Abstract
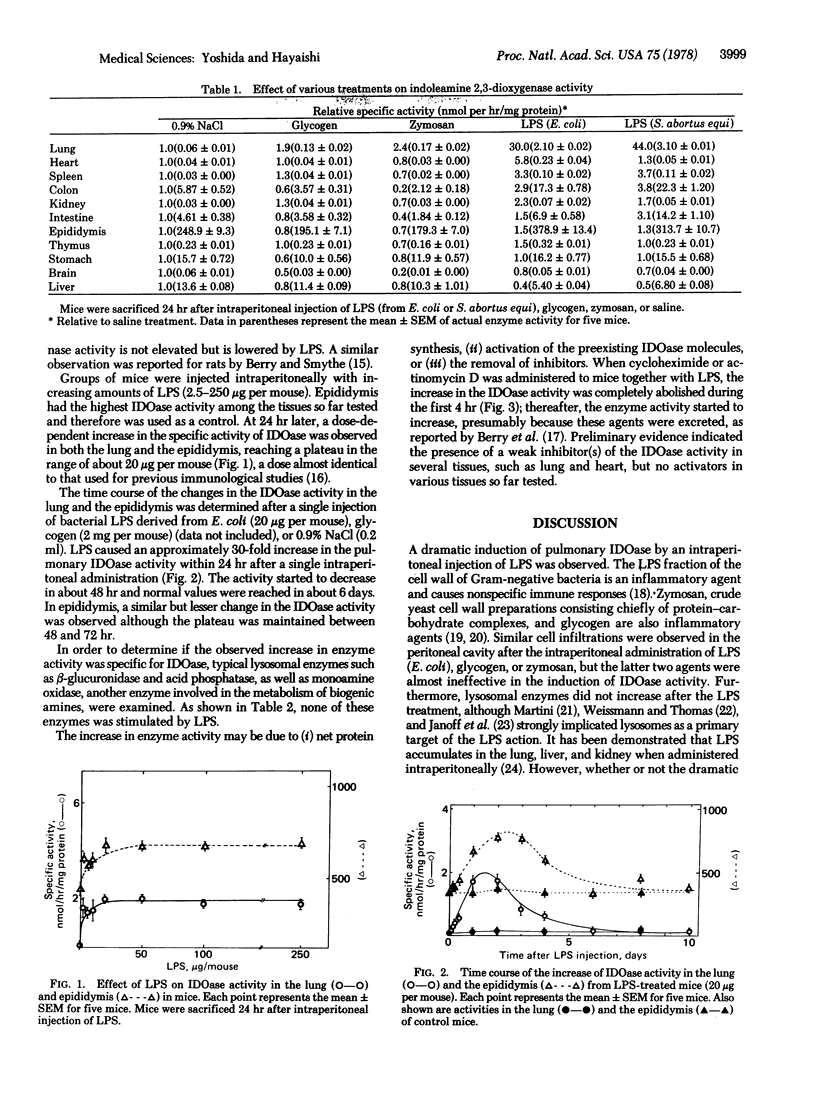
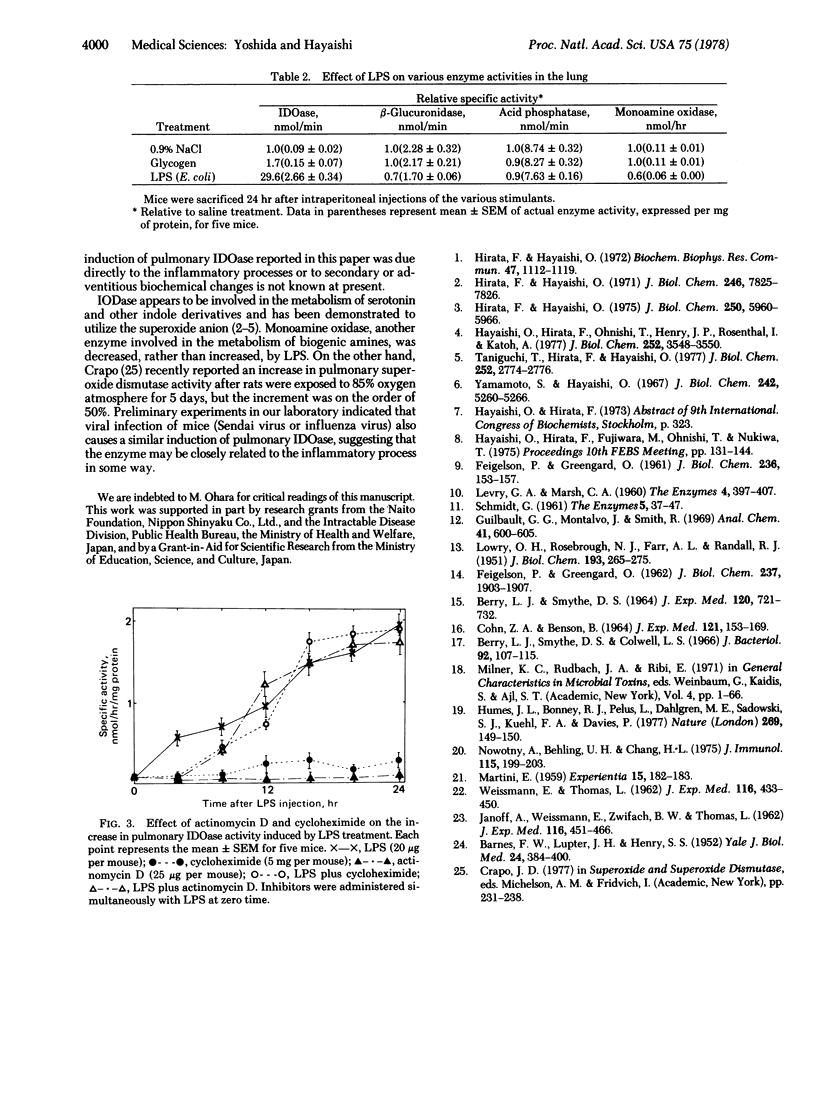
Indoleamine 2,3-dioxygenase [indoleamine: oxygen 2,3-oxidoreductase (decyclizing)] activity in the supernatant fraction (30,000 X g, 30 min) of the mice lung homogenate increased approximately 30- to 50-fold after an intraperitoneal administration of bacterial lipopolysaccharide. In all other tissues tested, no significant increase in enzyme activity was observed. The effect appeared to be specific for the lipopolysaccharide fraction because glycogen and zymosan were almost ineffective under the same experimental conditions. In the lung, the enzyme activity increased almost linearly during the first 24 hr after a single injection of the lipopolysaccharide fraction (20 microgram per mouse). The enzyme activity started to decrease after 48 hr and reached a normal value after about 6 days. The increase in enzyme activity was completely abolished by cycloheximide or actinomycin D. Other enzymes in the lung such as beta-glucuronidase, acid phosphatase, and monoamine oxidase did not change significantly with this treatment.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARNES F. W., Jr, LUPFER H., HENRY S. S. The biochemical target of Flexner dysentery somatic antigen; studies on the rat using antigen labeled with I131. Yale J Biol Med. 1952 Apr;24(5):384–400. [PMC free article] [PubMed] [Google Scholar]
- BERRY L. J., SMYTHE D. S. EFFECTS OF BACTERIAL ENDOTOXINS ON METABOLISM. VII. ENZYME INDUCTION AND CORTISONE PROTECTION. J Exp Med. 1964 Nov 1;120:721–732. doi: 10.1084/jem.120.5.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry L. J., Smythe D. S., Colwell L. S. Inhibition of inducible liver enzymes by endotoxin and actinomycin D. J Bacteriol. 1966 Jul;92(1):107–115. doi: 10.1128/jb.92.1.107-115.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN Z. A., BENSON B. THE DIFFERENTIATION OF MONONUCLEAR PHAGOCYTES. MORPHOLOGY, CYTOCHEMISTRY, AND BIOCHEMISTRY. J Exp Med. 1965 Jan 1;121:153–170. doi: 10.1084/jem.121.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FEIGELSON P., GREENGARD O. A microsomal iron-porphyrin activator of rat liver tryptophan pyrrolase. J Biol Chem. 1961 Jan;236:153–157. [PubMed] [Google Scholar]
- GREENGARD O., FEIGELSONP The purification and properties of liver tryptophan pyrrolase. J Biol Chem. 1962 Jun;237:1903–1907. [PubMed] [Google Scholar]
- Hayaishi O., Hirata F., Ohnishi T., Henry J. P., Rosenthal I., Katoh A. Indoleamine 2,3-dioxygenase: incorporation of 18O2-- and 18O2 into the reaction products. J Biol Chem. 1977 May 25;252(10):3548–3550. [PubMed] [Google Scholar]
- Hirata F., Hayaishi O. New degradative routes of 5-hydroxytryptophan and serotonin by intestinal tryptophan 2,3-dioxygenase. Biochem Biophys Res Commun. 1972 Jun 9;47(5):1112–1119. doi: 10.1016/0006-291x(72)90949-7. [DOI] [PubMed] [Google Scholar]
- Hirata F., Hayaishi O. Possible participation of superoxide anion in the intestinal tryptophan 2,3-dioxygenase reaction. J Biol Chem. 1971 Dec 25;246(24):7825–7826. [PubMed] [Google Scholar]
- Hirata F., Hayaishi O. Studies on indoleamine 2,3-dioxygenase. I. Superoxide anion as substrate. J Biol Chem. 1975 Aug 10;250(15):5960–5966. [PubMed] [Google Scholar]
- Humes J. L., Bonney R. J., Pelus L., Dahlgren M. E., Sadowski S. J., Kuehl F. A., Jr, Davies P. Macrophages synthesis and release prostaglandins in response to inflammatory stimuli. Nature. 1977 Sep 8;269(5624):149–151. doi: 10.1038/269149a0. [DOI] [PubMed] [Google Scholar]
- JANOFF A., WEISSMANN G., ZWEIOFACH B. W., THOMAS L. Pathogenesis of experimental shock. IV. Studies on lysosomes in normal and tolerant animals subjected to lethal trauma and endotoxemia. J Exp Med. 1962 Oct 1;116:451–466. doi: 10.1084/jem.116.4.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nowotny A., Behling U. H., Chang H. L. Relation of structure to function in bacterial endotoxins. VIII. Biological activities in a polysaccharide-rich fraction. J Immunol. 1975 Jul;115(1):199–203. [PubMed] [Google Scholar]
- Taniguchi T., Hirata F., Hayaishi O. Intracellular utilization of superoxide anion by indoleamine 2,3-dioxygenase of rabbit enterocytes. J Biol Chem. 1977 Apr 25;252(8):2774–2776. [PubMed] [Google Scholar]
- WEISSMANN G., THOMAS L. Studies on lysosomes. I. The effects of endotoxin, endotoxin tolerance, and cortisone on the release of acid hydrolases from a granular fraction of rabbit liver. J Exp Med. 1962 Oct 1;116:433–450. doi: 10.1084/jem.116.4.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S., Hayaishi O. Tryptophan pyrrolase of rabbit intestine. D- and L-tryptophan-cleaving enzyme or enzymes. J Biol Chem. 1967 Nov 25;242(22):5260–5266. [PubMed] [Google Scholar]


