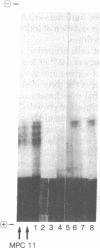
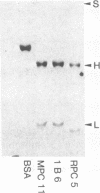
Abstract
We describe a procedure for the isolation of somatic cell variants in which gene products are expressed on the cell surface that are not expressed in the wild type. Cloned cells of the myeloma line MPC 11, which expresses an IgG2b protein, were incubated with an antiserum specific for IgGI and IgG2a. Cells reacting with this antiserum were stained with a fluorescent anti-antiserum and enriched in three cycles of sorting in the fluorescence-activated cell sorter and subsequent growth in vitro. From the enriched population two variants were isolated by cloning in soft agar. One of them expressed a variant immunoglobulin that types serologically as an IgG2a but whose variable portion was idiotypically related to that of the MPC 11 wild-type protein.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASKONAS B. A., FAHEY J. L. An investigation of closely related gamma-myeloma proteins and normal mouse gamma-globbulin by partial enzymic degradation and starch-gel electrophoresis. Nature. 1961 Jun 10;190:980–983. doi: 10.1038/190980a0. [DOI] [PubMed] [Google Scholar]
- Avrameas S., Ternynck T. The cross-linking of proteins with glutaraldehyde and its use for the preparation of immunoadsorbents. Immunochemistry. 1969 Jan;6(1):53–66. doi: 10.1016/0019-2791(69)90178-5. [DOI] [PubMed] [Google Scholar]
- Awdeh Z. L., Williamson A. R., Askonas B. A. Isoelectric focusing in polyacrylamide gel and its application to immunoglobulins. Nature. 1968 Jul 6;219(5149):66–67. doi: 10.1038/219066a0. [DOI] [PubMed] [Google Scholar]
- Bonner W. A., Hulett H. R., Sweet R. G., Herzenberg L. A. Fluorescence activated cell sorting. Rev Sci Instrum. 1972 Mar;43(3):404–409. doi: 10.1063/1.1685647. [DOI] [PubMed] [Google Scholar]
- Cancro M., Potter M. The requirement of an adherent cell substratum for the growth of developing plasmacytoma cells in vivo. J Exp Med. 1976 Dec 1;144(6):1554–1567. doi: 10.1084/jem.144.6.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffino P., Scharff M. D. Rate of somatic mutation in immunoglobulin production by mouse myeloma cells. Proc Natl Acad Sci U S A. 1971 Jan;68(1):219–223. doi: 10.1073/pnas.68.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichmann K., Kindt T. J. The inheritance of individual antigenic specificities of rabbit antibodies to streptococcal carbohydrates. J Exp Med. 1971 Aug 1;134(2):532–552. doi: 10.1084/jem.134.2.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henney C. S., Ishizaka K. A simplified procedure for the preparation of immunoglobulin-class-specific antisera. J Immunol. 1969 Jul;103(1):56–61. [PubMed] [Google Scholar]
- Jack R. S., Imanishi-Kari T., Rajewsky K. Idiotypic analysis of the response of C57BL/6 mice to the (4-hydroxy-3-nitrophenyl)acetyl group. Eur J Immunol. 1977 Aug;7(8):559–565. doi: 10.1002/eji.1830070813. [DOI] [PubMed] [Google Scholar]
- Koskimies S., Birshtein B. K. Primary and secondary variants in immunoglobulin heavy chain production. Nature. 1976 Dec 2;264(5585):480–482. doi: 10.1038/264480a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Loken M. R., Herzenber L. A. Analysis of cell populations with a fluorescence-activated cell sorter. Ann N Y Acad Sci. 1975 Jun 30;254:163–171. doi: 10.1111/j.1749-6632.1975.tb29166.x. [DOI] [PubMed] [Google Scholar]
- Morrison S. L., Baumal R., Birshtein B. K., Kuehl U. M., Preud'homme J. L., Frank L., Jasek T., Scharff M. D. The identification of mouse myeloma cells which have undergone mutations in immunoglobulin production. Soc Gen Physiol Ser. 1974;29:233–244. [PubMed] [Google Scholar]
- Preud'Homme J. L., Birshtein B. K., Scharff M. D. Variants of a mouse myeloma cell line that synthesize immunoglobulin heavy chains having an altered serotype. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1427–1430. doi: 10.1073/pnas.72.4.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seabright M. The use of proteolytic enzymes for the mapping of structural rearrangements in the chromosomes of man. Chromosoma. 1972;36(2):204–210. doi: 10.1007/BF00285214. [DOI] [PubMed] [Google Scholar]
- Secher D. S., Milstein C., Adetugbo K. Somatic mutants and antibody diversity. Immunol Rev. 1977;36:51–72. doi: 10.1111/j.1600-065x.1977.tb00382.x. [DOI] [PubMed] [Google Scholar]