Abstract
The increasing prevalence of microbial infections, especially those associated with impaired wound healing and biomedical implant failure has spurred the development of new materials having antimicrobial activity. Hydrogels are a class of highly hydrated material finding use in diverse medical applications such as drug delivery, tissue engineering, as wound fillers and as implant coatings, to name a few. The biocompatible nature of many gels make them a convenient starting platform to develop selectively active antimicrobial materials. Hydrogels with antimicrobial properties can be obtained through the encapsulation or covalent immobilization of known antimicrobial agents, or the material itself can be designed to possess inherent antimicrobial activity. In this review we present an overview of antimicrobial hydrogels that have recently been developed and when possible provide a discussion relevant to their mechanism of action.
1. Introduction
Microbial infections, caused by bacteria and fungi, are a serious health problem, especially with respect to wound healing and biomedical implant fouling.1–4 Staphylococcus aureus, Staphylococcus epidermidis, Escherichia coli, Pseudomonas aeruginosa and Candida species are examples of pathogens normally related to these types of infections.1–6 Infection can prolong or impair the wound healing process leading to tissue morbidity and depending on the severity of infection, sepsis can occur. Regarding biomedical implants, infection at the implant-tissue interface can lead to implant failure, which necessitates implant removal and replacement. Other devices such as catheters can act as vehicles that introduce infection from the nosocomial environment to the patient. Different strategies have emerged to develop materials having antimicrobial activity to prevent or treat infections at wound, implant, and device insertion sites. Materials can be impregnated with antimicrobial agents that are released over time7,8 or the surface of the material can be covalently modified to immobilize broad spectrum antimicrobial agents, such as antimicrobial peptides (AMPs), silver ions or polycationic groups,9–12 that confer antimicrobial properties to the material’s surface.
Hydrogels offer a useful starting point to engineer antimicrobial materials. They are a class of highly hydrated biomaterial, usually produced from natural or synthetic polymers. Polysaccharides such as alginate, dextran, and chitosan, along with the proteins gelatin and fibrin, are examples of natural polymers that form well-studied hydrogels. Poly(vinyl alcohol) (PVA), polyethylene oxide (PEO), and poly(acrylic acid) (PAA) are examples of hydrogel-forming synthetic polymers. Additionally, hydrogels can also be obtained from synthetic peptides and polypeptides. Many hydrogels are biocompatible and can be designed to have mechanical properties similar to natural tissues, and thus have been used in a myriad of applications including drug delivery, healing of chronic and traumatic wounds, surface coatings for implants, encapsulation of cells for three-dimensional cell culture, and tissue engineering, to name a few.13–17 Pertinent to this review, hydrogels with antimicrobial properties have been developed, further increasing the utility of this important class of biomaterial. Herein, we will review the use of hydrogels to impart antimicrobial action.
2. Antimicrobial hydrogels
Antimicrobial hydrogels are extremely attractive materials for use as wound dressings and fillers. Due to their high water content, gels provide a moist, heavily hydrated environment to the wound area, facilitating cellular immunological activity essential to the wound healing process. However, this same hydrated environment can also facilitate microbial infection. Thus, gels capable of imparting antimicrobial action in addition to serving their primary functional role (e.g. wound healing, drug, delivery, etc…) are desirable. The primary approaches to accomplish this are outlined below (Table 1).
Table 1.
List of antimicrobial hydrogels described in this review.
| Type of antimicrobial gels | Applications | References | |
|---|---|---|---|
| Loaded with drugs: | Silver NPs | Wound dressings and surface coatings | [27–39] |
| Gold NPs | Wound dressings | [42–44] | |
| Antibiotics | Wound dressings and implant coatings | [45–50] | |
| Antimicrobial agents | Wound dressings and surface coatings | [51–58] | |
|
| |||
| Inherently active based on: | Peptides | Wound dressings and surface coatings | [59–61,66–68] |
| Chitosan | Wound dressings and surface coatings | [73–85] | |
| Polymers | Surface coatings | [86–88] | |
2.1. Hydrogels for the controlled release of antimicrobial agents
Hydrogels can be used as controlled-release systems to deliver bioactive molecules such as small molecules, nucleic acids, peptides, and proteins. In addition, antimicrobials can be non-covalently encapsulated into the gel network for their controlled release locally to tissue.
2.1.1. Hydrogels loaded with silver and gold nanoparticles
Silver nanoparticles (NPs) have potential use in biomedical applications given their known antimicrobial properties against a broad range of bacteria and fungi.18–22 Although their mechanism of antimicrobial action is not completely understood it seems to involve the generation of reactive oxygen species and binding to bacterial cell membranes, leading to membrane damage. Additionally, silver ions released from the NP can also exert antimicrobial action independently.20,23–26
The incorporation of silver NPs into a given hydrogel allows the formation of hybrid materials that display antimicrobial properties and are advantageous for biomedical applications. Hydrogels of distinct composition have been used to prepare antimicrobial hydrogel-silver NPs systems. These include hydrogels derived from synthetic polymers such as PVA, PVP, and poly(acrylamide-co-acrylic acid), as well as natural polymers such as gelatin and alginate.27–31 With respect to synthesis, several methods have been used to incorporate silver NPs into a given hydrogel’s network. Thomas et al. prepared a poly(acrylamide-co-N-vinyl-2-pyrrolidone) hydrogel loaded with silver NPs through the use of a unique breathing-in/breathing out (BI-BO) method.32 In the BI-BO process, a nonionic hydrogel is exposed to different solution conditions that cause it to sequentially swell and shrink. When silver NPs are present during this process, they become encapsulated into the gel’s network. Resultant gels display antibacterial activity against E. coli in a manner that is dependent on the number of BI-BO cycles the gel had been subjected. As the number of cycles increase, the activity of the resultant gel increases.
Another approach is to form gels in the presence of NPs.27,31,33 For example, Travan et al. described the preparation of silver NPs in the presence of a chitosan-derived solution, Chitlac.33 The Chitlac-NP solution was then mixed with an alginate solution forming a hydrogel. The authors showed that the silver NPs were immobilized in the gel and that the gel conferred antimicrobial activity against S. aureus, S. epidermidis, E. coli, and P. aeruginosa. The antimicrobial activity of the material is most likely due to the destablization of the bacterial membrane when bacteria come into direct contact with the material. Importantly, the gels are selective in their action, being cytocompatible towards eukaryotic cells. Hydrogel-silver NPs systems can also be obtained through the production of NPs simultaneously with hydrogel formation, leading to the direct encapsulation of the NP in the hydrogel network.34,35 By using silver nitrate to mediate the oxidative crosslinking of branched catechol-functionalized poly(ethylene glycol) (cPEG), Fullenkamp et al. were able to form gels with the concomitant formation of encapsulated silver NPs,34 Figure 1. Resultant gels inhibit the growth of S. epidermidis and P. aeruginosa through the release of silver, while minimally influencing mammalian cell viability.
Figure 1.
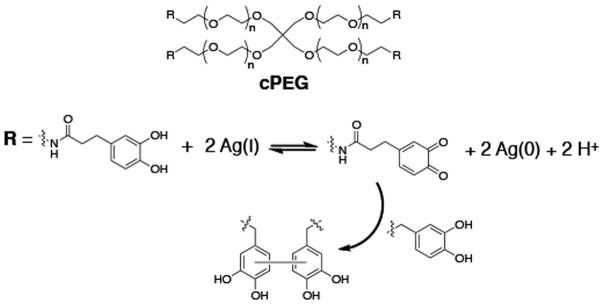
Branched catechol-functionalized PEG (cPEG) reduces Ag(I) leading to quinone-initiated radical cross linking and hydrogel formation with concomitant formation of silver nanoparticles.
A more common strategy to produce NP-laden gels is to directly reduce silver nitrate within a gel network.28,29,36–38 This is typically accomplished by the immersion of a hydrogel in a silver nitrate solution. Silver nitrate-loaded gels are then treated with a reducing agent, for example sodium borohydride, to reduce the silver and form the NPs directly in the gel network. Some gel networks can template the formation of the NP, providing a means to control NP shape and size.39 This is important since it has been shown that the size and shape of silver NPs influence their antimicrobial activity.29,40 Gold NPs are also known for their antibacterial properties.19,41 Similar to the approaches taken with silver NPs, antibacterial hydrogels have also been developed through the incorporation of gold NPs into their networks.42–44 Taking advantage of the known antimicrobial properties of both silver- and gold-NPs, Reddy et al. described a system where bi-metallic, silver-gold NPs were formed throughout the networks of acrylamide (AM)-2-acrylamido-2-methyl-1-propanesulfonic acid (AMPS) hydrogels.44 This bimetallic gel exhibits higher antibacterial activity when compared to gels containing either silver or gold NPs alone.
2.1.2. Hydrogels loaded with antibiotics
The hydrophilic nature of many gels provides a solubilizing environment for small molecule antimicrobials. For example, ciprofloxacin,45–47 gentamicin,48 teicoplanin,49 and amoxicillin50 have each been used to prepare active gels.
Marchesan et al. recently described an antimicrobial hydrogel that is formed via the self-assembly of the hydrophobic tripeptide (DLeu-Phe-Phe).47 When assembly occurs in the presence of ciprofloxacin, the authors report that the small molecule takes an active part in the assembly process, becoming directly incorporated in the gel’s structure. The same non-covalent interactions that are responsible for the integration of the molecule into the peptide network allow its eventual controlled release. The gel showed activity against S. aureus, E. coli and a clinical strain of Klebsiella pneumoniae. Important for its biological application, the hydrogel did not show significant cytotoxicity towards human red blood cells or mouse fibroblast cell cultures. This and other ciprofloxacin-loaded hydrogels have potential use as wound dressings.45,47
In another example, De Giglio et al. developed ciprofloxacin-modified hydrogels as titanium implant coatings to prevent implant-associated infections.46 Polyacrylic hydrogels, namely poly(2-hydroxyethyl methacrylate) (PHEMA) and a copolymer based on poly(ethylene-glycol diacrylate) (PEGDA) and acrylic acid (AA) (PEGDA-AA), were electrosynthesized onto titanium substrates containing the antibiotic ciprofloxacin. The resultant hydrogel coatings were investigated for their ability to release the antibiotic and to inhibit the growth of methicillin-resistant S. aureus (MRSA). It was shown that the PEGDA-AA hydrogel coating released a greater amount of ciprofloxacin and showed better antibacterial activity when compared to the PHEMA coating.
2.1.3. Hydrogels loaded with antimicrobials
There are also examples of hydrogels engineered to deliver more broadly acting antimicrobial agents.51–58 The use of antimicrobial agents, as opposed to frequently used antibiotics, can be advantageous with respect to the emergence of antibiotic resistance. With potential use as wound dressings, pHEMA-based hydrogels have been developed for the release of nitric oxide51 and poly(N-isopropylacrylamide) (PNIPAAm)-based hydrogels have been developed for the release of polyhexamethylene biguanide (PHMB).55 In this last example PEG-crosslinked PNIPAAm thermoresponsive hydrogels were developed for wound dressing applications where lysine acrylate was incorporated into the hydrogel to improve cell adhesion. The gel was shown to facilitate wound healing in a rat model. In addition, when assessed in a wound model that included P. aeruginosa infection, the PHMB-loaded hydrogels were able to reduce the infection as well as expedite healing. In other work described by Laverty et al., AMPs were incorporated into PHEMA hydrogels as promising surface coatings for the prevention of biomedical device-related infection.54 Three different AMPs, maximin-4, H-Orn-Orn-Trp-Trp-NH2, and C12-Orn-Orn-Trp-Trp-NH2, were used and shown to be released from the hydrogel network. All AMP-loaded hydrogels displayed anti-adherent properties when tested against S. epidermidis. The authors showed that the ability of each gel to inhibit cell adhesion is related to the amount of AMP released and that the lipopeptide C12-Orn-Orn-Trp-Trp-NH2 was the most effective AMP examined. Once released the AMPs act through their general accepted mechanism that includes bacterial membrane disruption.
Amphotericin B is a broad-spectrum antifungal agent, often used to treat medical device-derived infections. Thus, antifungal hydrogels employing amphotericin B may find use as surface coatings for devices as proposed by Zumbuehl et al. This group developed a dextran-based hydrogel containing amphotericin B.57 The hydrogel rapidly kills Candida albicans (C. albicans) by a mechanism involving direct fungi contact with the gel. In vivo studies demonstrated the hydrogel’s ability to prevent C. albicans infection in a mouse model. Amphotericin B was also used to develop injectable antifungal hydrogels for the treatment of localized infections.58
2.2. Hydrogels possessing inherent antimicrobial activity
Although the most common approach to constructing antimicrobial gels is to simply load them, non-covalently, with drugs, some hydrogel networks are, themselves, active against microbes. Inherently active gel networks can be produced by covalently ligating active agents to a polymer matrix or gels can be prepared by self-assembly mechanisms, where monomers can be engineered to assemble into hydrogel networks that display antimicrobial activity.
2.2.1. Peptide-based hydrogels
Inspired by the structure and function of AMPs, our lab developed a family of self-assembling β-hairpin peptides that form hydrogel networks that display inherent antibacterial activity.59–61 AMPs, which typically do not self-assemble to form gel networks, are characterized by their cationic net charge and amphiphilic structure, displaying broad-spectrum antimicrobial activities. In general, these peptides act through non-stereospecific mechanisms that involve initial binding to the outer leaflet of microbial cell membranes, typically though electrostatic interactions. This is followed by insertion of the peptides into the hydrophobic interior of the lipid membrane. Although the exact mechanism by which these events occur vary according to the exact AMP and microbe under attack, in general, these events ultimately lead to membrane disruption and cell lysis. Importantly, since AMPs do not bind to specific targets, such as cell surface receptors or enzymes, but rather act generally to destabilize membranes, it is difficult for microbes to gain resistance. As a result, AMPs are just recently enjoying a renaissance of interest for potentially treating multi-drug resistant infections.62
We designed a family of peptides whose folded structure resembles the amphiphilic, cationic nature of classical AMPs. Importantly and distinct from AMPs, these peptides are capable of self-assembling into a fibrillar network that constitutes the formation of a hydrogel. Our first design involved a twenty amino acid lysine-rich amphiphilic peptide, MAX1, which self-assembles into β-sheet rich fibrils that constitute the formation of a mechanically rigid hydrogel.59 Solvent exposed cationic lysines on the surface of the fibrils interact with negatively-charged bacterial cell surfaces, ultimately causing membrane disruption through a mechanism that involves the displacement of essential divalent metal ions from the bacteria cell wall. MAX1 gels are active towards both Gram-negative bacteria (E. coli and K. pneumoniae) and Gram-positive bacteria (S. aureus, S. epidermidis and Streptococcus pyogenes).
Given the known key role of arginine content in the activity of many AMPs,63–65 we recently developed a series of arginine-rich peptide gels. These gels are prepared from self-assembling peptides whose sequences are derived from MAX1, where this peptide’s lysine residues where systematically replaced with arginine. This approach resulted in a second-generation hydrogel prepared from the peptide MARG1, which contains two arginine residues. The MARG1 gel is highly active against the multi-drug resistant bacteria, MRSA.60 Further design culminated in an optimized peptide containing six arginine residues, PEP6R.61 This peptide forms a mechanically rigid hydrogel that is active against S. aureus, E. coli and P. aeruginosa, while being cytocompatible towards mammalian cells. Also important for its biomedical application, the PEP6R hydrogel exhibits shear thin-recovery rheological behavior making it suitable for simple syringe delivery. The mechanism of action for the PEP6R gel is similar to the other peptide gels studied and involves membrane disruption. Figure 2 shows AFM-derived micrographs of E. coli introduced to the surface of a PEP6R gel and to a poly-L-Lysine control surface. Membrane disruption is clearly evident for the bacterium that is in contact with the peptide gel surface.
Figure 2.
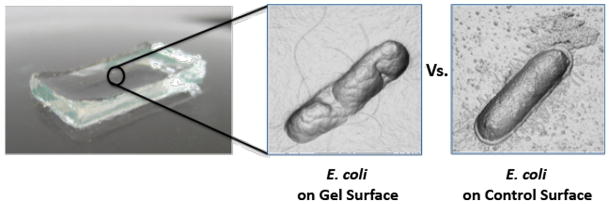
Micrographs showing E. coli bacteria in contact with a PEP6R hydrogel surface versus a poly-L-Lysine control surface. Membrane disruption from the gel surface is the principal cause of bacteria cell death. Micrographs were prepared from AFM height measurements.
Other groups have employed peptides to prepare active gels.66–68 For example, Zhou et al. use epsilon-poly-L-lysine (EPL), an AMP produced by Streptomyces albulus, to prepare antimicrobial hydrogels.67 Hydrogels were prepared from EPL-graft-methacrylamide (EPL-MA) using PEG diacrylate a crosslinker. The gels can be immobilized onto plastic surfaces for use as coatings for medical devices. These EPL-MA hydrogels are broadly active against bacteria and fungi, for example, E. coli, P. aeruginosa, S. aureus, C. albicans and Fusarium solani (F. solani). Song et al. provides another example with wound-healing hydrogels prepared from a series of polypeptides poly(Lys)x(Ala)y crosslinked with 6-arm PEG-amide succinimidyl glutarate. One particular formulation employing poly(Lys)60(Ala)40 showed superior mammalian cell adhesion and cell proliferation activities while exhibiting significant antibacterial activity.68
2.2.2. Chitosan-containing hydrogels
Chitosan is a linear polysaccharide derived from the naturally occurring biopolymer chitin. Chitosan is known for its wound healing and antimicrobial properties, which make it attractive for biomedical applications.69–71 The mechanism responsible for chitosan’s antimicrobial activity is not completely understood but is usually attributed to its polycationic nature.70 Aziz et al. used N-succinyl chitosan to prepared antibacterial chitosan/dextran-based hydrogels.72 However, the authors found that the material’s activity was not due to the N-succinyl chitosan component of the gel, but rather the dextran. Others have taken advantage of chitosan’s antimicrobial properties by either immobilizing the polysaccharide to an existing material’s surface or encapsulating it within a gel.73–85 For example, wound dressings were prepared by Chen et al. by immobilizing a chitosan hydrogel layer, using glutaraldehyde as the cross-linking agent, to the surface of PNIPAAm gel/polypropylene (PP) nonwoven composites. The authors showed that the chitosan hydrogel layer imbibed activity against E. coli and S. aureus, while being biocompatible towards fibroblast cells.73
With a proposed application as a coating for medical implants, Li et al. developed an antimicrobial hydrogel based on quaternized ammonium chitosan-graft-poly(ethylene glycol) methacrylate (qC-g-EM).79 A family of hydrogels was prepared differing in alkyl chain identity and in their degree of quaternization. The antimicrobial activity of the gels was assessed using four clinically important pathogens, namely the bacterial strains S. aureus, E. coli, P. aeruginosa and the fungus F. solani. Of all the hydrogels tested, the dimethyldecylammonium chitosan (with high quaternization)-graft-poly(ethylene glycol) methacrylate (DMDC-Q-g-EM) gel was the one that showed potent antimicrobial activity. It was proposed that the polycationic hydrogel is contact-active, acting like a molecular anionic sponge, attracting the anionic microbial membrane into the hydrogel nanopores, leading to membrane disruption and microbe death. The DMDC-Q-g-EM hydrogel proved biocompatible in in vitro and in vivo experiments and a further study, in which a model surface treated with this hydrogel was also active, suggests that the material may find use in coatings applications.
2.2.3. Other polymer-derived hydrogels
Several interesting hydrogels have been recently reported that are not only antimicrobial but also antifouling to prevent bacterial attachment.86,87 Liu et al.86 described the fabrication of PEG hydrogels that incorporate an antimicrobial polycarbonate (polycarbonate containing quaternary ammonium groups, APC), forming cationic PEG-APC hydrogels. These gels showed strong antimicrobial activity against S. aureus, E. coli, and C. albicans. One of their optimized gels displayed 99.9% killing efficiency against all microbes tested, including clinically isolated MRSA, vancomycin-resistant enterococci (VRE), Acinetobacter baumannii (A. baumannii), and Cryptococcus neoformans (C. neoformans). Impressively, the gel is able to maintain its activity over time even when challenged daily with S. aureus for 12 days. The gel was coated onto silicone rubber, mimicking the surface of a catheter. The hydrogel surface showed effective antimicrobial activity against S. aureus and E. coli, but also antifouling properties. The gel is thought to act via a contact-dependent mechanism involving membrane disruption.
Inspired by the antifouling properties of zwitterionic hydrogels, Cao et al.87 developed two hydrogels based on poly(2-((2-hydroxyethyl)(2-(methacryloyloxy)ethyl)(methyl) ammonio)acetate) (pCBOH1) and poly(2-(bis(2-hydroxyethyl)(2-(methacryloyloxy)ethyl) ammonio)acetate) (pCBOH2) that are zwitterionic when exposed to neutral or basic solution conditions. When the environmental pH is made acidic, these gels become cationic and are able to bind to and kill bacteria through a mechanism involving membrane damage. Thus, in their cationic form, the gels exhibit antimicrobial activity. However, once the material has carried out “the kill”, a change in environmental pH back to neutral or basic conditions, converts the material to its zwitterionic form and the dead bacteria are released from the gel. This antifouling property prevents the accumulation of dead bacteria at the gel’s surface, which can hamper its antimicrobial function.
If the bacterial attachment, growth and the resultant formation of a biofilm cannot be prevented, the answer can be the use of a hydrogel that kills microbial biofilms. In a recently published study Li et al.88 report the formation of temperature-responsive hydrogels formed through the stereocomplexation of triblock polymers, namely poly(L-lactide)-b-poly(ethylene glycol)-b-poly((L-lactide) (PLLA-PEG-PLLA), poly(D-lactide)-b-poly(ethylene glycol)-b-poly((D-lactide) (PDLA-PEG-PDLA), and the cationic triblock polymer poly(D-lactide)-b-cationic poly(carbonate)-b-poly(D-lactide) (PDLA-CPC-PDLA). Noncovalent interactions are involved in the gels formation providing them with shear-thinning properties. The antimicrobial activity of two gels formed by different amounts of PLLA-PEG-PLLA, PDLA-CPC-PDLA, and PDLA-PEG-PDLA was tested. The two gels were able to kill S. aureus and E. coli, but only the gel with an increased amount of the cationic polymer was able to completely eliminate C. albicans. In addition, these gels are active against several clinically isolated microbes such as MRSA, VRE, P. aeruginosa, A. baumannii, K. pneumoniae, C. neoformans, again, through a membrane lytic mechanism. The authors go on to demonstrate that these gels can disrupt microbial biofilms formed by S. aureus, MRSA, E. coli, and C. albicans. These gels were also shown to have no significant hemolytic activity or cytotoxicity towards mammalian cells, and animal studies were used to show their skin compatibility.
3. Conclusions
As described in this review, antimicrobial hydrogels can be prepared by either encapsulating known drugs into a given gel for eventual release or by covalently attaching therapeutics to the network. Some gels are inherently antimicrobial where the network itself displays activity. These materials are typically polycationic and act through non-stereospecific mechanisms that involve membrane disruption. As such, it’s difficult for bacteria to gain resistance and many of the materials are active against current strains of multi-drug resistant bacteria and may hold promise towards future bad actors. At any rate, the design of next generation materials that are active against virulent strains is proving important, especially in wound healing and implant applications.
Acknowledgments
ASV acknowledges Fundação para a Ciência e a Tecnologia – Ministério da Educação e Ciência (FCT-MEC, Portugal) for funding within the FCT Investigator Programme (IF/00803/2012).
References
- 1.Siddiqui AR, Bernstein JM. Clin Dermatol. 2010;28:519–526. doi: 10.1016/j.clindermatol.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 2.Edwards R, Harding KG. Curr Opin Infect Dis. 2004;17:91–96. doi: 10.1097/00001432-200404000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Schierholz JM, Beuth J. J Hosp Infect. 2001;49:87–93. doi: 10.1053/jhin.2001.1052. [DOI] [PubMed] [Google Scholar]
- 4.Montanaro L, Campoccia D, Arciola CR. Biomaterials. 2007;28:5155–5168. doi: 10.1016/j.biomaterials.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Sydnor ERM, Perl TM. Clin Microbiol Rev. 2011;24:141–173. doi: 10.1128/CMR.00027-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doyle JS, Buising KL, Thursky KA, Worth LJ, Richards MJ. Semin Respir Crit Care Med. 2011;32:115–138. doi: 10.1055/s-0031-1275525. [DOI] [PubMed] [Google Scholar]
- 7.Woo GLY, Mittelman MW, Santerre JP. Biomaterials. 2000;21:1235–1246. doi: 10.1016/s0142-9612(00)00003-x. [DOI] [PubMed] [Google Scholar]
- 8.Campbell AA, Song L, Li XS, Nelson BJ, Bottoni C, Brooks DE, DeJong ES. J Biomed Mater Res. 2000;53:400–407. doi: 10.1002/1097-4636(2000)53:4<400::aid-jbm14>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 9.Gao G, Lange D, Hilpert K, Kindrachuk J, Zou Y, Cheng JT, Kazemzadeh-Narbat M, Yu K, Wang R, Straus SK, Brooks DE, Chew BH, Hancock RE, Kizhakkedathu JN. Biomaterials. 2011;32:3899–3909. doi: 10.1016/j.biomaterials.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 10.Flores CY, Diaz C, Rubert A, Benitez GA, Moreno MS, Fernandez Lorenzo de Mele MA, Salvarezza RC, Schilardi PL, Vericat C. J Colloid Interface Sci. 2010;350:402–408. doi: 10.1016/j.jcis.2010.06.052. [DOI] [PubMed] [Google Scholar]
- 11.Kurt P, Wood L, Ohman DE, Wynne KJ. Langmuir. 2007;23:4719–4723. doi: 10.1021/la063718m. [DOI] [PubMed] [Google Scholar]
- 12.Murata H, Koepsel RR, Matyjaszewski K, Russell AJ. Biomaterials. 2007;28:4870–4879. doi: 10.1016/j.biomaterials.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 13.Peppas NA, Hilt JZ, Khademhosseini A, Langer R. Adv Mater. 2006;18:1345–1360. [Google Scholar]
- 14.Tibbitt MW, Anseth KS. Biotechnol Bioeng. 2009;103:655–663. doi: 10.1002/bit.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slaughter BV, Khurshid SS, Fisher OZ, Khademhosseini A, Peppas NA. Adv Mater. 2009;21:3307–3329. doi: 10.1002/adma.200802106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Censi R, Di Martino P, Vermonden T, Hennink WE. J Controlled Release. 2012;161:680–692. doi: 10.1016/j.jconrel.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Seliktar D. Science. 2012;336:1124–1128. doi: 10.1126/science.1214804. [DOI] [PubMed] [Google Scholar]
- 18.Panacek A, Kvitek L, Prucek R, Kolar M, Vecerova R, Pizurova N, Sharma VK, Nevecna T, Zboril R. J Phys Chem B. 2006;110:16248–16253. doi: 10.1021/jp063826h. [DOI] [PubMed] [Google Scholar]
- 19.Hernandez-Sierra JF, Ruiz F, Pena DC, Martinez-Gutierrez F, Martinez AE, Guillen AJP, Tapia-Perez H, Castanon GM. Nanomedicine. 2008;4:237–240. doi: 10.1016/j.nano.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 20.Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, Ramirez JT, Yacaman MJ. Nanotechnology. 2005;16:2346–2353. doi: 10.1088/0957-4484/16/10/059. [DOI] [PubMed] [Google Scholar]
- 21.Panacek A, Kolar M, Vecerova R, Prucek R, Soukupova J, Krystof V, Hamal P, Zboril R, Kvitek L. Biomaterials. 2009;30:6333–6340. doi: 10.1016/j.biomaterials.2009.07.065. [DOI] [PubMed] [Google Scholar]
- 22.Chaloupka K, Malam Y, Seifalian AM. Trends Biotechnol. 2010;28:580–588. doi: 10.1016/j.tibtech.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 23.Sondi I, Salopek-Sondi B. J Colloid Interface Sci. 2004;275:177–182. doi: 10.1016/j.jcis.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 24.Kim JS, Kuk E, Yu KN, Kim JH, Park SJ, Lee HJ, Kim SH, Park YK, Park YH, Hwang CY, Kim YK, Lee YS, Jeong DH, Cho MH. Nanomedicine. 2007;3:95–101. doi: 10.1016/j.nano.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 25.Lok CN, Ho CM, Chen R, He QY, Yu WY, Sun H, Tam PK, Chiu JF, Che CM. J Biol Inorg Chem. 2007;12:527–534. doi: 10.1007/s00775-007-0208-z. [DOI] [PubMed] [Google Scholar]
- 26.Li WR, Xie XB, Shi QS, Zeng HY, Ou-Yang YS, Chen YB. Appl Microbiol Biotechnol. 2010;85:1115–1122. doi: 10.1007/s00253-009-2159-5. [DOI] [PubMed] [Google Scholar]
- 27.Yu HJ, Xu XY, Chen XS, Lu TC, Zhang PB, Jing XB. J Appl Polym Sci. 2007;103:125–133. [Google Scholar]
- 28.Zan XJ, Kozlov M, McCarthy TJ, Su ZH. Biomacromolecules. 2010;11:1082–1088. doi: 10.1021/bm100048q. [DOI] [PubMed] [Google Scholar]
- 29.Thomas V, Yallapu MM, Sreedhar B, Bajpai SK. J Colloid Interface Sci. 2007;315:389–395. doi: 10.1016/j.jcis.2007.06.068. [DOI] [PubMed] [Google Scholar]
- 30.Singh R, Singh D. J Mater Sci Mater Med. 2012;23:2649–2658. doi: 10.1007/s10856-012-4730-3. [DOI] [PubMed] [Google Scholar]
- 31.Rattanaruengsrikul V, Pimpha N, Supaphol P. Macromol Biosci. 2009;9:1004–1015. doi: 10.1002/mabi.200900131. [DOI] [PubMed] [Google Scholar]
- 32.Thomas V, Yallapu MM, Sreedhar B, Bajpai SK. J Appl Polym Sci. 2009;111:934–944. [Google Scholar]
- 33.Travan A, Pelillo C, Donati I, Marsich E, Benincasa M, Scarpa T, Semeraro S, Turco G, Gennaro R, Paoletti S. Biomacromolecules. 2009;10:1429–1435. doi: 10.1021/bm900039x. [DOI] [PubMed] [Google Scholar]
- 34.Fullenkamp DE, Rivera JG, Gong YK, Lau KHA, He LH, Varshney R, Messersmith PB. Biomaterials. 2012;33:3783–3791. doi: 10.1016/j.biomaterials.2012.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hebeish A, Hashem M, Abd El-Hady MM, Sharaf S. Carbohydr Polym. 2013;92:407–413. doi: 10.1016/j.carbpol.2012.08.094. [DOI] [PubMed] [Google Scholar]
- 36.Vimala K, Sivudu KS, Mohan YM, Sreedhar B, Raju KM. Carbohydr Polym. 2009;75:463–471. [Google Scholar]
- 37.Varaprasad K, Mohan YM, Ravindra S, Reddy NN, Vimala K, Monika K, Sreedhar B, Raju KM. J Appl Polym Sci. 2010;115:1199–1207. [Google Scholar]
- 38.Murthy PSK, Mohan YM, Varaprasad K, Sreedhar B, Raju KM. J Colloid Interface Sci. 2008;318:217–224. doi: 10.1016/j.jcis.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 39.Mohan YM, Vimala K, Thomas V, Varaprasad K, Sreedhar B, Bajpai SK, Raju KM. J Colloid Interface Sci. 2010;342:73–82. doi: 10.1016/j.jcis.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 40.Pal S, Tak YK, Song JM. Appl Environ Microbiol. 2007;73:1712–1720. doi: 10.1128/AEM.02218-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cui Y, Zhao YY, Tian Y, Zhang W, Lu XY, Jiang XY. Biomaterials. 2012;33:2327–2333. doi: 10.1016/j.biomaterials.2011.11.057. [DOI] [PubMed] [Google Scholar]
- 42.Marsich E, Travan A, Donati I, Di Luca A, Benincasa M, Crosera M, Paoletti S. Colloids Surf B. 2011;83:331–339. doi: 10.1016/j.colsurfb.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 43.Jayaramudu T, Raghavendra GM, Varaprasad K, Sadiku R, Raju KM. Carbohydr Polym. 2013;92:2193–2200. doi: 10.1016/j.carbpol.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 44.Reddy PR, Varaprasad K, Reddy NN, Raju KM, Reddy NS. J Appl Polym Sci. 2012;125:1357–1362. [Google Scholar]
- 45.Tsou TL, Tang ST, Huang YC, Wu JR, Young JJ, Wang HJ. J Mater Sci Mater Med. 2005;16:95–100. doi: 10.1007/s10856-005-5954-2. [DOI] [PubMed] [Google Scholar]
- 46.De Giglio E, Cometa S, Ricci MA, Cafagna D, Savino AM, Sabbatini L, Orciani M, Ceci E, Novello L, Tantillo GM, Mattioli-Belmonte M. Acta Biomater. 2011;7:882–891. doi: 10.1016/j.actbio.2010.07.030. [DOI] [PubMed] [Google Scholar]
- 47.Marchesan S, Qu Y, Waddington LJ, Easton CD, Glattauer V, Lithgow TJ, McLean KM, Forsythe JS, Hartley PG. Biomaterials. 2013;34:3678–3687. doi: 10.1016/j.biomaterials.2013.01.096. [DOI] [PubMed] [Google Scholar]
- 48.Li HN, Yang J, Hu XN, Liang J, Fan YJ, Zhang XD. J Biomed Mater Res Part A. 2011;98A:31–39. doi: 10.1002/jbm.a.33045. [DOI] [PubMed] [Google Scholar]
- 49.Peng KT, Chen CF, Chu IM, Li YM, Hsu WH, Hsu RWW, Chang PJ. Biomaterials. 2010;31:5227–5236. doi: 10.1016/j.biomaterials.2010.03.027. [DOI] [PubMed] [Google Scholar]
- 50.Chang CH, Lin YH, Yeh CL, Chen YC, Chiou SF, Hsu YM, Chen YS, Wang CC. Biomacromolecules. 2010;11:133–142. doi: 10.1021/bm900985h. [DOI] [PubMed] [Google Scholar]
- 51.Halpenny GM, Steinhardt RC, Okialda KA, Mascharak PK. J Mater Sci Mater Med. 2009;20:2353–2360. doi: 10.1007/s10856-009-3795-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ji QX, Zhao QS, Deng J, Lu R. J Mater Sci Mater Med. 2010;21:2435–2442. doi: 10.1007/s10856-010-4098-1. [DOI] [PubMed] [Google Scholar]
- 53.Cheng G, Xue H, Li GZ, Jiang SY. Langmuir. 2010;26:10425–10428. doi: 10.1021/la101542m. [DOI] [PubMed] [Google Scholar]
- 54.Laverty G, Gorman SP, Gilmore BF. J Biomed Mater Res Part A. 2012;100A:1803–1814. doi: 10.1002/jbm.a.34132. [DOI] [PubMed] [Google Scholar]
- 55.Jiang B, Larson JC, Drapala PW, Perez-Luna VH, Kang-Mieler JJ, Brey EM. J Biomed Mater Res Part B. 2012;100B:668–676. doi: 10.1002/jbm.b.31991. [DOI] [PubMed] [Google Scholar]
- 56.Fallows SJ, Garland MJ, Cassidy CM, Tunney MM, Singh TRR, Donnelly RF. J Photochem Photobiol B. 2012;114:61–72. doi: 10.1016/j.jphotobiol.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 57.Zumbuehl A, Ferreira L, Kuhn D, Astashkina A, Long L, Yeo Y, Iaconis T, Ghannoum M, Fink GR, Langer R, Kohane DS. Proc Nat Acad Sci USA. 2007;104:12994–12998. doi: 10.1073/pnas.0705250104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hudson SP, Langer R, Fink GR, Kohane DS. Biomaterials. 2010;31:1444–1452. doi: 10.1016/j.biomaterials.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Salick DA, Kretsinger JK, Pochan DJ, Schneider JP. J Am Chem Soc. 2007;129:14793–14799. doi: 10.1021/ja076300z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Salick DA, Pochan DJ, Schneider JP. Adv Mater. 2009;21:4120–4123. [Google Scholar]
- 61.Veiga AS, Sinthuvanich C, Gaspar D, Franquelim HG, Castanho MARB, Schneider JP. Biomaterials. 2012;33:8907–8916. doi: 10.1016/j.biomaterials.2012.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fox JL. Nat Biotechnol. 2013;31:379–382. doi: 10.1038/nbt.2572. [DOI] [PubMed] [Google Scholar]
- 63.Chan DI, Prenner EJ, Vogel HJ. Biochim Biophys Acta. 2006;1758:1184–1202. doi: 10.1016/j.bbamem.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 64.Zou GZ, de Leeuw E, Li C, Pazgier M, Li CQ, Zeng PY, Lu WY, Lubkowski J, Lu WY. J Biol Chem. 2007;282:19653–19665. doi: 10.1074/jbc.M611003200. [DOI] [PubMed] [Google Scholar]
- 65.de Leeuw E, Rajabi M, Zou GZ, Pazgier M, Lu WY. Febs Lett. 2009;583:2507–2512. doi: 10.1016/j.febslet.2009.06.051. [DOI] [PubMed] [Google Scholar]
- 66.Yang Z, Liang G, Guo Z, Guo Z, Xu B. Angew Chem Int Ed. 2007;46:8216–8219. doi: 10.1002/anie.200701697. [DOI] [PubMed] [Google Scholar]
- 67.Zhou CC, Li P, Qi XB, Sharif ARM, Poon YF, Cao Y, Chang MW, Leong SSJ, Chan-Park MB. Biomaterials. 2011;32:2704–2712. doi: 10.1016/j.biomaterials.2010.12.040. [DOI] [PubMed] [Google Scholar]
- 68.Song AR, Rane AA, Christman KL. Acta Biomater. 2012;8:41–50. doi: 10.1016/j.actbio.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Park BK, Kim MM. Int J Mol Sci. 2010;11:5153–5165. [Google Scholar]
- 70.Kong M, Chen XG, Xing K, Park HJ. Int J Food Microbiol. 2010;144:51–63. doi: 10.1016/j.ijfoodmicro.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 71.Dai TH, Tanaka M, Huang YY, Hamblin MR. Expert Rev Anti Infect Ther. 2011;9:857–879. doi: 10.1586/eri.11.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Aziz MA, Cabral JD, Brooks HJL, Moratti SC, Hanton LR. Antimicrob Agents Chemother. 2012;56:280–287. doi: 10.1128/AAC.05463-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen KS, Ku YA, Lee CH, Lin HR, Lin FH, Chen TM. Mater Sci Eng C. 2005;25:472–478. [Google Scholar]
- 74.Yang SH, Lee YSJ, Lin FH, Yang JM, Chen KS. J Biomed Mater Res Part B. 2007;83B:304–313. doi: 10.1002/jbm.b.30796. [DOI] [PubMed] [Google Scholar]
- 75.Liu BH, Hu JL, Meng QH. J Biomed Mater Res Part B. 2009;89B:1–8. doi: 10.1002/jbm.b.31180. [DOI] [PubMed] [Google Scholar]
- 76.Zhao L, Mitomo H, Zhai ML, Yoshii F, Nagasawa N, Kume T. Carbohydr Polym. 2003;53:439–446. [Google Scholar]
- 77.Ji QX, Chen XG, Zhao QS, Liu CS, Cheng XJ, Wang LC. J Mater Sci Mater Med. 2009;20:1603–1610. doi: 10.1007/s10856-009-3729-x. [DOI] [PubMed] [Google Scholar]
- 78.Tsao CT, Chang CH, Lin YY, Wu MF, Wang JL, Han JL, Hsieh KH. Carbohydr Res. 2010;345:1774–1780. doi: 10.1016/j.carres.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 79.Li P, Poon YF, Li WF, Zhu HY, Yeap SH, Cao Y, Qi XB, Zhou CC, Lamrani M, Beuerman RW, Kang ET, Mu YG, Li CM, Chang MW, Leong SSJ, Chan-Park MB. Nature Mater. 2011;10:149–156. doi: 10.1038/nmat2915. [DOI] [PubMed] [Google Scholar]
- 80.Mohamed NA, Fahmy MM. Int J Mol Sci. 2012;13:11194–11209. doi: 10.3390/ijms130911194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mohamed NA, Abd El-Ghany NA. Cellulose. 2012;19:1879–1891. [Google Scholar]
- 82.Mohamed NA, Al-Mehbad NY. Int J Biol Macromol. 2013;57:111–117. doi: 10.1016/j.ijbiomac.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 83.Noppakundilograt S, Sonjaipanich K, Thongchul N, Kiatkamjornwong S. J Appl Polym Sci. 2013;127:4927–4938. [Google Scholar]
- 84.Niranjan R, Koushik C, Saravanan S, Moorthi A, Vairamani M, Selvamurugan N. Int J Biol Macromol. 2013;54:24–29. doi: 10.1016/j.ijbiomac.2012.11.026. [DOI] [PubMed] [Google Scholar]
- 85.Chang HW, Lin YS, Tsai YD, Tsai ML. J Appl Polym Sci. 2013;127:169–176. [Google Scholar]
- 86.Liu SQ, Yang C, Huang Y, Ding X, Li Y, Fan WM, Hedrick JL, Yang YY. Adv Mater. 2012;24:6484–6489. doi: 10.1002/adma.201202225. [DOI] [PubMed] [Google Scholar]
- 87.Cao B, Tang Q, Li L, Humble J, Wu H, Liu L, Cheng G. Adv Healthc Mater. 2013 doi: 10.1002/adhm.201200359. [DOI] [PubMed] [Google Scholar]
- 88.Li Y, Fukushima K, Coady DJ, Engler AC, Liu S, Huang Y, Cho JS, Guo Y, Miller LS, Tan JP, Ee PL, Fan W, Yang YY, Hedrick JL. Angew Chem Int Ed. 2013;52:674–678. doi: 10.1002/anie.201206053. [DOI] [PubMed] [Google Scholar]


