Abstract
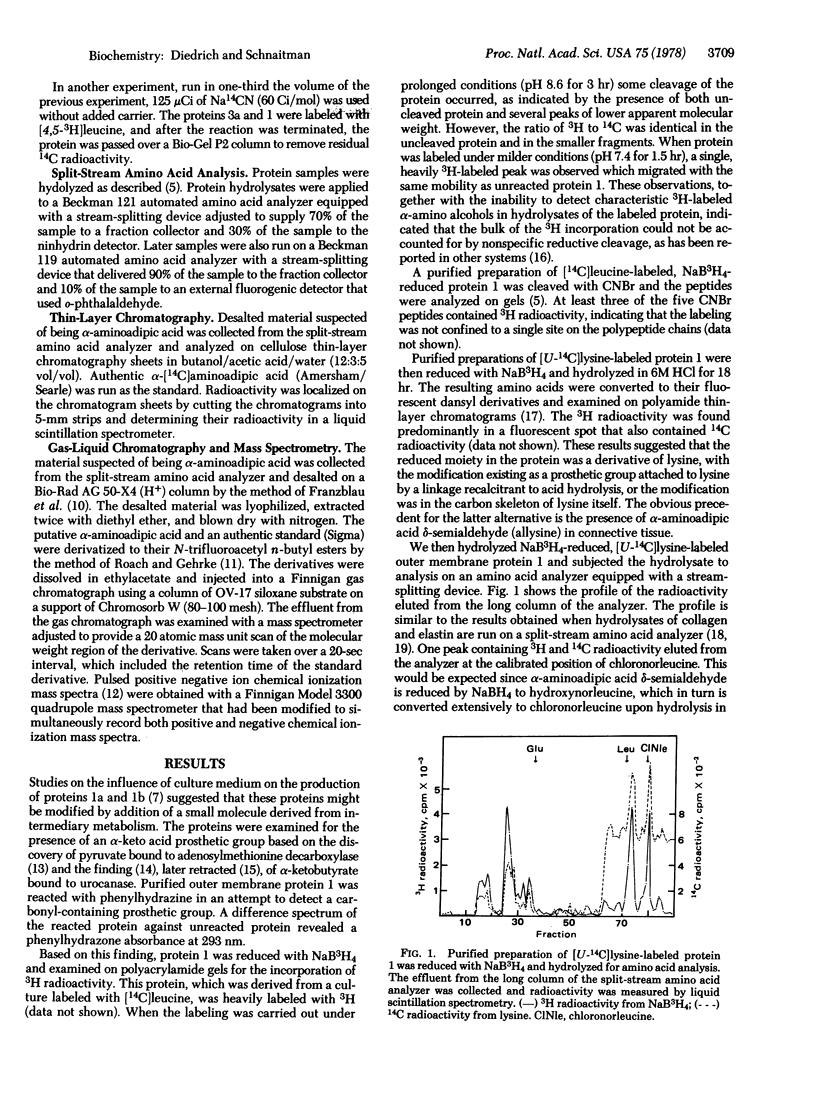
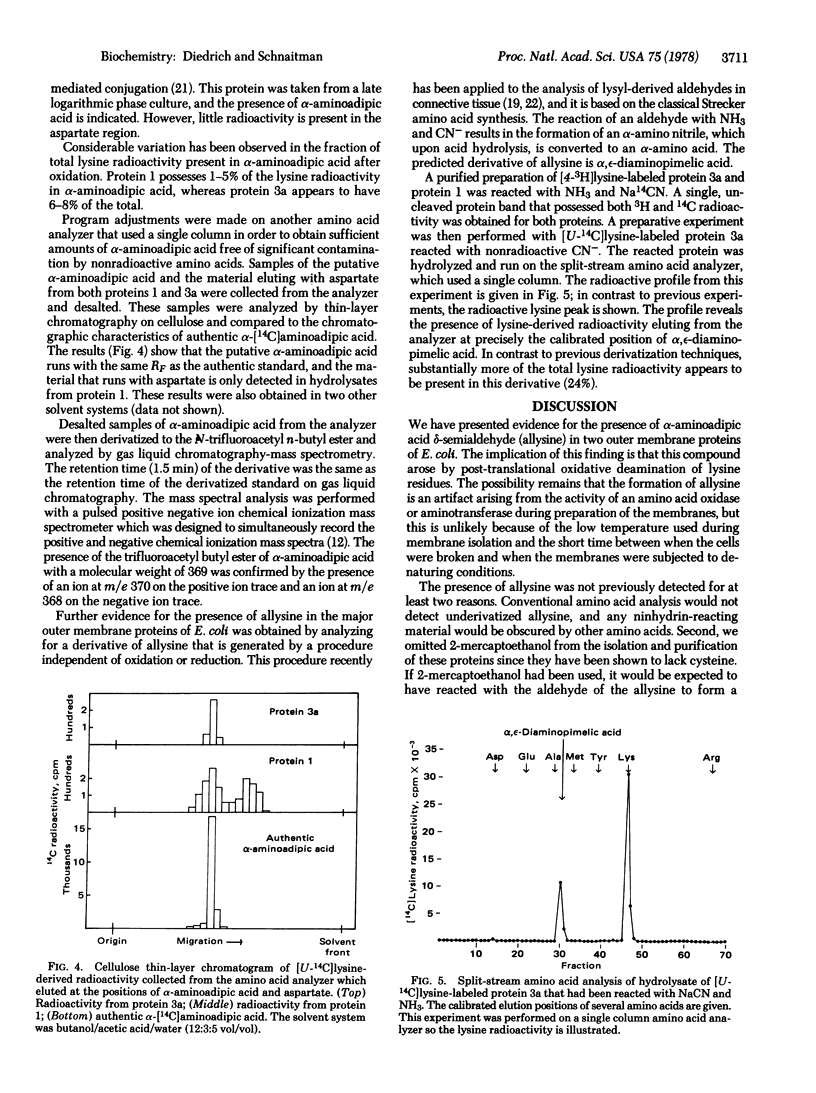
The major outer membrane proteins from Escherichia coli K-12 are modified to contain alpha-aminoadipic acid delta-semialdehyde (allysine). The allysine was found to be derived from lysine and it was identified by derivatizing it to chloronorleucine by reduction, alpha-aminoadipic acid by oxidation, and to alpha,epsilon-diaminopimelic acid by reacting it with CN- and NH3. The alpha-aminoadipic acid was identified by mass spectrometry. Two major outer membrane proteins were found to possess allysine, a modified lysine characteristically found to connective tissue.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bassford P. J., Jr, Diedrich D. L., Schnaitman C. L., Reeves P. Outer membrane proteins of Escherichia coli. VI. Protein alteration in bacteriophage-resistant mutants. J Bacteriol. 1977 Aug;131(2):608–622. doi: 10.1128/jb.131.2.608-622.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrich D. L., Summers A. O., Schnaitman C. A. Outer membrane proteins of Escherichia coli. V. Evidence that protein 1 and bacteriophage-directed protein 2 are different polypeptides. J Bacteriol. 1977 Aug;131(2):598–607. doi: 10.1128/jb.131.2.598-607.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan R. M., Phillips A. T. Presence of tightly bound NAD+ in urocanase of Pseudomonas putida. J Biol Chem. 1977 Aug 25;252(16):5701–5707. [PubMed] [Google Scholar]
- George D. J., Phillips A. T. Identification of alpha-ketobutyrate as the prosthetic group of urocanase from Pseudomonas putida. J Biol Chem. 1970 Feb 10;245(3):528–537. [PubMed] [Google Scholar]
- Kamio Y., Nikaido H. Outer membrane of Salmonella typhimurium: accessibility of phospholipid head groups to phospholipase c and cyanogen bromide activated dextran in the external medium. Biochemistry. 1976 Jun 15;15(12):2561–2570. doi: 10.1021/bi00657a012. [DOI] [PubMed] [Google Scholar]
- Lent R. W., Smith B., Salcedo L. L., Faris B., Franzblau C. Studies on the reduction of elastin. II. Evidence for the presence of alpha-aminoadipic acid delta-semialdehyde and its aldol condensation product. Biochemistry. 1969 Jul;8(7):2837–2845. doi: 10.1021/bi00835a022. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Miller E. J., Pinnell S. R., Martin G. R., Schiffmann E. Investigation of the nature of the intermediates involved in desmosine biosynthesis. Biochem Biophys Res Commun. 1967 Jan 23;26(2):132–137. doi: 10.1016/0006-291x(67)90224-0. [DOI] [PubMed] [Google Scholar]
- Mühlradt P. F., Golecki J. R. Asymmetrical distribution and artifactual reorientation of lipopolysaccharide in the outer membrane bilayer of Salmonella typhimurium. Eur J Biochem. 1975 Feb 21;51(2):343–352. doi: 10.1111/j.1432-1033.1975.tb03934.x. [DOI] [PubMed] [Google Scholar]
- Paz M. A., Henson E., Rombauer R., Abrash L., Blumenfeld O. O., Gallop P. M. Alpha-amino alcohols as products of a reductive side reaction of denatured collagen with sodium borohydride. Biochemistry. 1970 May 12;9(10):2123–2127. doi: 10.1021/bi00812a014. [DOI] [PubMed] [Google Scholar]
- Paz M. A., Keith D. A., Traverso H. P., Gallop P. M. Isolation, purification, and cross-linking profiles of elastin from lung and aorta. Biochemistry. 1976 Nov 2;15(22):4912–4918. doi: 10.1021/bi00667a025. [DOI] [PubMed] [Google Scholar]
- Pereyra B., Blumenfeld O. O., Paz M. A., Henson E., Gallop P. M. Maturation analysis in connective tissue proteins by (14C)cyanide incorporation. J Biol Chem. 1974 Apr 10;249(7):2212–2219. [PubMed] [Google Scholar]
- Rosenbusch J. P. Characterization of the major envelope protein from Escherichia coli. Regular arrangement on the peptidoglycan and unusual dodecyl sulfate binding. J Biol Chem. 1974 Dec 25;249(24):8019–8029. [PubMed] [Google Scholar]
- Schmitges C. J., Henning U. The major proteins of the Escherichia coli outer cell-envelope membrane. Heterogeneity of protein I. Eur J Biochem. 1976 Mar 16;63(1):47–52. doi: 10.1111/j.1432-1033.1976.tb10205.x. [DOI] [PubMed] [Google Scholar]
- Schnaitman C. A. Outer membrane proteins of Escherichia coli. 3. Evidence that the major protein of Escherichia coli O111 outer membrane consists of four distinct polypeptide species. J Bacteriol. 1974 May;118(2):442–453. doi: 10.1128/jb.118.2.442-453.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Outer membrane proteins of Escherichia coli. I. Effect of preparative conditions on the migration of protein in polyacrylamide gels. Arch Biochem Biophys. 1973 Aug;157(2):541–552. doi: 10.1016/0003-9861(73)90673-5. [DOI] [PubMed] [Google Scholar]
- Schnaitman C., Smith D., de Salsas M. F. Temperate Bacteriophage Which Causes the Production of a New Major Outer Membrane Protein by Escherichia coli. J Virol. 1975 May;15(5):1121–1130. doi: 10.1128/jvi.15.5.1121-1130.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skurray R. A., Hancock R. E., Reeves P. Con--mutants: class of mutants in Escherichia coli K-12 lacking a major cell wall protein and defective in conjugation and adsorption of a bacteriophage. J Bacteriol. 1974 Sep;119(3):726–735. doi: 10.1128/jb.119.3.726-735.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B., Tabor C. W., Tabor H. Purification of adenosylmethionine decarboxylase from Escherichia coli W: evidence for covalently bound pyruvate. J Biol Chem. 1970 Apr 25;245(8):2132–2139. [PubMed] [Google Scholar]


