Abstract
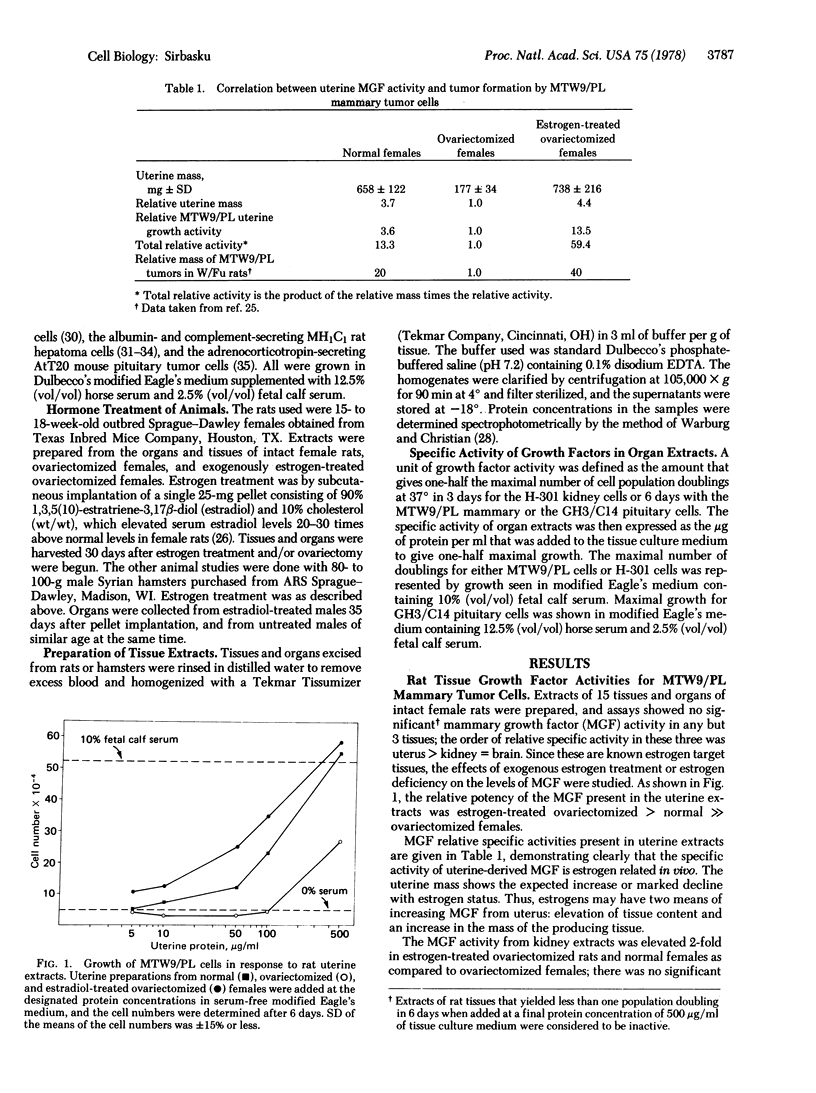
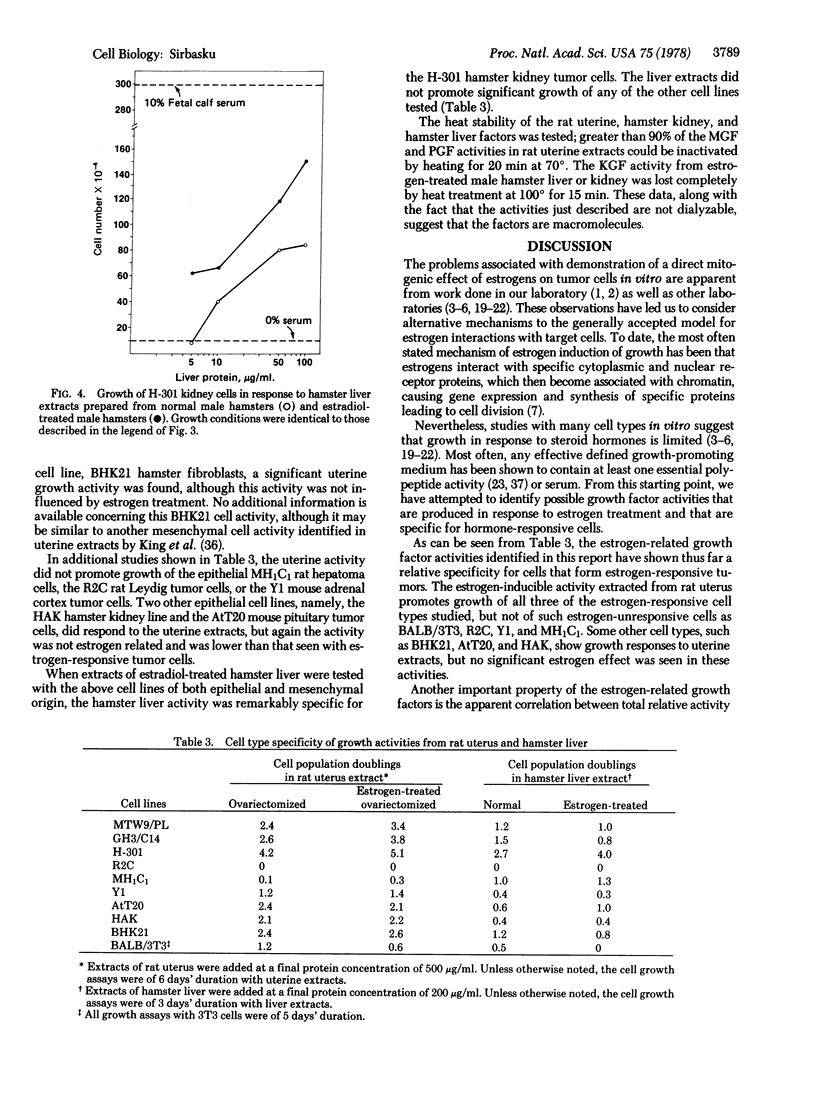
The problem of estrogen-promoted tumor cell growth has been studied extensively in an attempt to establish the direct mitogenic role of these steroid hormones. We have developed cell lines from three estrogen-responsive tumors or cell populations: the H-301 kidney tumor cells established from a parent estrogen-dependent hamster kidney tumor, the GH3/C14 rat pituitary tumor cell line established as a subline of the original GH3 population, and the MTW9/PL mammary cell line developed from a parent estrogen- and prolactin-responsive MT-W9A carcinogen-induced rat tumor. With all three of these cell lines, we have encountered a paradox: although estrogens are obligatory for tumor formation in vivo, no direct mitogenic effect of estrogens can be shown in culture when assayed by an increase in cell number. We have thus considered the possibility that estrogens may induce growth factors in vivo that are then responsible for tumor formation by the three cell lines described. Experiments presented in this report show that extracts of rodent uterus, kidney, or liver contain growth activity for these three tumor cell lines, that estrogen treatment causes an increase in tissue content of these activities, and that the estrogen-induced activities are specific for the estrogen-responsive cells. These studies suggest that estrogen-responsive tumor growth in vivo includes the mechanism of estrogen leads to uterus, kidney, or liver leads to specific growth factors leads to estrogen-responsive tumor cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALGARD F. T. Hormone-induced tumors. I. Hamster flankorgan and kidney tumors in vitro. J Natl Cancer Inst. 1960 Sep;25:557–571. [PubMed] [Google Scholar]
- BUFFETT R. F., CLIFTON K. H., FURTH J., GADSDEN E. L. Dependent and autonomous mammotropic pituitary tumors in rats; their somatotropic features. Cancer Res. 1956 Aug;16(7):608–616. [PubMed] [Google Scholar]
- BUONASSISI V., SATO G., COHEN A. I. Hormone-producing cultures of adrenal and pituitary tumor origin. Proc Natl Acad Sci U S A. 1962 Jul 15;48:1184–1190. doi: 10.1073/pnas.48.7.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan P. C., Tsuang J., Head J., Cohen L. A., Weisburger J. H. Effects of estradiol and prolactin on growth of rat mammary adenocarcinoma cells in monolayer cultures. Proc Soc Exp Biol Med. 1976 Feb;151(2):362–365. doi: 10.3181/00379727-151-39210. [DOI] [PubMed] [Google Scholar]
- Cohen L. A., Tsuang J., Chan P. C. Characteristics of rat normal mammary epithelial cells and dimethylbenzanthracene-induced mammary adenocarcinoma cells grown in monolayer culture. In Vitro. 1974 Jul-Aug;10:51–62. doi: 10.1007/BF02615338. [DOI] [PubMed] [Google Scholar]
- Hallowes R. C., Rudland P. S., Hawkins R. A., Lewis D. J., Bennet D., Durbin H. Comparison of the effects of hormones on DNA synthesis in cell cultures of nonneoplastic and neoplastic mammary epithelium from rats. Cancer Res. 1977 Aug;37(8 Pt 1):2492–2504. [PubMed] [Google Scholar]
- Hayashi I., Sato G. H. Replacement of serum by hormones permits growth of cells in a defined medium. Nature. 1976 Jan 15;259(5539):132–134. doi: 10.1038/259132a0. [DOI] [PubMed] [Google Scholar]
- Heuson J. C., Pasteels J. L., Legros N., Heuson-Stiennon J., Leclercq G. Estradiol-dependent collagenolytic enzyme activity in long-term organ culture of human breast cancer. Cancer Res. 1975 Aug;35(8):2039–2048. [PubMed] [Google Scholar]
- KIM U., FURTH J., YANNOPOULOS K. OBSERVATIONS ON HORMONAL CONTROL OF MAMMARY CANCER. I. ESTROGEN AND MAMMOTROPES. J Natl Cancer Inst. 1963 Aug;31:233–259. [PubMed] [Google Scholar]
- KIRKMAN H., ROBBINS M. Estrogen-induced tumors of the kidney. V. Histology and histogenesis in the Syrian hamster. Natl Cancer Inst Monogr. 1959 Dec;1:93–139. [PubMed] [Google Scholar]
- King R. J., Kaye A. M., Shodell M. J. Co-purification of an oestrogen-induced protein from rat uterus and a factor able to stimulate DNA synthesis in cultured cells. Exp Cell Res. 1977 Oct 1;109(1):1–8. doi: 10.1016/0014-4827(77)90037-4. [DOI] [PubMed] [Google Scholar]
- Kirkland W. L., Sorrentino J. M., Sirbasku D. A. Control of cell growth. III. Direct mitogenic effect of thyroid hormones on an estrogen-dependent rat pituitary tumor cell line. J Natl Cancer Inst. 1976 Jun;56(6):1159–1164. doi: 10.1093/jnci/56.6.1159. [DOI] [PubMed] [Google Scholar]
- Lippman M., Bolan G., Huff K. The effects of estrogens and antiestrogens on hormone-responsive human breast cancer in long-term tissue culture. Cancer Res. 1976 Dec;36(12):4595–4601. [PubMed] [Google Scholar]
- O'Malley B. W., Means A. R. Female steroid hormones and target cell nuclei. Science. 1974 Feb 15;183(4125):610–620. doi: 10.1126/science.183.4125.610. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D. Quantitation of parameters that determine the rate of ovalbumin synthesis. Cell. 1975 Mar;4(3):189–189. doi: 10.1016/0092-8674(75)90167-1. [DOI] [PubMed] [Google Scholar]
- Pasteels J. L., Heuson J. C., Heuson-Stiennon J., Legros N. Effects on insulin, prolactin, progesterone, and estradiol on DNA synthesis in organ culture of 7,12-dimethylbenz(a)anthracene-induced rat mammary tumors. Cancer Res. 1976 Jul;36(7 Pt 1):2162–2170. [PubMed] [Google Scholar]
- Redman L. W., Garland M. R., Thomson M., Ng T., Richards J. F. Nucleic acid metabolism of an estrogen-dependent carcinoma of the adrenal cortex of the rat. Cancer Res. 1971 Mar;31(3):265–269. [PubMed] [Google Scholar]
- Richardson U. I., Tashjian A. H., Jr, Levine L. Establishment of a clonal strain of hepatoma cells which secrete albumin. J Cell Biol. 1969 Jan;40(1):236–247. doi: 10.1083/jcb.40.1.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommel F. A., Goldlust M. B., Bancroft F. C., Mayer M. M., Tashjian A. H., Jr Synthesis of the ninth component of complement by a clonal strain of rat hepatoma cells. J Immunol. 1970 Aug;105(2):396–403. [PubMed] [Google Scholar]
- Rugstad H. E., Robinson S. H., Yannoni C., Tashjian A. H., Jr Transfer of bilirubin uridine diphosphate-glucuronyltransferase to enzyme-deficient rats. Science. 1970 Oct 30;170(3957):553–555. doi: 10.1126/science.170.3957.553. [DOI] [PubMed] [Google Scholar]
- Shin S. I., Yasumura Y., Sato G. H. Studies on interstitial cells in tissue culture. II. Steroid biosynthesis by a clonal line of rat testicular interstitial cells. Endocrinology. 1968 Mar;82(3):614–616. doi: 10.1210/endo-82-3-614. [DOI] [PubMed] [Google Scholar]
- Sirbasku D. A. Hormone-responsive growth in vivo of a tissue culture cell line established from the MT-W9A rat mammary tumor. Cancer Res. 1978 Apr;38(4):1154–1165. [PubMed] [Google Scholar]
- Sirbasku D. A., Kirkland W. L. Control of cell growth. IV. Growth properties of a new cell line established from an estrogen-dependent kidney tumor of the Syrian hamster. Endocrinology. 1976 May;98(5):1260–1272. doi: 10.1210/endo-98-5-1260. [DOI] [PubMed] [Google Scholar]
- Sorrentino J. M., Kirkland W. L., Sirbasku D. A. Control of cell growth. I. Estrogen-dependent growth in vivo of a rat pituitary tumor cell line. J Natl Cancer Inst. 1976 Jun;56(6):1149–1154. doi: 10.1093/jnci/56.6.1149. [DOI] [PubMed] [Google Scholar]
- Sorrentino J. M., Kirkland W. L., Sirbasku D. A. Control of cell growth. II. Requirement of thyroid hormones for the in vivo estrogen-dependent growth of rat pituitary tumor cells. J Natl Cancer Inst. 1976 Jun;56(6):1155–1158. doi: 10.1093/jnci/56.6.1155. [DOI] [PubMed] [Google Scholar]
- Tashjian A. H., Jr, Bancroft F. C., Richardson U. I., Goldlust M. B., Rommel F. A., Ofner P. Multiple differentiated functions in an unusual clonal strain of hepatoma cells. In Vitro. 1970 Jul-Aug;6(1):32–45. doi: 10.1007/BF02616132. [DOI] [PubMed] [Google Scholar]
- Welsch C. W., Rivera E. M. Differential effects of estrogen and prolactin on DNA synthesis in organ cultures of DMBA-induced rat mammary carcinoma. Proc Soc Exp Biol Med. 1972 Feb;139(2):623–626. doi: 10.3181/00379727-139-36201. [DOI] [PubMed] [Google Scholar]
- Yasumura Y., Buonassisi V., Sato G. Clonal analysis of differentiated function in animal cell cultures. I. Possible correlated maintenance of differentiated function and the diploid karyotype. Cancer Res. 1966 Mar;26(3):529–535. [PubMed] [Google Scholar]
- Zava D. T., Chamness G. C., Horwitz K. B., McGuire W. L. Human breast cancer: biologically active estrogen receptor in the absence of estrogen? Science. 1977 May 6;196(4290):663–664. doi: 10.1126/science.193182. [DOI] [PubMed] [Google Scholar]


