Abstract
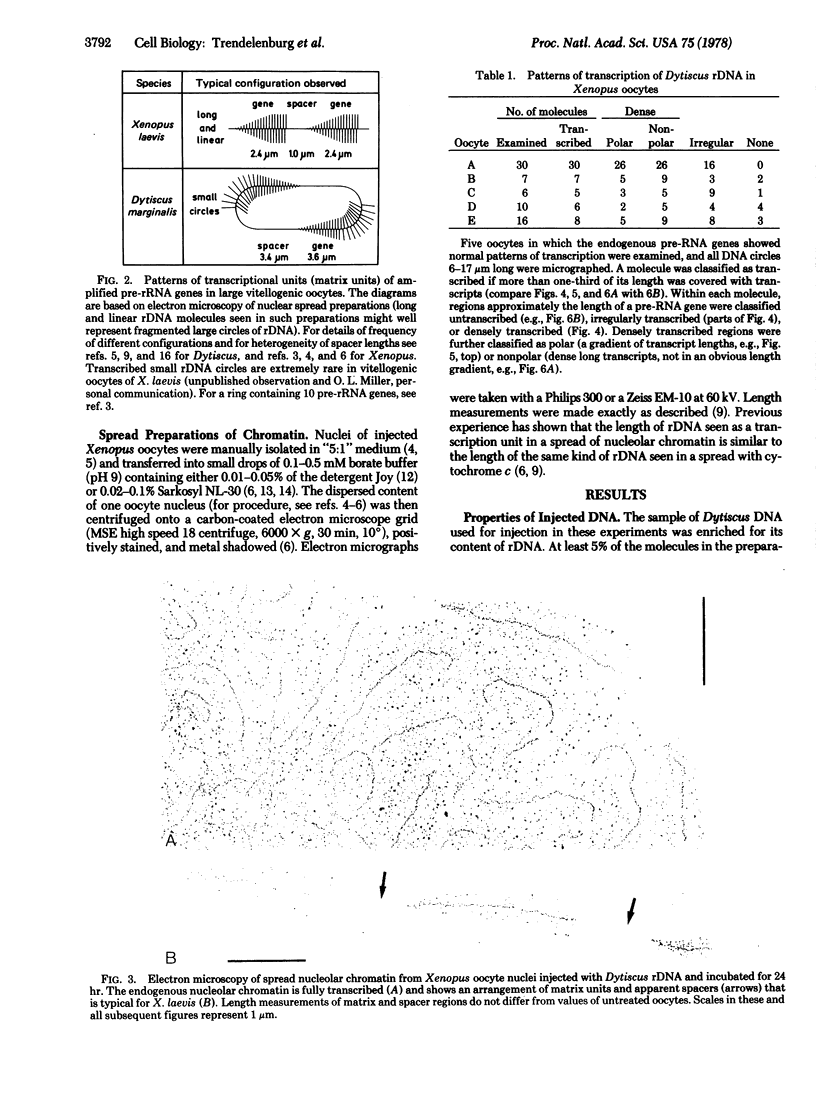
Oocytes of the frog Xenopus were injected with purified circular DNA containing amplified rRNA genes of the water beetle Dytiscus. Nuclear contents of injected oocytes were spread and examined by electron microscopy. Most of the Dytiscus DNA seen in injected nuclei contained regions indensely packed with polymerases and nascent transcripts. Apparently normal, as well as abnormal, patterns of transcription were observed. By this type of experiment, it may become possible to recognize the transcribed regions and immediate transcripts of cloned DNA molecules whose activity cannot be seen by electron microscopy of normal nuclei.
Full text
PDF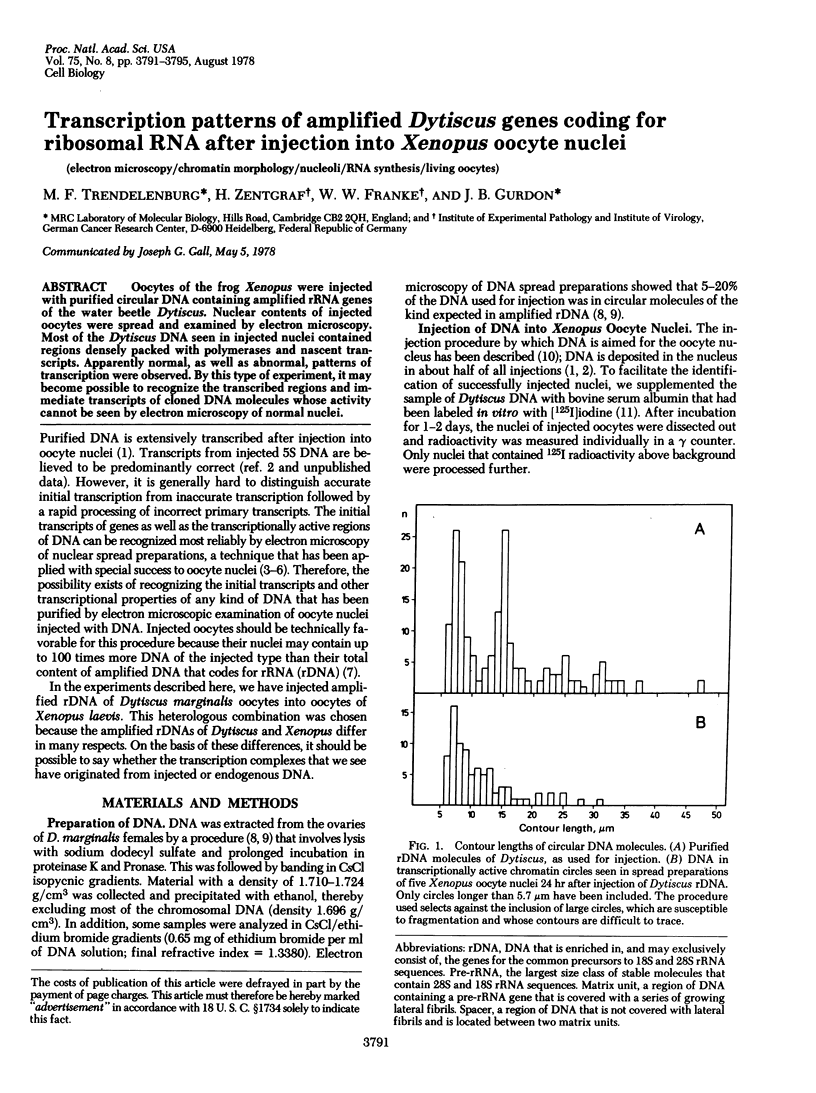



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson E. D., Woodland H. R. Histone synthesis in early amphibian development: histone and DNA syntheses are not co-ordinated. J Mol Biol. 1974 Sep 15;88(2):263–285. doi: 10.1016/0022-2836(74)90481-1. [DOI] [PubMed] [Google Scholar]
- Brown D. D., Gurdon J. B. High-fidelity transcription of 5S DNA injected into Xenopus oocytes. Proc Natl Acad Sci U S A. 1977 May;74(5):2064–2068. doi: 10.1073/pnas.74.5.2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke W. W., Scheer U., Spring H., Trendelenburg M. F., Krohne G. Morphology of transcriptional units of rDNA. Evidence for transcription in apparent spacer intercepts and cleavages in the elongating nascent RNA. Exp Cell Res. 1976 Jul;100(2):233–244. doi: 10.1016/0014-4827(76)90143-9. [DOI] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall J. G., Rochaix J. D. The amplified ribosomal DNA of dytiscid beetles. Proc Natl Acad Sci U S A. 1974 May;71(5):1819–1823. doi: 10.1073/pnas.71.5.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurdon J. B. Injected nuclei in frog oocytes: fate, enlargement, and chromatin dispersal. J Embryol Exp Morphol. 1976 Dec;36(3):523–540. [PubMed] [Google Scholar]
- Gurdon J. B. The croonian lecture, 1976. Egg cytoplasm and gene control in development. Proc R Soc Lond B Biol Sci. 1977 Sep 5;198(1132):211–247. doi: 10.1098/rspb.1977.0095. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D., Morris N. R. Assembly of SV40 chromatin in a cell-free system from Xenopus eggs. Cell. 1977 Feb;10(2):237–243. doi: 10.1016/0092-8674(77)90217-3. [DOI] [PubMed] [Google Scholar]
- Mertz J. E., Gurdon J. B. Purified DNAs are transcribed after microinjection into Xenopus oocytes. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1502–1506. doi: 10.1073/pnas.74.4.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller O. L., Jr, Bakken A. H. Morphological studies of transcription. Acta Endocrinol Suppl (Copenh) 1972;168:155–177. doi: 10.1530/acta.0.071s155. [DOI] [PubMed] [Google Scholar]
- Roeder R. G. Multiple forms of deoxyribonucleic acid-dependent ribonucleic acid polymerase in Xenopus laevis. Levels of activity during oocyte and embryonic development. J Biol Chem. 1974 Jan 10;249(1):249–256. [PubMed] [Google Scholar]
- Scheer U. Changes of nucleosome frequency in nucleolar and non-nucleolar chromatin as a function of transcription: an electron microscopic study. Cell. 1978 Mar;13(3):535–549. doi: 10.1016/0092-8674(78)90327-6. [DOI] [PubMed] [Google Scholar]
- Scheer U., Trendelenburg F., Franke W. W. Effects of actinomycin D on the association of newly formed ribonucleoproteins with the cistrons of ribosomal RNA in Triturus oocytes. J Cell Biol. 1975 Apr;65(1):163–179. doi: 10.1083/jcb.65.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer U., Trendelenburg M. F., Franke W. W. Regulation of transcription of genes of ribosomal rna during amphibian oogenesis. A biochemical and morphological study. J Cell Biol. 1976 May;69(2):465–489. doi: 10.1083/jcb.69.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer U., Trendelenburg M. F., Franke W. W. Transcription of ribosomal RNA cistrons. Correlation of morphological and biochemical data. Exp Cell Res. 1973 Jul;80(1):175–190. doi: 10.1016/0014-4827(73)90289-9. [DOI] [PubMed] [Google Scholar]
- Scheer U., Trendelenburg M. F., Krohne G., Franke W. W. Lengths and patterns of transcriptional units in the amplified nucleoli of oocytes of Xenopus laevis. Chromosoma. 1977 Mar 16;60(2):147–167. doi: 10.1007/BF00288462. [DOI] [PubMed] [Google Scholar]
- Stanfield S., Helinski D. R. Small circular DNA in Drosophila melanogaster. Cell. 1976 Oct;9(2):333–345. doi: 10.1016/0092-8674(76)90123-9. [DOI] [PubMed] [Google Scholar]
- Trendelenburg M. F., Franke W. W., Scheer U. Frequencies of circular units of nucleolar DNA in oocytes of two insects, Acheta domesticus and dytiscus marginalis, and changes of nucleolar morphology during oogenesis. Differentiation. 1977;7(3):133–158. doi: 10.1111/j.1432-0436.1977.tb01506.x. [DOI] [PubMed] [Google Scholar]
- Trendelenburg M. F. Morphology of ribosomal RNA cistrons in oocytes of the water beetle, Dytiscus marginalis L. Chromosoma. 1974;48(2):119–135. doi: 10.1007/BF00283959. [DOI] [PubMed] [Google Scholar]
- Trendelenburg M. F., Scheer U., Franke W. W. Structural organization of the transcription of ribosomal DNA in oocytes of the house cricket. Nat New Biol. 1973 Oct 10;245(145):167–170. doi: 10.1038/newbio245167a0. [DOI] [PubMed] [Google Scholar]
- Trendelenburg M. F., Scheer U., Zentgraf H., Franke W. W. Heterogeneity of spacer lengths in circles of amplified ribosomal DNA of two insect species, Dytiscus marginalis and Acheta domesticus. J Mol Biol. 1976 Dec;108(2):453–470. doi: 10.1016/s0022-2836(76)80130-1. [DOI] [PubMed] [Google Scholar]






