Abstract
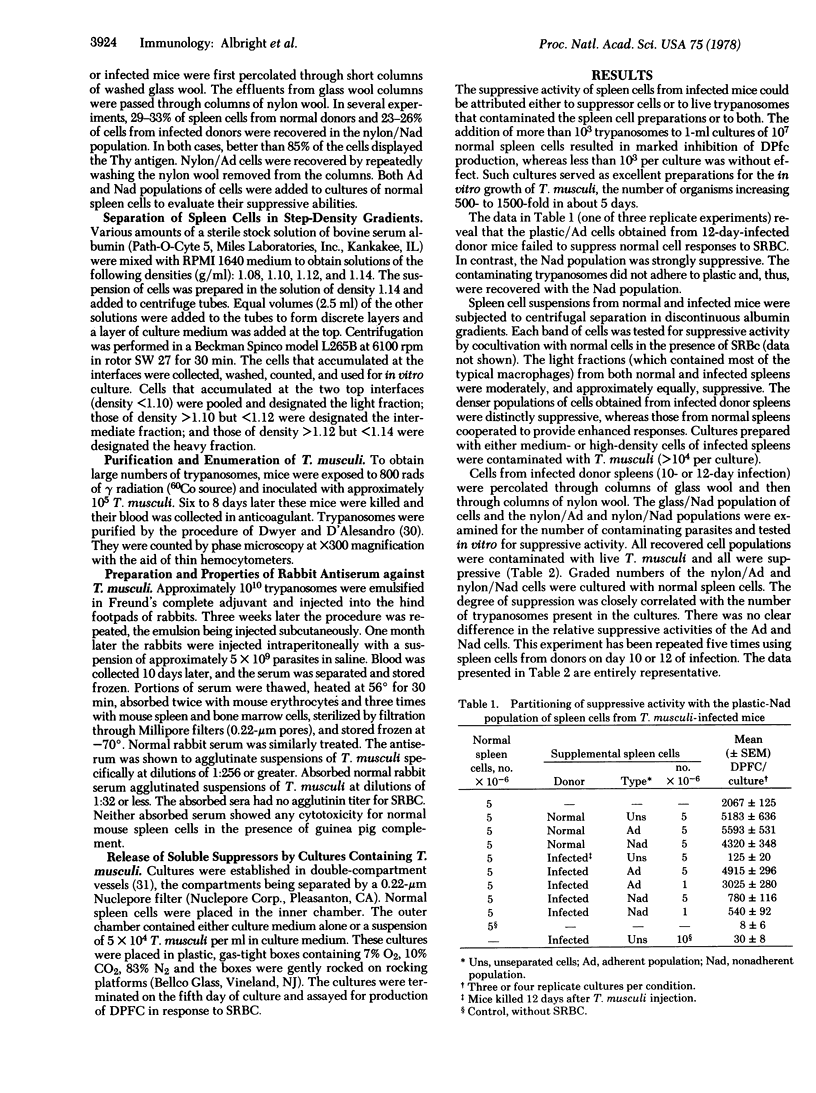
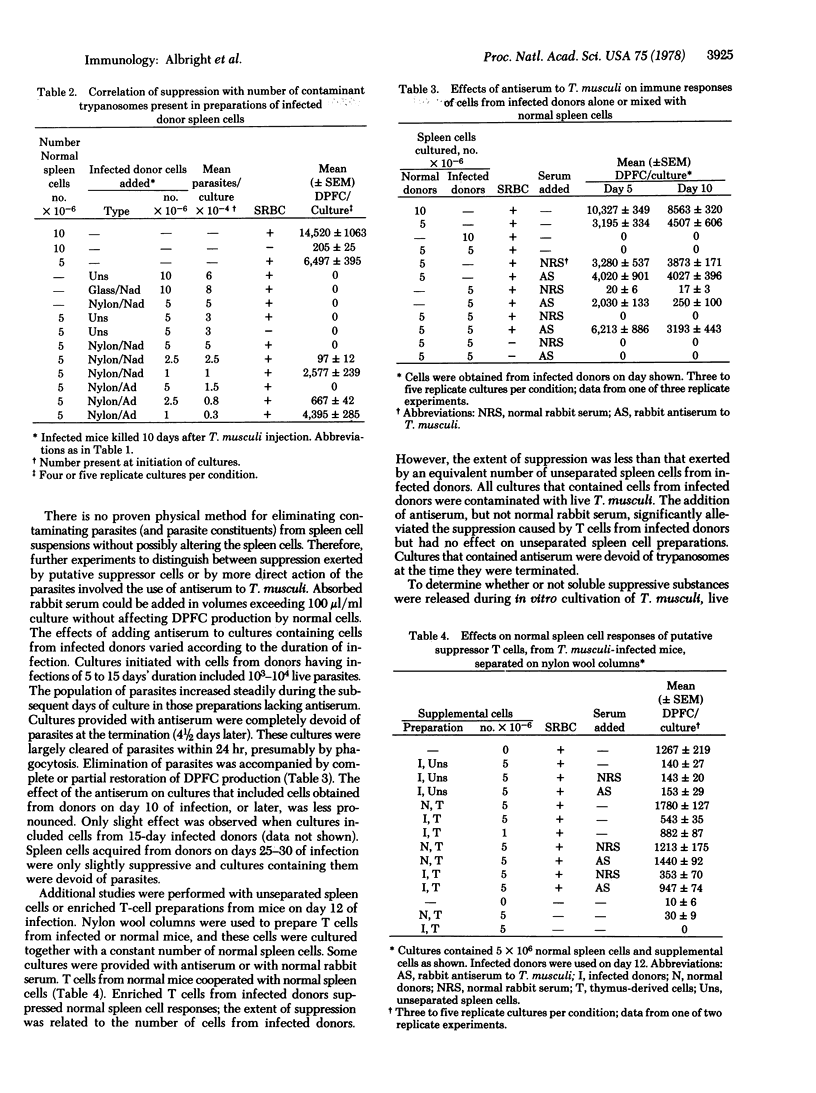
Spleen cells from Trypanosoma musculi-infected mice were unable to respond to sheep erythrocyte antigen in vitro; moreover, they suppressed the responses of normal spleen cell cultures in dose-dependent fashion. Suppression was maximal with spleen cells obtained during maximal parasitemia and waned as the donors recovered from infection. A macrophage-enriched population of spleen cells from infected mice, prepared either by adherence to plastic or by density gradient centrifugation, was not contaminated by the parasites and was not suppressive, whereas the plastic-nonadherent population was both contaminated and suppressive. Adherent and nonadherent cells of infected mice, separated by use of nylon wool columns, were almost equally suppressive and equally contaminated by trypanosomes. Addition of specific rabbit antiserum against T. musculi to cultures containing cells from infected mice eliminated contaminating parasites and alleviated suppression exerted by cells obtained from mice early during the course of infection but not after 12-15 days of infection. The suppression exerted by enriched T-cells obtained from mice on day 12 of infection was largely alleviated by use of the antiserum. A large portion of cells obtained on day 14 of infection could be killed by the antiserum and complement. It appeared that soluble substances derived from the parasites acted directly on B lymphocytes or essential assistant cells rather than by activating suppressor T cells or macrophages.
Keywords: suppressor T cells, macrophages, in vitro culture
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albright J. F., Albright J. W., Dusanic D. G. Trypanosome-induced splenomegaly and suppression of mouse spleen cell responses to antigen and mitogens. J Reticuloendothel Soc. 1977 Jan;21(1):21–31. [PubMed] [Google Scholar]
- Albright J. F., Deitchman J. W., Hassell S. A., Ozato K. Differential antibody production of adherent and nonadherent spleen cells transferred to irradiated and cyclophosphamide-treated recipient mice. J Reticuloendothel Soc. 1975 Apr;17(4):195–209. [PubMed] [Google Scholar]
- Barriga O. O. Selective immunodepression in mice by Trichinella spiralis extracts and infections. Cell Immunol. 1975 May;17(1):306–309. doi: 10.1016/s0008-8749(75)80031-1. [DOI] [PubMed] [Google Scholar]
- Clinton B. A., Ortiz-Ortiz L., Garcia W., Martinez T., Capin R. Trypanosoma cruzi: early immune responses in infected mice. Exp Parasitol. 1975 Jun;37(3):417–425. doi: 10.1016/0014-4894(75)90012-0. [DOI] [PubMed] [Google Scholar]
- Corsini A. C., Clayton C., Askonas B. A., Ogilvie B. M. Suppressor cells and loss of B-cell potential in mice infected with Trypanosoma brucei. Clin Exp Immunol. 1977 Jul;29(1):122–131. [PMC free article] [PubMed] [Google Scholar]
- Coulis P. A., Lewert R. M., Fitch F. W. [Splenic suppressor cells and cell-mediated cytotoxicity in murine schistosomiasis]. J Immunol. 1978 Jan;120(1):58–60. [PubMed] [Google Scholar]
- Dessaint J. P., Camus D., Fischer E., Capron A. Inhibition of lymphocyte proliferation by factor(s) produced by Schistosoma mansoni. Eur J Immunol. 1977 Sep;7(9):624–629. doi: 10.1002/eji.1830070909. [DOI] [PubMed] [Google Scholar]
- Dusanic D. G. Trypanosoma musculi infections in complement-deficient mice. Exp Parasitol. 1975 Apr;37(2):205–210. doi: 10.1016/0014-4894(75)90071-5. [DOI] [PubMed] [Google Scholar]
- Dwyer D. M., D'alesandro P. A. The cell surface of Trypanosoma musculi bloodstream forms. I. Fine structure and cytochemistry. J Protozool. 1976 Feb;23(1):75–83. doi: 10.1111/j.1550-7408.1976.tb05248.x. [DOI] [PubMed] [Google Scholar]
- Eardley D. D., Jayawardena A. N. Suppressor cells in mice infected with Trypanosoma brucei. J Immunol. 1977 Sep;119(3):1029–1033. [PubMed] [Google Scholar]
- Greenwood B. M., Brown J. C., De Jesus D. G., Holborow E. J. Immunosuppression in murine malaria. II. The effect on reticulo-endothelial and germinal centre function. Clin Exp Immunol. 1971 Sep;9(3):345–354. [PMC free article] [PubMed] [Google Scholar]
- Greenwood B. M., Playfair J. H., Torrigiani G. Immunosuppression in murine malaria. I. General characteristics. Clin Exp Immunol. 1971 Mar;8(3):467–478. [PMC free article] [PubMed] [Google Scholar]
- Hudson K. M., Byner C., Freeman J., Terry R. J. Immunodepression, high IgM levels and evasion of the immune response in murine trypanosomiasis. Nature. 1976 Nov 18;264(5583):256–258. doi: 10.1038/264256a0. [DOI] [PubMed] [Google Scholar]
- JERNE N. K., NORDIN A. A. Plaque formation in agar by single antibody-producing cells. Science. 1963 Apr 26;140(3565):405–405. [PubMed] [Google Scholar]
- Jayawardena A. N., Waksman B. H. Suppressor cells in experimentally trypanosomiasis. Nature. 1977 Feb 10;265(5594):539–541. doi: 10.1038/265539a0. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kirchner H., Holden H. T., Herberman Splenic suppressor macrophages induced in mice by injection of Corynebacterium parvum. J Immunol. 1975 Nov;115(5):1212–1216. [PubMed] [Google Scholar]
- Kirchner H., Muchmore A. V., Chused T. M., Holden H. T., Herberman R. B. Inhibition of proliferation of lymphoma cells and T lymphocytes by suppressor cells from spleens of tumor-bearing mice. J Immunol. 1975 Jan;114(1 Pt 1):206–210. [PubMed] [Google Scholar]
- Loose L. D., di Luzio N. R. A temporal relationship between reticuloendothelial system phagocytic alterations and antibody responses in mice infected with Plasmodium berghei (NYU-2 strain). Am J Trop Med Hyg. 1976 Mar;25(2):221–228. doi: 10.4269/ajtmh.1976.25.221. [DOI] [PubMed] [Google Scholar]
- Marbrook J. Primary immune response in cultures of spleen cells. Lancet. 1967 Dec 16;2(7529):1279–1281. doi: 10.1016/s0140-6736(67)90393-5. [DOI] [PubMed] [Google Scholar]
- Mishell R. I., Dutton R. W. Immunization of dissociated spleen cell cultures from normal mice. J Exp Med. 1967 Sep 1;126(3):423–442. doi: 10.1084/jem.126.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray P. K., Jennings F. W., Murray M., Urquhart G. M. The nature of immunosuppression in Trypanosoma brucei infections in mice. I. The role of the macrophage. Immunology. 1974 Nov;27(5):815–824. [PMC free article] [PubMed] [Google Scholar]
- Murray P. K., Jennings F. W., Murray M., Urquhart G. M. The nature of immunosuppression in Trypanosoma brucei infections in mice. II. The role of the T and B lymphocytes. Immunology. 1974 Nov;27(5):825–840. [PMC free article] [PubMed] [Google Scholar]
- Oehler J. R., Herberman R. B., Campbell D. A., Jr, Djeu J. Y. Inhibition of rat mixed lymphocyte cultures by suppressor macrophages. Cell Immunol. 1977 Mar 15;29(2):238–250. doi: 10.1016/0008-8749(77)90319-7. [DOI] [PubMed] [Google Scholar]
- Pelley R. P., Ruffier J. J., Warren K. S. Suppressive effect of a chronic helminth infection, schistosomiasis mansoni, on the in vitro responses of spleen and lymph node cells to the T cell mitogens phytohemagglutinin and concanavalin A. Infect Immun. 1976 Apr;13(4):1176–1183. doi: 10.1128/iai.13.4.1176-1183.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotz P. H., Talal N., Asofsky R. Assignment of direct and facilitated hemolytic plaques in mice to specific immunoglobulin classes. J Immunol. 1968 Apr;100(4):744–751. [PubMed] [Google Scholar]
- Salaman M. H., Wedderburn N., Bruce-Chwatt L. J. The immunodepressive effect of a murine plasmodium and its interaction with murine oncogenic viruses. J Gen Microbiol. 1969 Dec;59(3):383–391. doi: 10.1099/00221287-59-3-383. [DOI] [PubMed] [Google Scholar]
- Shimp R. G., Crandall R. B., Crandall C. A. Heligmosomoides polygyrus (=Nematospiroides dubius): suppression of antibody response to orally administered sheep erythrocytes in infected mice. Exp Parasitol. 1975 Oct;38(2):257–269. doi: 10.1016/0014-4894(75)90028-4. [DOI] [PubMed] [Google Scholar]
- Spira D. T., Golenser J., Gery I. The reactivity of spleen cells from malarious rats to non-specific mitogens. Clin Exp Immunol. 1976 Apr;24(1):139–145. [PMC free article] [PubMed] [Google Scholar]
- Warren H. S., Weidanz W. P. Malarial immunodepression in vitro: adherent spleen cells are functionally defective as accessory cells in the response to horse erythrocytes. Eur J Immunol. 1976 Nov;6(11):816–819. doi: 10.1002/eji.1830061112. [DOI] [PubMed] [Google Scholar]
- Wedderburn N., Dracott B. N. The immune reponse to type III pneumococcal polysaccharide in mice with malaria. Clin Exp Immunol. 1977 Apr;28(1):130–137. [PMC free article] [PubMed] [Google Scholar]



