Abstract
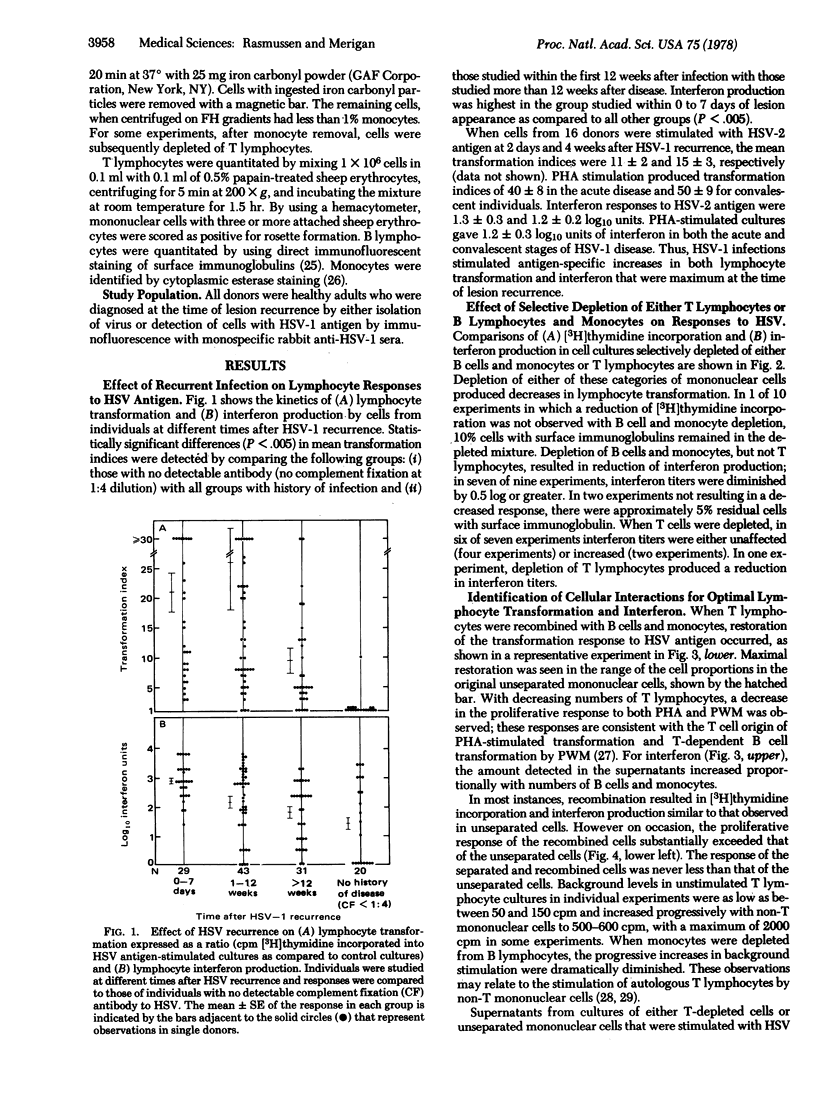
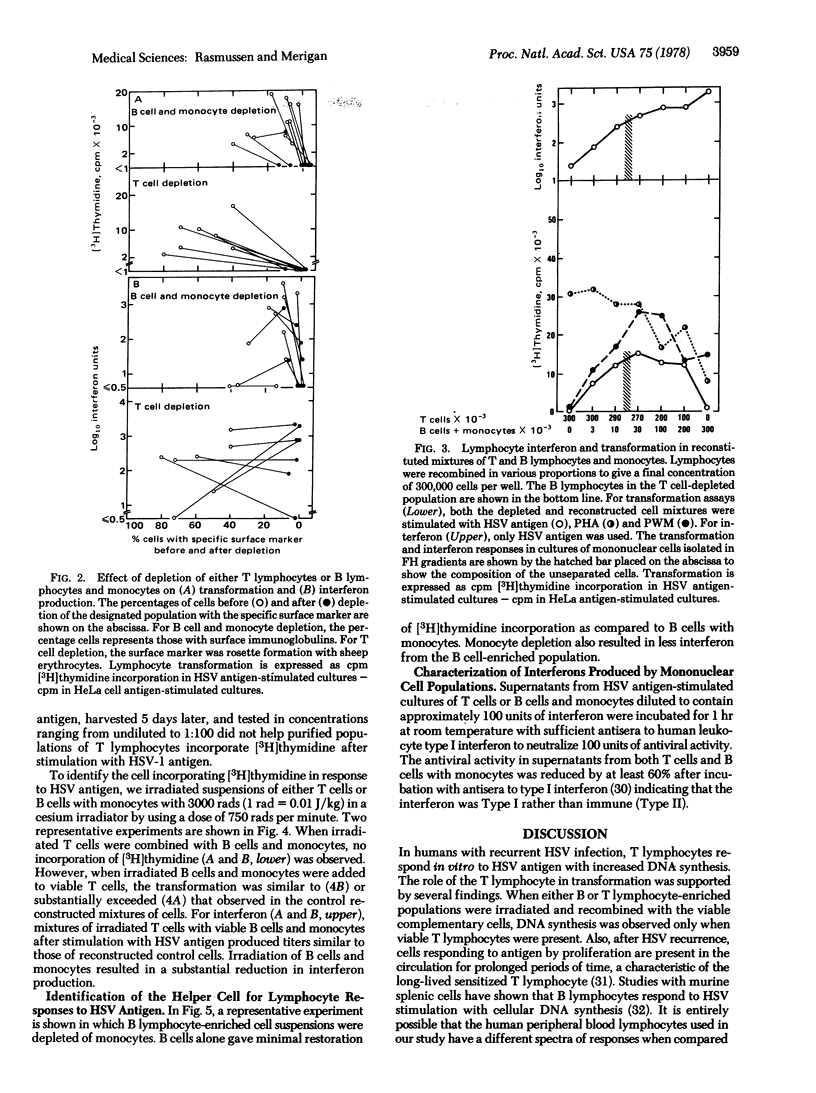
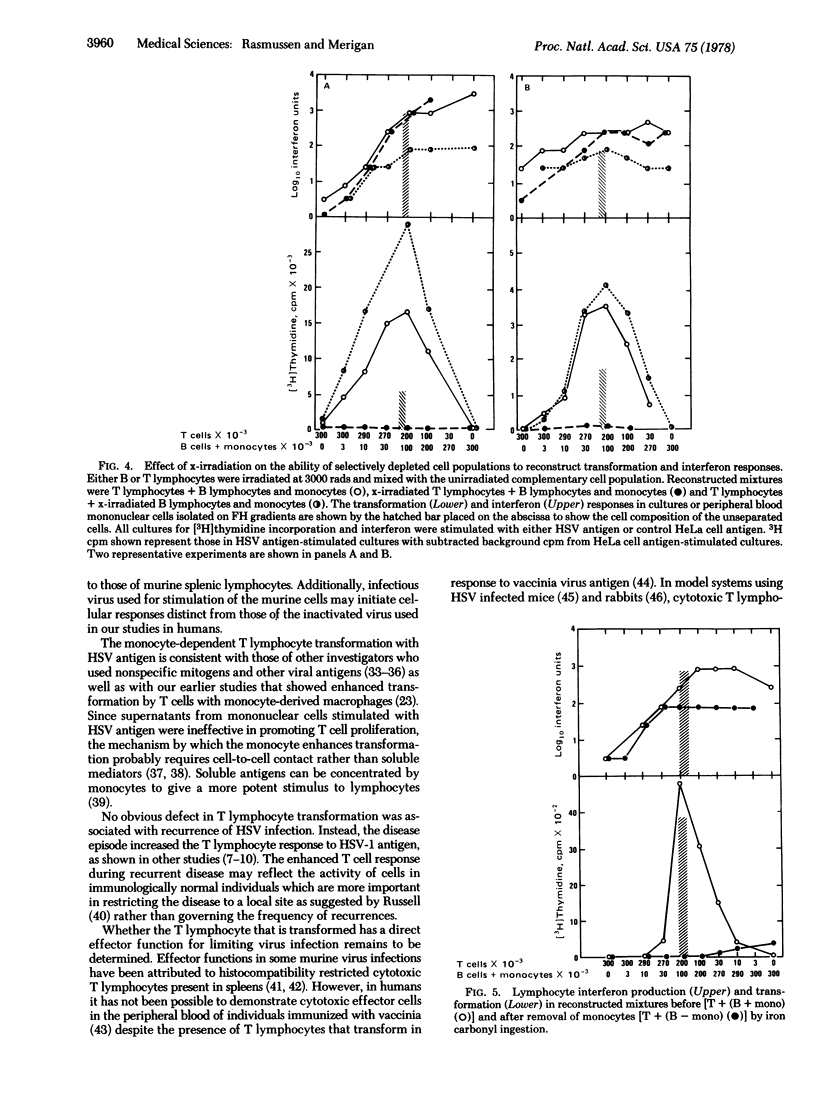
Lymphocyte blast transformation and interferon production in mononuclear cell culture prepared on Ficoll-Hypaque gradients from individuals with herpes simplex virus-I infection were enhanced by a disease recurrence. Responses to both herpes simplex virus-2 and phytohemagglutinin were unaltered. Transformation to herpes simplex virus-I antigen was adversely affected by depleting either thymus-derived (T) lymphocytes or bone marrow-derived (B) lymphocytes together with monocytes from cultures. The transformation response was reconstructed when the selectively depleted lymphocyte populations were recombined. X-irradiation of either T or B lymphocytes and monocytes showed that T lymphocytes incorporated [3H]thymidine with the aid of a radioresistant non-rosetting cell, probably a monocyte. Depletion of B lymphocytes and monocytes, but not of T lymphocytes, resulted in reduction in interferon production. Irradiated B lymphocytes and monocytes failed to produce significant quantities of interferon, suggesting that a radiosensitive B cell was a major interferon source.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASKONAS B. A., RHODES J. M. IMMUNOGENICITY OF ANTIGEN-CONTAINING RIBONUCLEIC ACID PREPARATIONS FROM MACROPHAGES. Nature. 1965 Jan 30;205:470–474. doi: 10.1038/205470a0. [DOI] [PubMed] [Google Scholar]
- Adler W. H., Rabinowitz S. G. Host defenses during primary Venezuelan equine encephalomyelitis virus infection in mice. II. In vitro methods for the measurement and qualitation of the immune response. J Immunol. 1973 May;110(5):1354–1362. [PubMed] [Google Scholar]
- Bobrove A. M., Strober S., Herzenberg L. A., DePamphilis J. D. Identification and quantitation of thymus-derived lymphocytes in human peripheral blood. J Immunol. 1974 Feb;112(2):520–527. [PubMed] [Google Scholar]
- Clancy R., Rawls W. E., Jagannath S. Appearanc of cytotoxic cells within the bronchus after local infection with herpes simplex virus. J Immunol. 1977 Sep;119(3):1102–1105. [PubMed] [Google Scholar]
- Doherty P. C., Blanden R. V., Zinkernagel R. M. Specificity of virus-immune effector T cells for H-2K or H-2D compatible interactions: implications for H-antigen diversity. Transplant Rev. 1976;29:89–124. doi: 10.1111/j.1600-065x.1976.tb00198.x. [DOI] [PubMed] [Google Scholar]
- Elfenbein G. J., Rosenberg G. L. In vitro proliferation of rabbit bone marrow-derived and thymus-derived lymphocytes in response to vaccinia virus. Cell Immunol. 1973 Jun;7(3):516–521. doi: 10.1016/0008-8749(73)90216-5. [DOI] [PubMed] [Google Scholar]
- Ennis F. A. Host defense mechanisms against herpes simplex virus. II. Protection conferred by sensitized spleen cells. J Infect Dis. 1973 Jun;127(6):632–638. doi: 10.1093/infdis/127.6.632. [DOI] [PubMed] [Google Scholar]
- Ennis F. A., Wells M. Immune control of herpes simplex virus infections. Cancer Res. 1974 May;34(5):1140–1145. [PubMed] [Google Scholar]
- Epstein L. B., Kreth H. W., Herzenberg L. A. Fluorescence-activated cell sorting of human T and B lymphocytes. II. Identification of the cell type responsible for interferon production and cell proliferation in response to mitogens. Cell Immunol. 1974 Jun;12(3):407–421. doi: 10.1016/0008-8749(74)90097-5. [DOI] [PubMed] [Google Scholar]
- Epstein L. B., Salmon S. E. The production of interferon by malignant plasma cells from patients with multiple myeloma. J Immunol. 1974 Mar;112(3):1131–1138. [PubMed] [Google Scholar]
- Epstein L. B., Stevens D. A., Merigan T. C. Selective increase in lymphocyte interferon response to vaccinia antigen after revaccination. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2632–2636. doi: 10.1073/pnas.69.9.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford W. L., Gowans J. L. The traffic of lymphocytes. Semin Hematol. 1969 Jan;6(1):67–83. [PubMed] [Google Scholar]
- Gery I., Gershon R. K., Waksman B. H. Potentiation of the T-lymphocyte response to mitogens. I. The responding cell. J Exp Med. 1972 Jul 1;136(1):128–142. doi: 10.1084/jem.136.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger G. A., Moore G. E., White J. G., Matzinger P., Sundsmo J. S., Shupe S., Kolb W. P., Kramer J., Glade P. R. Production of lymphotoxin and migration inhibitory factor by established human lymphocytic cell lines. J Immunol. 1970 Jun;104(6):1476–1485. [PubMed] [Google Scholar]
- Gresser I., Tovey M. G., Maury C., Bandu M. T. Role of interferon in the pathogenesis of virus diseases in mice as demonstrated by the use of anti-interferon serum. II. Studies with herpes simplex, Moloney sarcoma, vesicular stomatitis, Newcastle disease, and influenza viruses. J Exp Med. 1976 Nov 2;144(5):1316–1323. doi: 10.1084/jem.144.5.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haahr S., Rasmussen L., Merigan T. C. Lymphocyte transformation and interferon production in human mononuclear cell microcultures for assay of cellular immunity to herpes simplex virus. Infect Immun. 1976 Jul;14(1):47–54. doi: 10.1128/iai.14.1.47-54.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersh E. M., Harris J. E. Macrophage-lymphocyte interaction in the antigen-induced blastogenic response of human peripheral blood leukocytes. J Immunol. 1968 Jun;100(6):1184–1194. [PubMed] [Google Scholar]
- Hirsch M. S., Murphy F. A. Effects of anti-lymphoid sera on viral infections. Lancet. 1968 Jul 6;2(7558):37–40. doi: 10.1016/s0140-6736(68)92904-8. [DOI] [PubMed] [Google Scholar]
- Hirsch M. S., Zisman B., Allison A. C. Macrophages and age-dependent resistance to Herpes simplex virus in mice. J Immunol. 1970 May;104(5):1160–1165. [PubMed] [Google Scholar]
- Jevitz M. A., Ekstedt R. D. Correlation of lymphocyte transformation with the in vivo immune responsiveness of rabbits. J Immunol. 1971 Feb;106(2):494–505. [PubMed] [Google Scholar]
- Kuntz M. M., Innes J. B., Weksler M. E. Lymphocyte transformation induced by autologous cells. IV. Human T-lymphocyte proliferation induced by autologous or allogeneic non-T lymphocytes. J Exp Med. 1976 May 1;143(5):1042–1054. doi: 10.1084/jem.143.5.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodmell D. L., Niwa A., Hayashi K., Notkins A. L. Prevention of cell-to-cell spread of herpes simplex virus by leukocytes. J Exp Med. 1973 Mar 1;137(3):706–720. doi: 10.1084/jem.137.3.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohrmann H. P., Novikovs L., Graw R. G., Jr Cellular interactions in the proliferative response of human T and B lymphocytes to phytomitogens and allogeneic lymphocytes. J Exp Med. 1974 Jun 1;139(6):1553–1567. doi: 10.1084/jem.139.6.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez C., O'Reilly R. J. Cell-mediated immune responses in recurrent herpesvirus infections. I. Lymphocyte proliferation assay. J Immunol. 1977 Mar;118(3):895–902. [PubMed] [Google Scholar]
- Mackler B. F., Altman L. C., Rosenstreich D. L., Oppenheim J. J. Induction of lymphokine production by EAC and of blastogenesis by soluble mitogens during human B-cell activation. Nature. 1974 Jun 28;249(460):834–837. doi: 10.1038/249834a0. [DOI] [PubMed] [Google Scholar]
- Mochizuki D., Hedrick S., Watson J., Kingsbury D. T. The interaction of Herpes Simplex Virus with murine lymphocytes. I. Mitogenic properties of herpes simplex virus. J Exp Med. 1977 Dec 1;146(6):1500–1510. doi: 10.1084/jem.146.6.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly R. J., Chibbaro A., Anger E., Lopez C. Cell-mediated immune responses in patients with recurrent Herpes Simplex infections. II. Infection-associated deficiency of lymphokine production in patients with recurrent herpes labialis or herpes progenitalis. J Immunol. 1977 Mar;118(3):1095–1102. [PubMed] [Google Scholar]
- Oakes J. E. Role for cell-mediated immunity in the resistance of mice to subcutaneous herpes simplex virus infection. Infect Immun. 1975 Jul;12(1):166–172. doi: 10.1128/iai.12.1.166-172.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opelz G., Kiuchi M., Takasugi M., Terasaki P. I. Autologous stimulation of human lymphocyte subpopulation. J Exp Med. 1975 Nov 1;142(5):1327–1333. doi: 10.1084/jem.142.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim J. J., Leventhal B. G., Hersh E. M. The transformation of column-purified lymphocytes with nonspecific and specific antigenic stimuli. J Immunol. 1968 Aug;101(2):262–267. [PubMed] [Google Scholar]
- Perrin L. H., Zinkernagel R. M., Oldstone M. B. Immune response in humans after vaccination with vaccinia virus: generation of a virus-specific cytotoxic activity by human peripheral lymphocytes. J Exp Med. 1977 Oct 1;146(4):949–969. doi: 10.1084/jem.146.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfizenmaier K., Jung H., Starzinski-Powitz A., Röllinghoff M., Wagner H. The role of T cells in anti-herpes simplex virus immunity. I. Induction of antigen-specific cytotoxic T lymphocytes. J Immunol. 1977 Sep;119(3):939–944. [PubMed] [Google Scholar]
- Rand K. H., Rasmussen L. E., Pollard R. B., Arvin A., Merigan T. C. Cellular immunity and herpesvirus infections in cardiac-transplant patients. N Engl J Med. 1977 Jun 16;296(24):1372–1377. doi: 10.1056/NEJM197706162962402. [DOI] [PubMed] [Google Scholar]
- Rasmussen L. E., Jordan G. W., Stevens D. A., Merigan T. C. Lymphocyte interferon production and transformation after Herpes simplex infections in humans. J Immunol. 1974 Feb;112(2):728–736. [PubMed] [Google Scholar]
- Rocklin R. E., MacDermott R. P., Chess L., Schlossman S. F., David J. R. Studies on mediator production by highly purified human T and B lymphocytes. J Exp Med. 1974 Nov 1;140(5):1303–1316. doi: 10.1084/jem.140.5.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg G. L., Snyderman R., Notkins A. L. Production of chemotactic factor and lymphotoxin by human leukocytes stimulated with Herpes simplex virus. Infect Immun. 1974 Jul;10(1):111–115. doi: 10.1128/iai.10.1.111-115.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell A. S. Cell-mediated immunity to herpes simplex virus in man. J Infect Dis. 1974 Feb;129(2):142–146. doi: 10.1093/infdis/129.2.142. [DOI] [PubMed] [Google Scholar]
- Russell A. S. Cell-mediated immunity to herpes simplex virus in man. Am J Clin Pathol. 1973 Dec;60(6):826–830. doi: 10.1093/ajcp/60.6.826. [DOI] [PubMed] [Google Scholar]
- Seeger R. C., Oppenheim J. J. Synergistic interaction of macrophages and lymphocytes in antigen-induced transformation of lymphocytes. J Exp Med. 1970 Jul 1;132(1):44–65. doi: 10.1084/jem.132.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shillitoe E. J., Wilton J. M., Lehner T. Sequential changes in cell-mediated immune responses to herpes simplex virus after recurrent herpetic infection in humans. Infect Immun. 1977 Oct;18(1):130–137. doi: 10.1128/iai.18.1.130-137.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele R. W., Vincent M. M., Hensen S. A., Fuccillo D. A., Chapa I. A., Canales L. Cellular immune responses to Herpes simplex virus type 1 in recurrent herpes labialis: in vitro blastogenesis and cytotoxicity to infected cell line. J Infect Dis. 1975 May;131(5):528–534. doi: 10.1093/infdis/131.5.528. [DOI] [PubMed] [Google Scholar]
- Valle M. J., Bobrove A. M., Strober S., Merigan T. C. Immune specific production of interferon by human T cells in combined macrophage-lymphocyte cultures in response to Herpes simplex antigen. J Immunol. 1975 Jan;114(1 Pt 2):435–441. [PubMed] [Google Scholar]
- Waldman S. R., Gottlieb A. A. Macrophage regulation of DNA synthesis in lymphoid cells: effects of a soluble factor from macrophages. Cell Immunol. 1973 Oct;9(1):142–156. doi: 10.1016/0008-8749(73)90175-5. [DOI] [PubMed] [Google Scholar]
- Waldron J. A., Jr, Horn R. G., Rosenthal A. S. Antigen-induced proliferation of guinea pig lymphocytes in vitro: obligatory role of macrophages in the recognition of antigen by immune T-lymphocytes. J Immunol. 1973 Jul;111(1):58–64. [PubMed] [Google Scholar]
- Wilson A. B., Gurner B. W., Coombs R. R. Observations on rabbit thymocytes and peripheral T cells. II. Rosette formation with rabbit erythrocytes. Int Arch Allergy Appl Immunol. 1975;48(3):383–394. doi: 10.1159/000231323. [DOI] [PubMed] [Google Scholar]
- Wilton J. M., Ivanyi L., Lehner T. Cell-mediated immunity in Herpesvirus hominis infections. Br Med J. 1972 Mar 18;1(5802):723–726. doi: 10.1136/bmj.1.5802.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T., Handa K., Shimizu Y., Abo T., Kumagai K. Target cells for interferon production in human leukocytes stimulated by sendai virus. J Immunol. 1977 Jun;118(6):1931–1935. [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. Restriction of in vitro T cell-mediated cytotoxicity in lymphocytic choriomeningitis within a syngeneic or semiallogeneic system. Nature. 1974 Apr 19;248(5450):701–702. doi: 10.1038/248701a0. [DOI] [PubMed] [Google Scholar]


