (blue), and 4 (green). Inset: the conversion of 3 (blue) into 4 (green) in CH3CN at 0 8C. b) ESI mass spectrum of 2. Insets: the observed isotope distribution patterns for [Co(15-TMC)(O2)]+ at m/z 361.1 (bottom panel) and [Co(15-TMC)-(18O2)]+ at m/z 365.1 (top panel).
Figure 3.
a) Normalized Co K-edge XAS spectra of 1 (black), 2 (red), and 3 (blue). The inset shows the pre-edge region. FEFF fits (black) to the non-phase-shift-corrected Fourier transforms (red) and the corresponding EXAFS data (insets, red) for b) 2 and c) 3.
Addition of HClO4 (10 equiv) to a solution of 2 in CH3CN at 0 °C immediately generated the violet intermediate 3 with electronic absorption bands at 566 and 805 nm; this was followed by the conversion (t1/2≈90 s) of 3 to a new purple species, 4, with electronic absorption bands at 531 and 740 nm (Figure 2 a). In the conversion of 3 to 4, the rate, kobs, was determined to be 3.7(3) × 10−3 s−1 at 0 °C (Figure S3 in the Supporting Information), and isosbestic points were observed at 555, 640, and 820 nm (Figure 2 a, inset). In addition, the temperature dependence of the kobs value was determined in the range of 253–283 °C, and the results gave rise to an Eyring plot that afforded activation parameters of ΔH‡ = 104(6) kJ−1mol−1 and ΔS‡= 90(5) Jmol−1K−1 (Figure S4 in the Supporting Information). Complex 3 was EPR silent, and the spin state of the cobalt ion was determined to be S = 0 by using the Evans 1H NMR method.[12] In addition, the similarity in the Co K-edge XAS rising-edge energy positions (Figure 3 a; Table S4 in the Supporting Information) indicates that the oxidation state of 3 is the same as that of 2 (namely, Co3+). FEFF program fits to the EXAFS data are consistent with one short Co–O bond length of 1.86 Å, four Co–N bond lengths of 2.04 Å, and one Co–N bond length of 2.19 Å, which supports a cobalt(III) complex with an ‘end-on’ hydroperoxo binding and a trans-axial acetonitrile ligation, [CoIII(15-TMC)(O2H)(CH3CN)]2+.
Intermediate 3 rapidly reverted back to 2 upon addition of tetramethylammonium hydroxide (TMAH; 3 equiv), and addition of HClO4 to the resulting solution of 2 regenerated 3 quantitatively. This reversible cycle could be repeated several times, which suggests that 2 and 3 are interconverted through the acid–base chemistry proposed in Scheme 3; such an interconversion has been well demonstrated in non-heme iron(III)–peroxo and iron(III)–hydroperoxo species.[13] Thus, the above evidence for this acid–base interconversion further supports the identification of intermediates 2 and 3 as the cobalt(III)–peroxo, [CoIII(15-TMC)(O2)]+, and cobalt-(III)–hydroperoxo species, [CoIII(15-TMC)(O2H)]2+, respectively.
scheme 3.
A reversible acid–base equilibrium between the cobalt(III)–peroxo (2) and cobalt(III)–hydroperoxo (3) complexes.
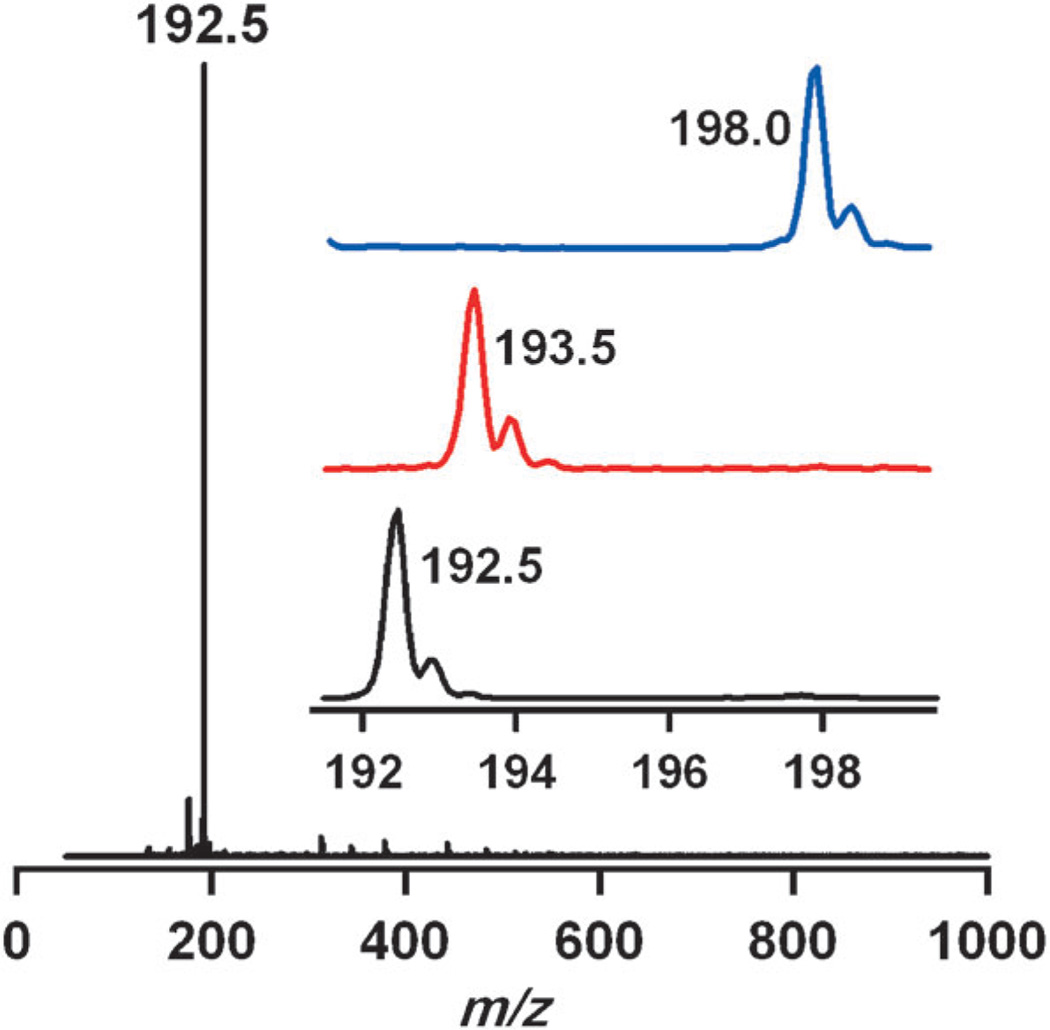
As mentioned above, the cobalt(III)–hydroperoxo species, 3, cleanly converts to the thermally stable product 4. We thus characterized 4 with various spectroscopic methods and X-ray crystallography. The ESI mass spectrum of 4 exhibits a signal at m/z 192.5 (Figure 4), and the mass and isotope distribution pattern correspond to [CoIII(15-TMC-CH2-O)-(CH3CN)]2+ (calcd: m/z 192.6). When the reaction was carried out with 3 prepared with isotopically labeled H2 18O2, the mass peak corresponding to 4 shifted to m/z 193.5 (calcd: m/z 193.6; Figure 4), which indicates that 4 contains an oxygen atom. Furthermore, when the reaction was carried out with deuterated [CoII(15-TMC-(CD3)4)]2+ (1-CD3), a mass peak corresponding to [CoIII(15-TMC-(CD3)3-CD2-O)(CH3CN)]2+ (4-CD3) appeared at m/z 198.0 (calcd: m/z 198.1; Figure 4; see below). Complex 4 was EPR silent at 4.3 K, and the spin state of the cobalt ion was determined to be S = 0 by the Evans 1H NMR method.[12]
Figure 4.
ESI mass spectrum of 4. The insets show the observed isotope distribution patterns for 4-16O, 4-18O, and 4-CD3.
Complex 4 was further characterized by X-ray diffraction analysis (Tables S1 and S2 in the Supporting Information). As shown in the molecular structure in Figure 1b, complex 4 has a six-coordinate cobalt ion with four nitrogen atoms from the macrocyclic 15-TMC ligand in equatorial positions, one axial hydroxide ligand, and a trans-axial oxygen atom from an alkoxide group. Thus, the crystal structure clearly illustrates the hydroxylation of a ligand methyl group and the ligation of the resulting O atom of the alkoxide to the cobalt ion.
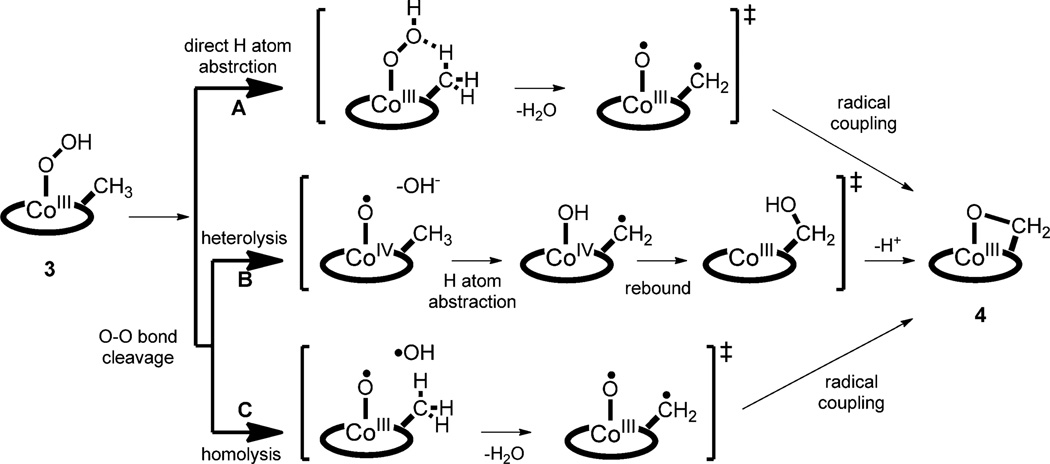
We then investigated the mechanism of the conversion of 3 to 4 (i.e., the hydroxylation of the N-methyl group by a cobalt(III)–hydroperoxo species). Three possible mechanisms were considered (Scheme 4): One is the direct C–H bond activation of the methyl group by the hydroperoxo ligand in 3, which would generate H2O, CoIII–O•, and N– CH2• (pathway A).[14] Radical coupling between CoIII–O• and N–CH2• would produce the oxygenated product 4. In this direct C–H bond activation by the hydroperoxo group, a high kinetic isotope effect (KIE) value (>1) would be expected with the deuterated N-methyl group. Since the KIE value of 1 was obtained in the conversion of 3 to 4 (kobs = 3.7(3) × 10−3 s−1 for 3-(CH3) and 3.4(4) × 10–3 s–1 for 3-(CD3)), we ruled out the mechanism of direct C–H bond activation by the hydroperoxo ligand in 3.[15] The other possibilities are O–O bond cleavage of 3 by heterolysis or homolysis. Heterolytic cleavage of the O–O bond would generate CoIV–O• and OH− (pathway B). H-atom abstraction by CoIV–O• and a rebound process would produce 4. Addition of a proton would facilitate the heterolytic cleavage of the O–O bond, in which one of the oxygen atoms forms an oxyl radical and the other is released as water.[1b, 16] However, we found no proton-concentration effect on the rate of the O–O bond cleavage of 3 (Table S5 in the Supporting Information). Thus, homolytic cleavage is the most likely mechanism (pathway C), because it is consistent with KIE = 1 and a lack of proton-concentration effect in the kinetic experiments. The homolytic cleavage of the hydroperoxo O–O bond would produce CoIII–O• and • OH species, which is proposed to be a rate-determining step. The • OH species abstracts an H atom from the methyl group to afford H2O; this is followed by radical coupling between CoIII–O• and N–CH2• to yield 4. As a precedent, we have recently shown an example of O–O bond homolysis of an iron(III)–hydroperoxo complex that leads to the generation of an iron(IV)–oxo complex.[7a]
scheme 4.
Possible mechanisms for the conversion of 3 to 4
The reactivity of 3 toward external substrates was investigated as well. Upon addition of substrates, such as xanthene, anthracene, cyclohexadiene, and cyclohexane, to the solution of 3, we could not observe any difference in the kobs value. Furthermore, product analysis of the reaction solutions revealed that no oxidized products were formed in these reactions. The results demonstrate that 3 is not capable of reacting with external substrates because the intramolecular hydroxylation occurs at a fast rate under the reaction conditions.
Conclusion
We have shown the synthesis of a cobalt(III)–peroxo (2) complex with a macrocyclic 15-TMC ligand and its conversion to a cobalt(III)–hydroperoxo (3) complex upon protonation. The latter species was further converted to give an oxygenated product, 4, in which an Nmethyl C–H bond of the 15-TMC ligand bound to the Co center was hydroxylated. Kinetic studies support a homolytic O–O bond cleavage of the hydroperoxo ligand in 3 through the formation of a cobalt(III)–oxyl species, which is responsible for the hydroxylation of the N-methyl group of the 15-TMC ligand. This study is another step towards understanding formation processes through structural and spectroscopic studies and ligand oxidation reactions, which provide important fundamental information about the aliphatic hydroxylation by metal–O2(H) and metal–O intermediates.
Experimental Section
Materials
All chemicals obtained from Aldrich Chemical Co. were the best available purity and were used without further purification unless otherwise indicated. Solvents were dried according to published procedures and distilled under Ar prior to use.[17] H2 18O2 (90% 18O-enriched, 2% H2 18O2 in water) and H2 18O (95% 18O-enriched) were purchased from ICON Services Inc. (Summit, NJ, USA). Iodosylbenzene (PhIO) and 15-TMC (1,4,8,12-tetramethyl-1,4,8,12-tetraazacyclopentadecane) were prepared according to the literature methods.[18, 19] The [D12]-15-TMC ligand was prepared by mixing an excess amount of [D2]formaldehyde (20 mmol, 2800 µL) and [D2]formic acid (20 mmol, 770 µL) with 1,4,8,12-tetraazacyclopentadecane (1.5 mmol, 33 mg). The mixture was heated to reflux for 12 h. After cooling, an aqueous solution of NaOH was added to the mixture. The resulting solution was extracted with CHCl3 (3 × 20 µL). The combined extracts were dried over Na2SO4 and CHCl3 was removed by evaporation under reduced pressure: ESI-MS (CH3CN): m/z 283.3 [[D12]-15-TMC+H]+; 1H NMR (CD3CN, 400 MHz): δ = 2.46 (s, 4 H), 2.39 (t, 12H), 1.60 ppm (q, 6 H).
Instrumentation
UV/Vis spectra were recorded on a Hewlett Packard 8453 diode array spectrophotometer equipped with a UNISOKU scientific instrument for low-temperature experiments. Electrospray-ionization mass spectra were collected on a Thermo Finnigan LCQ Advantage MAX quadrupole-ion-trap instrument (San Jose, CA, USA), by infusing samples directly into the source with a manual method. The spray voltage was set at 4.2 kV and the capillary temperature at 80°C. 1H NMR spectra were measured with Bruker DPX-400 spectrometer. The effective magnetic moments were determined by using the modified 1H NMR method of Evans at −20 °C.[12] A WILMAD coaxial insert (sealed capillary) tube containing the blank [D3]acetonitrile solvent (with 1.0% tetramethylsilane (TMS)) only was inserted into the normal NMR tubes containing the complexes (8.0 mm) dissolved in [D3]acetonitrile (with 0.05% TMS). The chemical shift of the TMS peak (and/or solvent peak) in the presence of the paramagnetic metal complexes was compared to that of the TMS peak (and/or solvent peak) in the inner coaxial insert tube. The effective magnetic moment (µ) was calculated by using the equation µ = 0.0618(ΔυT/2fM)1/2, in which f is the oscillator frequency (MHz) of the superconducting spectrometer, T is the absolute temperature, M is the molar concentration of the metal ion, and Δ υ is the difference in frequency (Hz) between the two reference signals.[12c] CW-EPR spectra were taken at 4.3 K by using an X-band Bruker EMX-plus spectrometer equipped with a dual mode-cavity (ER4116DM). Low temperatures were achieved and controlled by using an Oxford Instruments ESR900 liquid He quartz cryostat with an Oxford Instruments ITC503 temperature and gas-flow controller.
Generation and characterization of [CoII(15-TMC)(CH3CN)2](ClO4)2 ([1•(CH3CN)2](ClO4)2)
15-TMC (0.33 g, 1.5 mmol) was added to a CH3CN (50 mL) solution of Co(ClO4)2•6H2O (0.71 g, 2.6 mmol). The mixture was heated to reflux for 12 h. After the reaction mixture had cooled, the solvent was removed under vacuum to give a red solid, which was collected by filtration and then washed with methanol several times to remove remaining Co(ClO4)2•6H2O: Yield: 1.11 g (70%); ESI-MS (CH3CN; Figure S1 in the Supporting Information): m/z 164.8 [Co(15-TMC)]2+, 184.9 [Co(15-TMC)(CH3CN)]2+, 205.0 [Co(15-TMC)-(CH3CN)2]2+, 428.1 [Co(15-TMC)(ClO4)]+ ; µeff = 4.3 µB; elemental analysis: calcd for C19H40N6O8Cl2Co: C 37.39, H 6.61, N 13.77; found: C 37.12, H 6.73, N 13.48; X-ray crystallographically suitable crystals were obtained by slow diffusion of Et2O into concentrated solutions of 1.
Generation and characterization of [CoIII(15-TMC)(O2)]+ (2)
Treatment of 1 (4 mm) with H2O2 (10 equiv) in the presence of TEA (5 equiv) in CH3CN (2 mL) afforded the formation of a green solution. [Co(15-TMC)(18O2)]+ was prepared by adding H2 18O2 (10 equiv; 32 µL; 90% 18O-enriched 2% H2 18O2 in water) to a solution containing 1 (4 mm) and TEA (5 equiv) in CH3CN (2 mL) at 0°C: UV/Vis (CH3CN at 0°C; Figure 2a): λ = 464 (ε = 120), 615 nm (90 m−1cm−1); ESI-MS (CH3CN; see Figure 2b): m/z 361.1 [Co(15-TMC)(O2)]+.
Generation and characterization of [CoIII(15-TMC)(OOH)]2+ (3)
Treatment of 2 (4 mm) with HClO4 (10 equiv) in CH3CN (2 mL) at 0°C afforded the formation of a purple solution. Titration experiments showed that ten equivalents of HClO4 were required for the full formation of 3 (data not shown): UV/Vis (CH3CN at 0°C; Figure 2a): λ = 566 (ε = 100), 805 nm (30 m−1cm−1).
Generation and characterization of [CoIII(15-TMC-CH2-O)(OH)](ClO4)-(CH3CN) ([4• (OH)](ClO4)(CH3CN))
A solution of 3 in CH3CN was allowed to stand for several hours at ambient temperature to change the solution color from purple to violet. Slow diffusion of Et2O into the resulting solution afforded violet crystals: Yield: 0.26 g (45%); UV/Vis (CH3CN at 0°C; Figure 2a): λ = 531 (ε = 120), 749 nm (70 m−1cm−1); ESI-MS (CH3CN; Figure 4): m/z 192.5 [Co(15-TMC-CH2-O)(CH3CN)]2+ ; elemental analysis: calcd for C17H37N5O6ClCo: C 40.68, H 7.43, N 13.95; found: C 40.29, H 7.28, N 13.73; crystals suitable for X-ray crystallo-graphic analysis were obtained by CH3CN/Et2O layering of 4.
Kinetic measurements
All reactions were run in an 1 cm UV cuvette by monitoring UV/Vis spectral changes of reaction solutions, and rate constants were determined by fitting the changes in absorbances at 550 nm for 2, 566 nm for 3, and 531 nm for 4. Reactions were run at least in triplicate, and the data reported represent the average of these reactions. After the completion of the reactions, pseudo-first-order fitting of the kinetic data allowed the kobs values to be determined.
X-ray crystallography
Single crystals of [Co(15-TMC)(CH3CN)2](ClO4)2 and [Co(15-TMC-CH2-O)(OH)](ClO4)(CH3CN) were picked from solutions by a nylon loop (Hampton Research Co.) on a handmade copper plate mounted inside a liquid N2 Dewar vessel at approximately −40°C and mounted on a goniometer head in an N2 cryostream. Data collections were carried out on a Bruker SMART APEX II CCD diffractometer equipped with a monochromator in the MoKα (λ= 0.71073 Å) incident beam. The CCD data were integrated and scaled by using the Bruker SAINT software package, and the structure was solved and refined by using SHELXTL Version 6.12 software.[20] Hydrogen atoms were located in the calculated positions for [Co(15-TMC-CH2-O)(OH)](ClO4)-(CH3CN). Due to the high degree of disorder, however, hydrogen atoms could not be placed in ideal positions for [Co(15-TMC)(CH3CN)2]-(ClO4)2. Crystal data for [Co(15-TMC)(CH3CN)2](ClO4)2 : C19H0Cl2CoN6O8 ; monoclinic; P21/n; Z= 2; a= 8.6493(8), b= 15.6174(13), c= 10.7612(9) Å; b= 111.2190(10)°; V= 1355.1(2) Å3 ; µ = 0.880 mm−1; ρcalcd = 1.397 gcm−3 ; R1 = 0.0865, wR2 = 0.2603 for 2636 unique reflections; 179 variables. Crystal data for [Co(15-TMC-CH2-O)(OH)](ClO4)-(CH3CN): C17H37ClCoN5O6 ; monoclinic; P21/c; Z= 4; a= 9.6291(3), b= 19.6761(6), c= 12.0095(4) Å; b= 98.974(2)8; V= 2247.51(12) Å3 ; m= 0.925 mm−1; ρcalcd = 1.483 g cm−3 ; R1 = 0.0699, wR2 = 0.2470 for 4263 unique reflections, 276 variables. The crystallographic data for [Co-(15-TMC)(CH3CN)2](ClO4)2 and [Co(15-TMC-CH2-O)(OH)](ClO4)-(CH3CN) and selected bond distances and angles are listed in Tables S1 and S2, respectively, in the Supporting Information. CCDC 882840 ([Co-(15-TMC)(CH3CN)2](ClO4)2) and 882839 ([Co(15-TMC-CH2-O)(OH)]-(ClO4)(CH3CN)) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
X-ray absorption spectroscopy
The Co K-edge X-ray absorption spectra of 1–3 were measured at the Stanford Synchrotron Radiation Laboratory (SSRL) on the unfocussed 20-pole 2T wiggler side-station beam line 7–3 under standard ring conditions of 3 GeV and approximately 100 mA. A Si(220) double crystal monochromator was used for energy selection. A Rh-coated harmonic rejection mirror was used on beam line 7–3 to reject components of higher harmonics. The solid sample for 1 was finely ground with BN into a homogeneous mixture and pressed into a 1 mm aluminum spacer between X-ray transparent 37 µm Kapton tape. The solution samples for 2 and 3 (≈120 µL) were transferred into 2 mm delrin XAS cells with 37 µm Kapton tape windows under synthesis conditions. Both solid and solution samples were immediately frozen after preparation and stored under liquid N2. During data collection, the samples were maintained at a constant temperature of 15 K by using an Oxford Instruments CF 1208 liquid helium cryostat. Data were measured to k= 15 Å−1 on 1 (transmission mode) by using an ionization chamber detector and to k= 15 Å−1 on 2 and 3 (fluorescence mode) by using a Canberra Ge 30-element array detector. Internal energy calibration was accomplished by simultaneous measurement of the absorption of a Co foil placed between two ionization chambers situated after the sample. The first inflection point of the foil spectrum was fixed at 7709.5 eV. Data presented here are 2-scan (1), 15-scan (2), and 15-scan (3) average spectra, which were processed by fitting a second-order polynomial to the pre-edge region and subtracting this from the entire spectrum as background. A four-region spline of orders 2, 3, 3, and 3 was used to model the smoothly decaying post-edge region. The data were normalized by subtracting the cubic spline and assigning the edge jump to 1.0 at 7725 eV by using the Pyspline program.[21] Theoretical EXAFS signals λ(k) were calculated by using the FEFF program (Macintosh Version 8.4),[22–24] the crystal structure of 1, and structural models of 2 and 3 built in Avogadro.[25] The theoretical models were fitted to the data by using the EXAFSPAK program.[26] The structural parameters varied during the fitting process were the bond distance (R), the bond variance (σ2), which is related to the Debye–Waller factor resulting from thermal motion, and the static disorder of the absorbing and scattering atoms. The nonstructural parameter E0 (the energy at which k= 0) was also allowed to vary but was restricted to a common value for every component in a given fit. Coordination numbers were systematically varied in the course of the fit but were fixed within a given fit. A comparison of the k3-weighted Co K-edge EXAFS data for 2 and 3, along with their non-phase-shift-corrected Fourier transforms (k= 2–14 Å−1) is shown in Figure 3. The FEFF program fits to the data are presented in Figure 3 and Table S3 in the Supporting Information. On going from 2 to 3, the first-shell Fourier transform peak intensity decreases, which indicates a decrease in the coordination number in 3. The second-shell and third-shell features were fitted with single- and multiple-scattering components from the macrocyclic ligand (Table S4 in the Supporting Information).
Supplementary Material
Acknowledgements
W.N. at Ewha Womans University acknowledges financial support from the NRF/MEST of Korea through the CRI (2-2012-1794-001-1) and GRL (2010-00353) and from the 2011 KRICT OASIS Project. J.C. at DGIST acknowledges the R&D program of the MEST of Korea (13-BD-0403) for financial support. Portions of this research were carried out at the Stanford Synchrotron Radiation Lightsource, a Directorate of the SLAC National Accelerator Laboratory and an Office of Science User Facility operated for the U.S. Department of Energy, Office of Science by Stanford University. The SSRL Structural Molecular Biology Program is supported by the Department of Energy, Office of Biological and Environmental Research and by the National Institutes of Health, National Institute of General Medical Sciences (including P41M103393), and the National Center for Research Resources (P41RR001209).
Footnotes
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/chem.201300107.
References
- 1.a) Ortiz de Montellano PR. Cytochrome P450: Structure, Mechanism, and Biochemistry. 3rd ed. New York: Kluwer Academic/Plenum; 2005. [Google Scholar]; b) Denisov IG, Makris TM, Sligar SG, Schlichting I. Chem. Rev. 2005;105:2253–2277. doi: 10.1021/cr0307143. [DOI] [PubMed] [Google Scholar]; c) Abu-Omar MM, Loaiza A, Hontzeas N. Chem. Rev. 2005;105:2227–2252. doi: 10.1021/cr040653o. [DOI] [PubMed] [Google Scholar]; d) Itoh S. Curr. Opin. Chem. Biol. 2006;10:115–122. doi: 10.1016/j.cbpa.2006.02.012. [DOI] [PubMed] [Google Scholar]; e) Chufán EE, Puiu SC, Karlin KD. Acc. Chem. Res. 2007;40:563–572. doi: 10.1021/ar700031t. [DOI] [PubMed] [Google Scholar]; f) Krebs C, Fujimori DG, Walsh CT, Bollinger JM., Jr Acc. Chem. Res. 2007;40:484–492. doi: 10.1021/ar700066p. [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Bruijnincx PCA, van Koten G, Klein Gebbink RJM. Chem. Soc. Rev. 2008;37:2716–2744. doi: 10.1039/b707179p. [DOI] [PubMed] [Google Scholar]; h) Green MT. Curr. Opin. Chem. Biol. 2009;13:84–88. doi: 10.1016/j.cbpa.2009.02.028. [DOI] [PubMed] [Google Scholar]; i) Ortiz de Montellano PR. Chem. Rev. 2010;110:932–948. doi: 10.1021/cr9002193. [DOI] [PMC free article] [PubMed] [Google Scholar]; j) Shaik S, Cohen S, Wang Y, Chen H, Kumar D, Thiel W. Chem. Rev. 2010;110:949–1017. doi: 10.1021/cr900121s. [DOI] [PubMed] [Google Scholar]
- 2. Nam W. Acc. Chem. Res. 2007;40:465. doi: 10.1021/ar700027f. and review articles in this special issue; van Eldik R. Coord. Chem. Rev. 2007;251:1649–1662. Nam W. Acc. Chem. Res. 2007;40:522–531. doi: 10.1021/ar700027f. Cramer CJ, Tolman WB. Acc. Chem. Res. 2007;40:601–608. doi: 10.1021/ar700008c. Himes RA, Karlin KD. Curr. Opin. Chem. Biol. 2009;13:119–131. doi: 10.1016/j.cbpa.2009.02.025. Gunay A, Theopold KH. Chem. Rev. 2010;110:1060–1081. doi: 10.1021/cr900269x. Yao S, Driess M. Acc. Chem. Res. 2012;45:276–287. doi: 10.1021/ar200156r. Cho J, Sarangi R, Nam W. Acc. Chem. Res. 2012;45:1321–1330. doi: 10.1021/ar3000019. Hohenberger J, Ray K, Meyer K. Nature Commun. 2012;3:720. doi: 10.1038/ncomms1718. de Visser J-U, Rohde Y-M, Lee SP, Cho J, Nam W. Coord. Chem. Rev. 2013;257:381–393. McDonald AR, Que L., Jr Coord. Chem. Rev. 2013;257:414–428.
- 3.Kent Barefield E. Coord. Chem. Rev. 2010;254:1607–1627. [Google Scholar]
- 4.Abbreviations used for n-TMC: 12-TMC: 1,4,7,10-tetramethyl-1,4,7,10-tetraazacyclododecane; 13-TMC: 1,4,7,10-tetramethyl-1,4,7,10-tetraazacyclotridecane; 14-TMC: 1,4,8,11-tetramethyl-1,4,8,11-tetraazacyclotetradecane; 15-TMC: 1,4,8,12-tetramethyl-1,4,8,12-tetraazacyclopentadecane.
- 5.a) Cho J, Woo J, Nam W. J. Am. Chem. Soc. 2010;132:5958–5959. doi: 10.1021/ja1015926. [DOI] [PubMed] [Google Scholar]; b) Cho J, Woo J, Han JE, Kubo M, Ogura T, Nam W. Chem. Sci. 2011;2:2057–2062. [Google Scholar]; c) Yokoyama A, Han JE, Cho J, Kubo M, Ogura T, Siegler MA, Karlin KD, Nam W. J. Am. Chem. Soc. 2012;134:15269–15272. doi: 10.1021/ja307384e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.a) Seo MS, Kim JY, Annaraj J, Kim Y, Lee Y-M, Kim SJ, Kim J, Nam W. Angew. Chem. 2007;119:381–384. Angew. Chem. Int. Ed.2007, 46, 377 –380; [Google Scholar]; b) Annaraj J, Cho J, Lee YM, Kim SY, Latifi R, de Visser SP, Nam W. Angew. Chem. 2009;121:4214–4217. doi: 10.1002/anie.200900118. Angew. Chem. Int. Ed.2009, 48, 4150 –4153. [DOI] [PubMed] [Google Scholar]
- 7.a) Cho J, Jeon S, Wilson SA, Liu LV, Kang EA, Braymer JJ, Lim MH, Hedman B, Hodgson KO, Valentine JS, Solomon EI, Nam W. Nature. 2011;478:502–505. doi: 10.1038/nature10535. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Annaraj J, Suh Y, Seo MS, Kim SO, Nam W. Chem. Commun. 2005:4529–4531. doi: 10.1039/b505562h. [DOI] [PubMed] [Google Scholar]
- 8.a) Jo Y, Annaraj J, Seo MS, Lee Y-M, Kim SY, Cho J, Nam W. J. Inorg. Biochem. 2008;102:2155–2159. doi: 10.1016/j.jinorgbio.2008.08.008. [DOI] [PubMed] [Google Scholar]; b) Cho J, Sarangi R, Kang HY, Lee JY, Kubo M, Ogura T, Solomon EI, Nam W. J. Am. Chem. Soc. 2010;132:16977–16986. doi: 10.1021/ja107177m. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Sarangi R, Cho J, Nam W, Solomon EI. Inorg. Chem. 2011;50:614–620. doi: 10.1021/ic101730r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.a) Kieber-Emmons MT, Annaraj J, Seo MS, Van Heuvelen KM, Tosha T, Kitagawa T, Brunold TC, Nam W, Riordan CG. J. Am. Chem. Soc. 2006;128:14230–14231. doi: 10.1021/ja0644879. [DOI] [PubMed] [Google Scholar]; b) Cho J, Sarangi R, Annaraj J, Kim SY, Kubo M, Ogura T, Solomon EI, Nam W. Nat. Chem. 2009;1:568–572. doi: 10.1038/nchem.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.a) Rohde J-U, In J-H, Lim MH, Brennessel WW, Bukowski MR, Stubna A, Münck E, Nam W, Que L., Jr Science. 2003;299:1037–1039. doi: 10.1126/science.299.5609.1037. [DOI] [PubMed] [Google Scholar]; b) Fukuzumi S, Morimoto Y, Kotani H, Naumov P, Lee Y-M, Nam W. Nat. Chem. 2010;2:756–759. doi: 10.1038/nchem.731. [DOI] [PubMed] [Google Scholar]
- 11.Some examples of cobalt complexes with peroxo and hydroperoxo ligands: Busch DH, Alcock NW. Chem. Rev. 1994;94:585–623. Hikichi S, Akita M, Moro-oka Y. Coord. Chem. Rev. 2000;198:61–87. Hu X, Castro-Rodriguez I, Meyer K. J. Am. Chem. Soc. 2004;126:13464–13473. doi: 10.1021/ja046048k. Hikichi S, Komatsuzaki H, Akita M, Moro-oka Y. J. Am. Chem. Soc. 1998;120:4699–4710.
- 12.a) Evans DF. J. Chem. Soc. 1959:2003–2005. [Google Scholar]; b) Loliger J, Scheffold R. J. Chem. Educ. 1972;49:646–647. [Google Scholar]; c) Evans DF, Jakubovic DA. J. Chem. Soc. Dalton Trans. 1988:2927–2933. [Google Scholar]
- 13.a) Jensen KB, McKenzie CJ, Nielsen LP, Pedersen JZ, Svendsen HM. Chem. Commun. 1999:1313–1314. [Google Scholar]; b) Ho RYN, Roelfes G, Hermant R, Hage R, Feringa BL, Que L., Jr Chem. Commun. 1999:2161–2162. [Google Scholar]; c) Nebe T, Beitat A, Würtele C, Dücker-Benfer C, van Eldik R, McKenzie CJ, Schindler S. Dalton Trans. 2010;39:7768–7773. doi: 10.1039/c0dt00247j. [DOI] [PubMed] [Google Scholar]
- 14.A cobalt – oxo complex was reported recently: Pfaff FF, Kundu S, Risch M, Pandian S, Heims F, Pryjomska-Ray I, Haack P, Met-zinger R, Bill E, Dau H, Comba P, Ray K. Angew. Chem. 2011;123:1749–1753. doi: 10.1002/anie.201005869. Angew. Chem. Int. Ed.2011, 50, 1711–1715.
- 15.The involvement of metal–hydroperoxo complexes in oxidation reactions has been the topic of much interest: Franke A, Fertinger C, van Eldik R. Chem. Eur. J. 2012;18:6935–6949. doi: 10.1002/chem.201103036. Song YJ, Hyun MY, Lee JH, Lee HG, Kim JH, Jang SP, Noh JY, Kim Y, Kim S-J, Lee SJ, Kim C. Chem. Eur. J. 2012;18:6094–6101. doi: 10.1002/chem.201103916. Song YJ, Lee SH, Park HM, Kim SH, Goo HG, Eom GH, Lee JH, Lah MS, Kim Y, Kim S-J, Lee JE, Lee H-I, Kim C. Chem. Eur. J. 2011;17:7336–7344. doi: 10.1002/chem.201003202. Fertinger C, Hessenauer-Ilicheva N, Franke A, van Eldik R. Chem. Eur. J. 2009;15:13435–13440. doi: 10.1002/chem.200901804. Seo MS, Kamachi T, Kouno T, Murata K, Park MJ, Yoshizawa K, Nam W. Angew. Chem. 2007;119:2341–2344. doi: 10.1002/anie.200604219. Angew. Chem. Int. Ed.2007, 46, 2291 – 2294; Park MJ, Lee J, Suh Y, Kim J, Nam W. J. Am. Chem. Soc. 2006;128:2630–2634. doi: 10.1021/ja055709q. Ogliaro F, de Visser SP, Cohen S, Sharma PK, Shaik S. J. Am. Chem. Soc. 2002;124:2806–2817. doi: 10.1021/ja0171963. Shaik S, de Visser SP, Ogliaro F, Schwarz H, Schröder D. Curr. Opin. Chem. Biol. 2002;6:556–567. doi: 10.1016/s1367-5931(02)00363-0. Newcomb M, Shen R, Choi S-Y, Toy PH, Hollenberg PF, Vaz ADN, Coon MJ. J. Am. Chem. Soc. 2000;122:2677–2686. Liu LV, Hong S, Cho J, Nam W, Solomon EI. J. Am. Chem. Soc. 2013;135:3286–3299. doi: 10.1021/ja400183g. Kim YM, Cho K-B, Wang B, Li C, Shaik S, Na W. J. Am. Chem. Soc. 2013;135:8838–8841. doi: 10.1021/ja404152q.
- 16.a) Watanabe Y, Nakajima H, Ueno T. Acc. Chem. Res. 2007;40:554–562. doi: 10.1021/ar600046a. [DOI] [PubMed] [Google Scholar]; b) Groves JT, Watanab Y. J. Am. Chem. Soc. 1988;110:8443–8452. [Google Scholar]
- 17.Armarego WLF, Cha CLL. Purification of Laboratory Chemicals. 6th ed. Pergamon, Oxford: 2009. [Google Scholar]
- 18.Saltzman H, Sharefki JG. Organic Syntheses, Collect. Vol. V. Wiley: New York; 1973. p. 658. [Google Scholar]
- 19.Barefield EK, Wargne F. Inorg. Chem. 1973;12:2435–2439. [Google Scholar]
- 20.Sheldick GM. SHELXTL/PC, Version 6.12 for Windows XP. Madison, WI, USA: Bruker AXS Inc.; 2001. [Google Scholar]
- 21.Tenderholt A. Pyspline and QMForge. 2007 [Google Scholar]
- 22.Mustre de Leon J, Rehr JJ, Zabinsky SI, Albers RC. Phys. Rev. B: Condens. Matter. 1991;44:4146–4156. doi: 10.1103/physrevb.44.4146. [DOI] [PubMed] [Google Scholar]
- 23.Rehr JJ, Albers RC. Rev. Mod. Phys. 2000;72:621–624. [Google Scholar]
- 24.Rehr JJ, Mustre de Leon J, Zabinsky SI, Albers RC. J. Am. Chem. Soc. 1991;113:5135–5140. [Google Scholar]
- 25.Avogadro: an open-source molecular builder and visualization tool, Version 1.XX, http://avogadro.openmolecules.net/.
- 26.George GN. EXAFSPAK and EDG – FIT. 2000 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.