Abstract
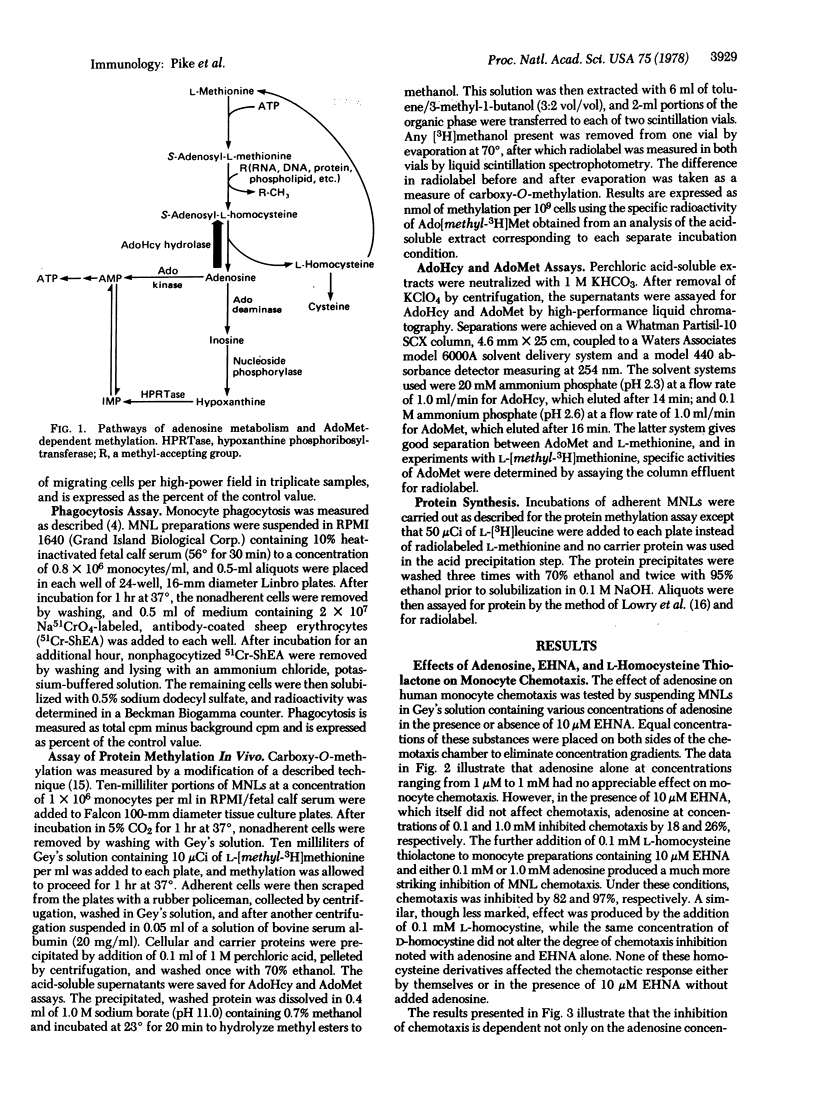
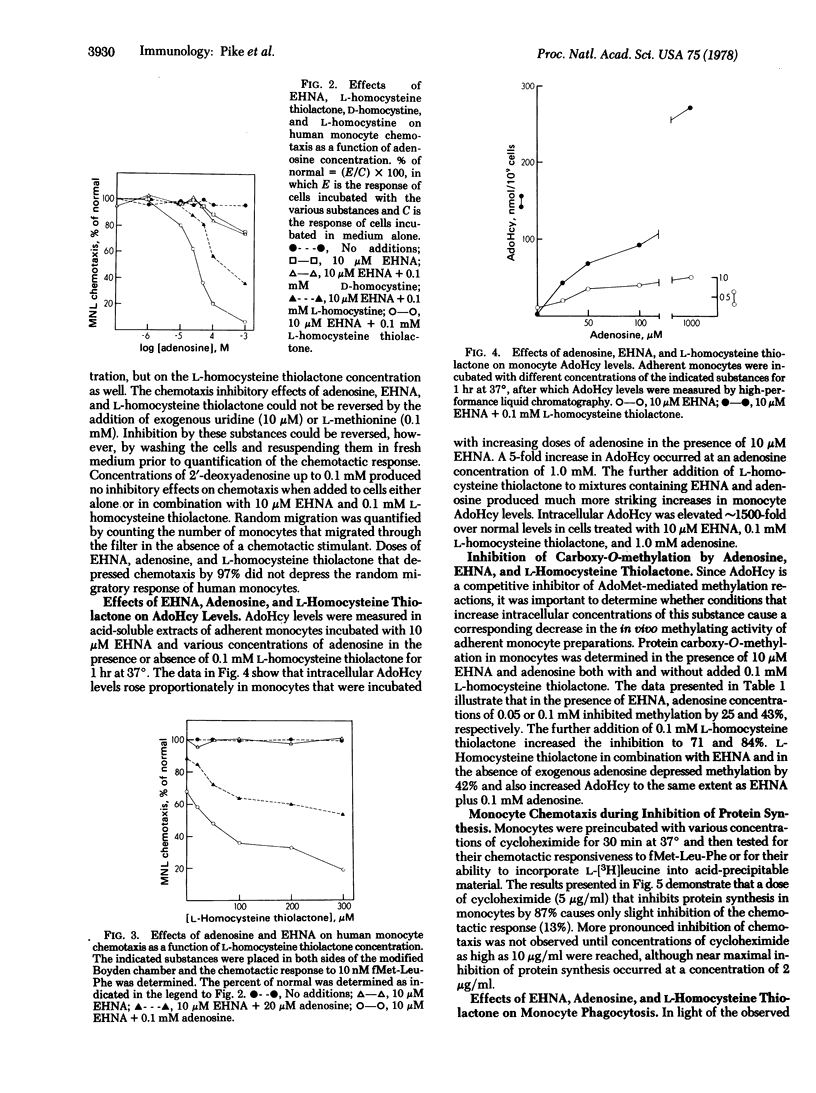
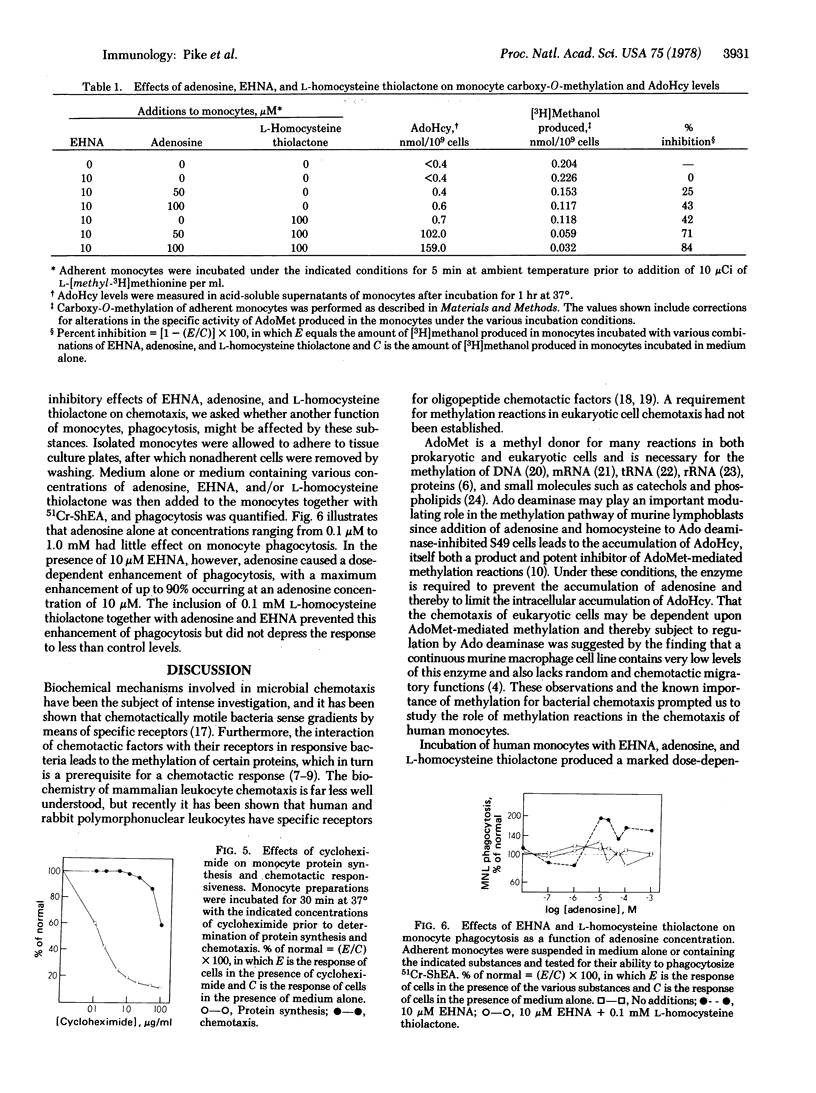
The chemotactic response of motile bacteria requires the methylation of specific proteins by S-adenosyl-L-methionine. To determine whether methylation is required for the chemotaxis of human leukocytes, we studied the effects of inhibition of S-adenosyl-L-methionine-mediated methylation on monocyte chemotactic responsiveness. Methylation was inhibited in monocytes by treating the cells with substances that produced elevations in intracellular S-adenosyl-L-homocysteine, a competitive inhibitor of S-adenosyl-L-methionine methylation. Treatment of isolated monocytes with the adenosine deaminase inhibitor, erythro-9-(2-hydroxy-3-nonyl)adenine, plus exogenous adenosine and L-homocysteine thiolactone increased intracellular S-adenosyl-L-homocysteine levels by as much as 1500-fold. Concomitant with increases in S-adenosyl-L-homocysteine were a decrease in monocyte protein carboxy-O-methylation as well as a marked inhibition of monocyte chemotactic responsiveness. Conditions that almost completely inhibited methylation and chemotaxis did not depress monocyte phagocytosis, indicating that this latter function either is independent of S-adenosyl-L-methionine-mediated methylation or is extremely resistant to inhibition of such reactions by S-adenosyl-L-homocysteine. These studies indicate that S-adenosyl-L-methionine-mediated methylation is required for the chemotaxis of eukaryotic cells and that the chemotactic and phagocytic functions of human monocytes have different requirements for methylation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler J. Chemotaxis in bacteria. Annu Rev Biochem. 1975;44:341–356. doi: 10.1146/annurev.bi.44.070175.002013. [DOI] [PubMed] [Google Scholar]
- Aswanikumar S., Corcoran B., Schiffmann E., Day A. R., Freer R. J., Showell H. J., Becker E. L. Demonstration of a receptor on rabbit neutrophils for chemotactic peptides. Biochem Biophys Res Commun. 1977 Jan 24;74(2):810–817. doi: 10.1016/0006-291x(77)90375-8. [DOI] [PubMed] [Google Scholar]
- Boucek M. M., Snyderman R. Calcium influx requirement for human neutrophil chemotaxis: inhibition by lanthanum chloride. Science. 1976 Sep 3;193(4256):905–907. doi: 10.1126/science.948752. [DOI] [PubMed] [Google Scholar]
- Brown G. M., Attardi G. Methylation of nucleic acids in HeLa cells. Biochem Biophys Res Commun. 1965 Jul 26;20(3):298–302. doi: 10.1016/0006-291x(65)90363-3. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Cantoni G. L. Biological methylation: selected aspects. Annu Rev Biochem. 1975;44:435–451. doi: 10.1146/annurev.bi.44.070175.002251. [DOI] [PubMed] [Google Scholar]
- Diliberto D. J., Jr, Veiveros O. H., Axelrod J. Subcellualr distribution of protein carboxymethylase and its endogenous substrates in the adrenal medulla: possible role in excitation-secretion coupling. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4050–4054. doi: 10.1073/pnas.73.11.4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer D., Van der Weyden M. B., Snyderman R., Kelley W. N. A role for adenosine deaminase in human monocyte maturation. J Clin Invest. 1976 Aug;58(2):399–407. doi: 10.1172/JCI108484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENBERG D. M. BIOLOGICAL METHYLATION. Adv Enzymol Relat Areas Mol Biol. 1963;25:395–431. doi: 10.1002/9780470122709.ch8. [DOI] [PubMed] [Google Scholar]
- Giblett E. R., Anderson J. E., Cohen F., Pollara B., Meuwissen H. J. Adenosine-deaminase deficiency in two patients with severely impaired cellular immunity. Lancet. 1972 Nov 18;2(7786):1067–1069. doi: 10.1016/s0140-6736(72)92345-8. [DOI] [PubMed] [Google Scholar]
- Huxley H. E. Muscular contraction and cell motility. Nature. 1973 Jun 22;243(5408):445–449. doi: 10.1038/243445a0. [DOI] [PubMed] [Google Scholar]
- Kerr S. J., Borek E. The tRNA methyltransferases. Adv Enzymol Relat Areas Mol Biol. 1972;36:1–27. doi: 10.1002/9780470122815.ch1. [DOI] [PubMed] [Google Scholar]
- Knudsen B. B., Dissing J. Adenosine deaminase deficiency in a child with severe combined immunodeficiency. Clin Genet. 1973;4(4):344–347. doi: 10.1111/j.1399-0004.1973.tb01929.x. [DOI] [PubMed] [Google Scholar]
- Kredich N. M., Martin D. V., Jr Role of S-adenosylhomocysteine in adenosinemediated toxicity in cultured mouse T lymphoma cells. Cell. 1977 Dec;12(4):931–938. doi: 10.1016/0092-8674(77)90157-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Paik W. K., Kim S. Protein methylation: chemical, enzymological, and biological significance. Adv Enzymol Relat Areas Mol Biol. 1975;42:227–286. doi: 10.1002/9780470122877.ch5. [DOI] [PubMed] [Google Scholar]
- Schiffmann E., Corcoran B. A., Wahl S. M. N-formylmethionyl peptides as chemoattractants for leucocytes. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1059–1062. doi: 10.1073/pnas.72.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Showell H. J., Freer R. J., Zigmond S. H., Schiffmann E., Aswanikumar S., Corcoran B., Becker E. L. The structure-activity relations of synthetic peptides as chemotactic factors and inducers of lysosomal secretion for neutrophils. J Exp Med. 1976 May 1;143(5):1154–1169. doi: 10.1084/jem.143.5.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyderman R., Altman L. C., Hausman M. S., Mergenhagen S. E. Human mononuclear leukocyte chemotaxis: a quantitative assay for humoral and cellular chemotactic factors. J Immunol. 1972 Mar;108(3):857–860. [PubMed] [Google Scholar]
- Snyderman R., Pike M. C., Fischer D. G., Koren H. S. Biologic and biochemical activities of continuous macrophage cell lines P388D1 and J774.1. J Immunol. 1977 Dec;119(6):2060–2066. [PubMed] [Google Scholar]
- Springer M. S., Goy M. F., Adler J. Sensory transduction in Escherichia coli: two complementary pathways of information processing that involve methylated proteins. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3312–3316. doi: 10.1073/pnas.74.8.3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer W. R., Koshland D. E., Jr Identification of a protein methyltransferase as the cheR gene product in the bacterial sensing system. Proc Natl Acad Sci U S A. 1977 Feb;74(2):533–537. doi: 10.1073/pnas.74.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watterson D. M., Harrelson W. G., Jr, Keller P. M., Sharief F., Vanaman T. C. Structural similarities between the Ca2+-dependent regulatory proteins of 3':5'-cyclic nucleotide phosphodiesterase and actomyosin ATPase. J Biol Chem. 1976 Aug 10;251(15):4501–4513. [PubMed] [Google Scholar]
- Williams L. T., Snyderman R., Pike M. C., Lefkowitz R. J. Specific receptor sites for chemotactic peptides on human polymorphonuclear leukocytes. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1204–1208. doi: 10.1073/pnas.74.3.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]


