Abstract
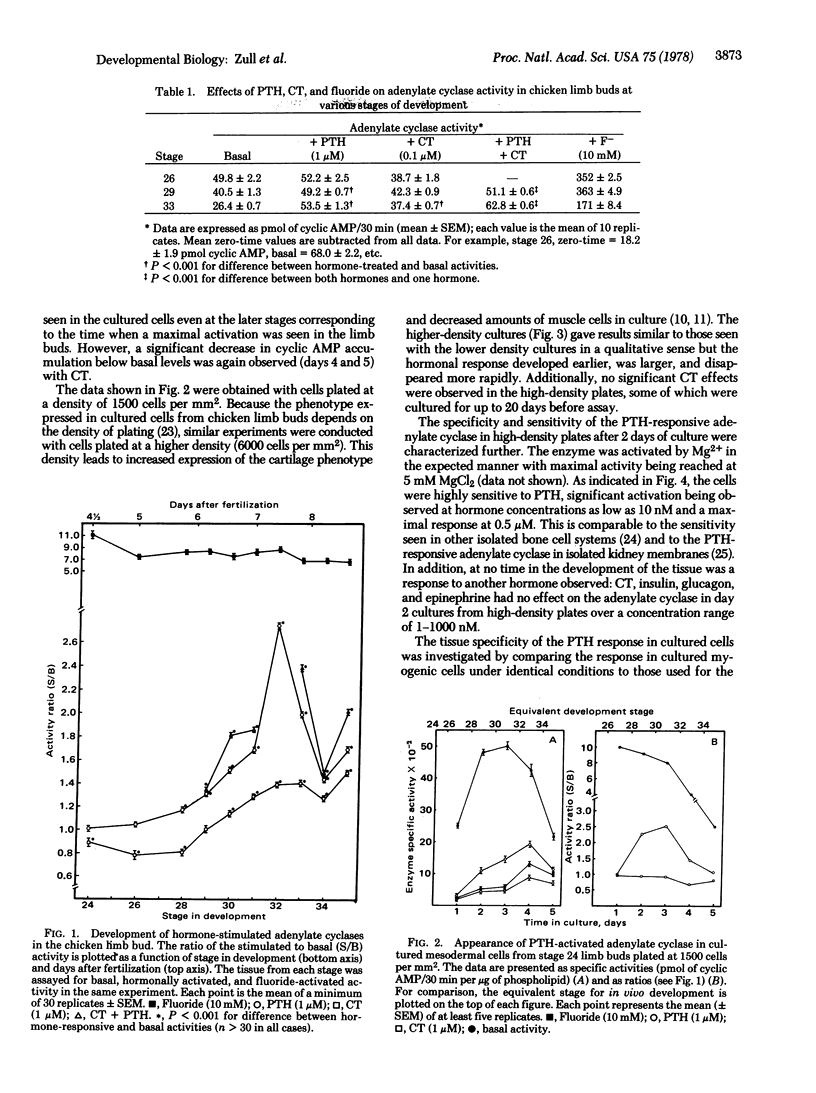
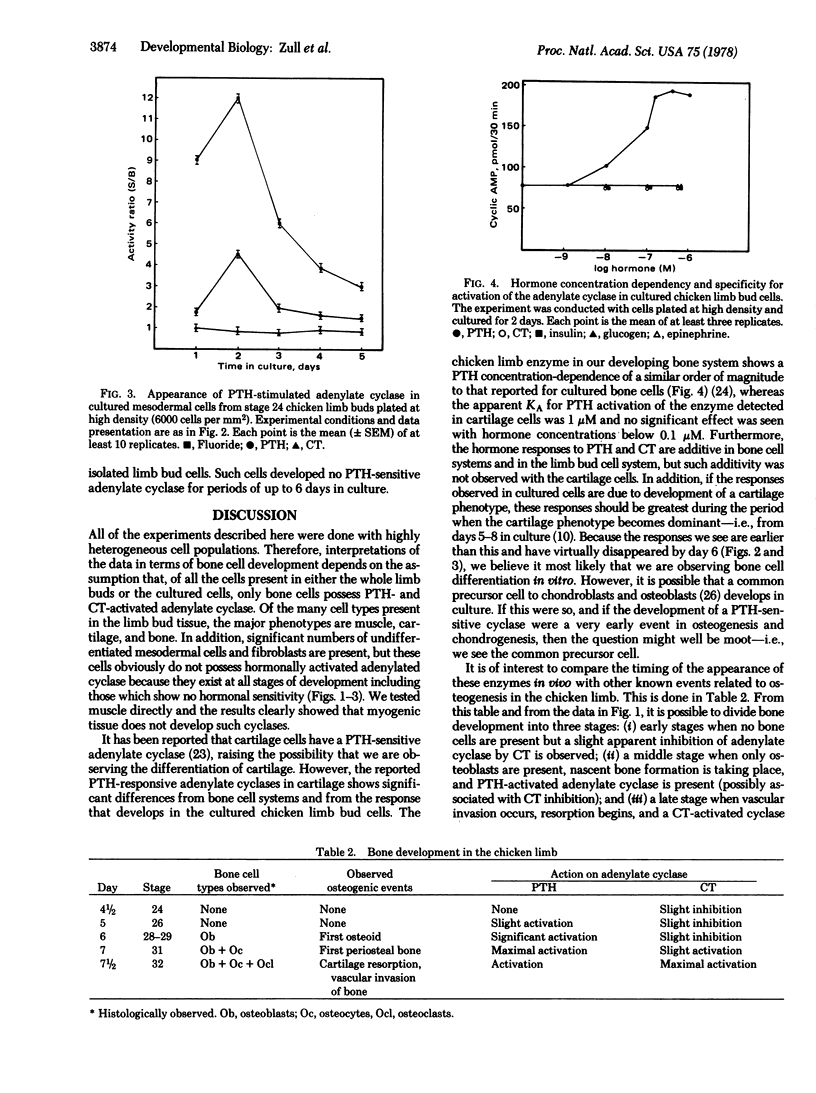
Activation of adenylate cyclase [ATP pyrophosphate-lyase (cyclizing), EC 4.6.1.1] by parathyroid hormone (PTH) and calcitonin was measured as a function of stage of development in embryonic chicken limb buds. Responsiveness to both hormones develops in the tissue at the time when nascent bone is forming. In addition, a temporal sequence of development of hormone response was observed, with a PTH-activated adenylate cyclase appearing earlier than the calcitonin-activated enzyme. The responsiveness to the two hormones was additive, indicating the presence of two receptor populations. Undifferentiated cells obtained from limb buds prior to appearance of hormonal responsiveness were cultured and were found to develop a PTH-activated adenylate cyclase in vitro. However, a calcitonin-stimulated enzyme did not appear in such cultures. The PTH-activated enzyme was found to be similar to that present in bone in regard to its sensitivity to PTH. The enzyme did not respond to other hormones, and myoblast cultures did not develop a PTH-activated adenylate cyclase, indicating that a true bone adenylate cyclase was being measured.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caplan A. I. Effects of the nicotinamide-sensitive teratogen3-acetylpyridine on chick limb cells in culture. Exp Cell Res. 1970 Oct;62(2):341–355. doi: 10.1016/0014-4827(70)90564-1. [DOI] [PubMed] [Google Scholar]
- Caplan A. I., Zwilling E., Kaplan N. O. 3-acetylpyridine: effects in vitro related to teratogenic activity in chicken embryos. Science. 1968 May 31;160(3831):1009–1010. doi: 10.1126/science.160.3831.1009. [DOI] [PubMed] [Google Scholar]
- Dedman J. R., Jackson R. L., Schreiber W. E., Means A. R. Sequence homology of the Ca2+-dependent regulator of cyclic nucleotide phosphodiesterase from rat testis with other Ca2+-binding proteins. J Biol Chem. 1978 Jan 25;253(2):343–346. [PubMed] [Google Scholar]
- JACKSON S. F. The fine structure of developing bone in the embryonic fowl. Proc R Soc Lond B Biol Sci. 1956 Mar 26;146(923):270–280. doi: 10.1098/rspb.1957.0010. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Luben R. A., Wong G. L., Cohn D. V. Biochemical characterization with parathormone and calcitonin of isolated bone cells: provisional identification of osteoclasts and osteoblasts. Endocrinology. 1976 Aug;99(2):526–534. doi: 10.1210/endo-99-2-526. [DOI] [PubMed] [Google Scholar]
- Luben R. A., Wong G. L., Cohn D. V. Parathormone-stimulated resorption of devitalised bone by cultured osteoclast-type bone cells. Nature. 1977 Feb 17;265(5595):629–630. doi: 10.1038/265629a0. [DOI] [PubMed] [Google Scholar]
- Marcus R., Aurbach G. D. Adenyl cyclase from renal cortex. Biochim Biophys Acta. 1971 Aug 20;242(2):410–421. doi: 10.1016/0005-2744(71)90232-4. [DOI] [PubMed] [Google Scholar]
- Osdoby P., Caplan A. I. The possible differentiation of osteogenic elements in vitro from chick limb mesodermal cells. I. Morphological evidence. Dev Biol. 1976 Sep;52(2):283–299. doi: 10.1016/0012-1606(76)90246-3. [DOI] [PubMed] [Google Scholar]
- Peck W. A., Burks J. K., Wilkins J., Rodan S. B., Rodan G. A. Evidence for preferential effects of parathyroid hormone, calcitonin and adenosine on bone and periosteum. Endocrinology. 1977 May;100(5):1357–1364. doi: 10.1210/endo-100-5-1357. [DOI] [PubMed] [Google Scholar]
- Peck W. A., Carpenter J., Messinger K., DeBra D. Cyclic 3'5'-adenosine monophosphate in isolated bone cells. Response to low concentrations of parathyroid hormone. Endocrinology. 1973 Mar;92(3):692–697. doi: 10.1210/endo-92-3-692. [DOI] [PubMed] [Google Scholar]
- Ramachandran J., Lee V. Divergent effects of o-nitrophenyl sulfenyl ACTH on rat and rabbit fat cell adenyl cyclases. Biochem Biophys Res Commun. 1970 Oct 23;41(2):358–366. doi: 10.1016/0006-291x(70)90512-7. [DOI] [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Searls R. L., Janners M. Y. The stabilization of cartilage properties in the cartilage-forming mesenchyme of the embryonic chick limb. J Exp Zool. 1969 Mar;170(3):365–375. doi: 10.1002/jez.1401700313. [DOI] [PubMed] [Google Scholar]
- Smith D. M., Roth L. M., Johnston C. C., Jr Hormonal responsiveness of adenylate cyclase activity in cartilage. Endocrinology. 1976 Jan;98(1):242–246. doi: 10.1210/endo-98-1-242. [DOI] [PubMed] [Google Scholar]
- Walker D. G. Bone resorption restored in osteopetrotic mice by transplants of normal bone marrow and spleen cells. Science. 1975 Nov 21;190(4216):784–785. doi: 10.1126/science.1105786. [DOI] [PubMed] [Google Scholar]
- Wong G., Cohn D. V. Separation of parathyroid hormone and calcitonin-sensitive cells from non-responsive bone cells. Nature. 1974 Dec 20;252(5485):713–715. doi: 10.1038/252713a0. [DOI] [PubMed] [Google Scholar]
- Zull J. E., Chuang J. Further studies on acetamidination as a technique for preparation of a biologically valid 3-H-labeled tracer for parathyroid hormone. J Biol Chem. 1975 Mar 10;250(5):1668–1675. [PubMed] [Google Scholar]
- Zull J. E., Malbon C. C., Chuang J. Binding of tritiated bovine parathyroid hormone to plasma membranes from bovine kidney cortex. J Biol Chem. 1977 Feb 10;252(3):1071–1078. [PubMed] [Google Scholar]


