Abstract
Fear-conditioning testing paradigms have been used to study differences in memory formation between inbred mouse strains, including numerous mouse models of human diseases. In this study, we characterized the conditioned fear memory of 3 inbred strains: C57BL/6NCrl, 129S2/SvPasCrl, and FVB/NCrl, obtained from Charles River Laboratories. We used 2 training paradigms: delay conditioning, in which an unconditional stimulus coterminates with the presentation of a conditional stimulus, and trace conditioning, in which the conditional and unconditional stimuli are separated by a trace interval. In each paradigm, we evaluated the recent (3 d) and remote (25 d) memory of the mice by using a longitudinal design. Our results showed that both C57BL/6NCrl and 129S2/SvPasCrl mice developed strong and long-lasting context and tone memories in both paradigms, but FVB/NCrl mice showed a weaker but nevertheless consistent tone memory after delay training. Tone memory in the FVB strain was stronger in male than female mice. The remote tone memory of 129S2/SvPasCrl mice diminished after delay training but was stable and stronger than that of C57BL/6NCrl mice after trace training. In conclusion, both C57BL/6NCrl and 129S2/SvPasCrl mice showed reliable and long-lasting fear memory after delay or trace training, with 129 mice showing particularly strong tone memory after trace conditioning. The FVB/NCrl strain, especially male mice, showed reliable tone fear memory after delay training. Our findings confirm that both C57BL/6NCrl and 129S2/SvPasCrl mice develop strong context and tone memory in delay and trace fear-conditioning paradigms.
Abbreviations: FC, fear conditioning
Fear conditioning (FC), a form of Pavlovian associative learning, can be rapidly acquired by animals and is long-lasting.44,66 In this test, animals develop an association between an initially neutral explicit cue (a tone or light) and the context of the training chamber, as a conditional stimulus (CS), with an aversive unconditional stimulus (US), such as a foot shock.26,45 Subsequently, they express signs of fear response in the presence of one or both CS. The fear response manifests itself behaviorally as cessation of movements (that is, freezing), which represents a defensive response in rodents.9,10 FC paradigms have frequently been used to identify neural and molecular mechanisms of memory formation in rats,8,22,27,45,62 to identify the genetic underpinnings that modulate memory in mutant mice,2,3,15,21,29,43,65 and to evaluate cognitive decline in genetic mouse models of human disorders.1,18,23,35
One of the important issues in studies using laboratory inbred strains of mice relates to their broad behavioral variability, including cognitive function.5,6,11,12,58,59 This variability is confounded further by the differences between substrains related to the origin of the stock or a supplier. For example, 129/SvEvTac mice are good learners in the Morris water maze spatial reference memory task and show reliable startle reflexes, whereas 129/SvJ mice are poor learners and show reduced startle response (reviewed by 20, 59, 60). Such variability in cognitive function between strains or their origin might present serious confounds during the interpretation of results related to memory decline in mutant mice or mouse models of human diseases.74,75 For example, the genetic background of mouse models of Alzheimer disease-like amyloidosis may significantly affect amyloid-β brain pathology,46,50 metal metabolism,51 immune response,67 and behavior.32,39 Less is known about the variability between differences of subtypes of strains coming from different suppliers. We recently obtained C57BL/6NCrl, 129S2/SvPasCrl, and FVB/NCrl inbred strains of mice with the purpose of using these strains in the generation of somatic transgenic lines of mouse models of neurodegeneration.41,48 In the present study, we evaluated their baseline cognitive function in FC testing, which has been used in our laboratory to assess the mnemonic function of mouse models of Alzheimer disease-like amyloidosis.35
We used delay and trace FC tests to evaluate the efficacy of each paradigm in eliciting conditioned fear memory in each strain. The delay paradigm, which most frequently is used to test memory in mouse strains,5,6,11,12,58,59 has recently been used to evaluate the 129 and C57BL/6J strains,16 whereas the trace paradigm has been used to compare the C57BL/6J and FVB/NJ strains.28 Recently other colleagues compared C57BL/6J and 129S1/SvImJ strains and reported that 129S1/SvImJ did not develop fear memory after trace conditioning training.70 However, the strains used in all of these previously studies originated from a single vendor, which was different from the supplier from which we purchased the mice for the current study. We chose the C57BL/6 strain as the most suitable for behavioral studies,19,20 the 129 strain because it is often used as the original background for targeted mutations,30,68 and the FVB strain, because some of the available mouse models are still generated or maintained, at least initially, on this genetic background.17,49 FVB mice often are used for pronuclear injections,13 and although they are known to be visually impaired,31 they reportedly develop reliable memory in nonvisual hippocampal tasks, including contextual memory following trace FC training.28 We tested the contextual and tone fear memories of the mice after 3 to 4 d and after 24 to 25 d after CS–US pairing, thus corresponding to recent and remote memories, respectively,4,5,7,29,42 by using a longitudinal experimental design. We report that C57BL/6NCrl mice showed the strongest and most stable contextual memory in both paradigms and strong tone memory following delay training. In comparison, 129S2/SvPasCrl mice showed overall less robust contextual memory but showed the strongest and stable tone-fear memory following trace training. The FVB/NCrl mice were the poorest performers and showed reliable and stable tone memory, which was significantly stronger in male than in female mice, only in the delay paradigm.
Materials and Methods
Mice.
The FVB/NCrl, C57BL/6NCrl, and 129S2/SvPasCrl strains (n = 96, 32 mice per strain, 16 mice of each sex per strain) were purchased from Charles River Laboratories (Wilmington, MA) at the age of 10 wk. Mice were housed in same-sex groups of 2 to 4 in ventilated mouse cages (29 × 18 × 13 cm) under standard laboratory conditions (12:12-h light:dark cycle; lights on, 0600) with a room temperature of 22 °C, and water and food available ad libitum. After 2 wk of the acclimation to the colony facility, the cohort was divided randomly into 2 groups (balanced according to strain and sex), which then were allocated randomly to delay and trace FC training paradigms. During the week before the tests, mice were handled twice daily according to the ‘cupped hand’ method. In this method mice were scooped up, allowed to stay on the experimenter's cupped hand and were transferred to the holding cage, without direct physical restraint.37 Physical restraint associated with picking up the mice and restraining them by their tail increases anxiety responses in mice,37 which might affect their exploration during training and subsequent freezing in contextual and tone tests. During the period of handling, male mice in some cages (1 B6 cage in delay, and 1 129 and 1 FVB cage in trace conditions) showed signs of aggressive interactions and consequently were split into groups of 2 mice per cage. This procedure prevented the escalation of aggressive interactions. All female mice were kept in groups of 4 per cage. Tests were performed during the light phase between 0900 and 1400. All experimental procedures were approved by the IACUC of the University of Florida and were in accordance with guidelines from AAALAC and in accordance with all applicable federal regulatory policies.
FC test.
Apparatus.
Conditioning was completed in 4 identical chambers (25.3 × 29.5 × 29.5 cm; Coulbourn Instruments, Harvard Apparatus, Holliston, MA) as described.35 The total floor area of each chamber was 746 cm2. The chambers were constructed from aluminum (sidewalls and ceiling) and acrylic (rear and front walls) materials. The chambers were placed individually in sound-attenuated cabinets with black interior walls (interior dimensions, 43.3 × 55.3 × 58.5 cm), which were located in a dedicated experimental room. A ventilation fan in each cabinet provided 50 dB of background noise, and a 24-V DC white light, mounted on the wall of each chamber, provided illumination (65 lx at the floor level). A speaker mounted in the wall opposite to the light delivered an acoustic CS. The floor of each chamber consisted of 26 stainless steel rods (diameter, 3 mm in diameter; spaced 11 mm center to center) and was wired to a precision-regulated shocker (H13-15, Coulbourn Instruments). A camera mounted above the chamber enabled recording of mouse activity. Freezing response, defined as the cessation of all movements other than respiratory activity,26 was evaluated with the aid of a software program (version 3.06, FreezeFrame, Actimetrics, Wilmette, IL). The program monitors movements of a mouse as many as 4 times per second and analyses differences in image sequences by comparing pixel activation in subsequent frames. The software can detect the minute movements of grooming, sniffing, turning, and rearing and exclude them from freezing analysis. A proprietary motion-detection algorithm filters out shadows, light flicker, and camera noise and detects movements as small as 1 mm. The threshold of the minimal duration of freezing bout was set to 0.75 s, after observations that shorter bouts were not scored as freezing by human observers and usually reflected brief pauses during exploration or short sniffing bouts. The final freezing score was expressed as the percentage of the time spent freezing throughout the test period.
Conditioning procedure and memory tests.
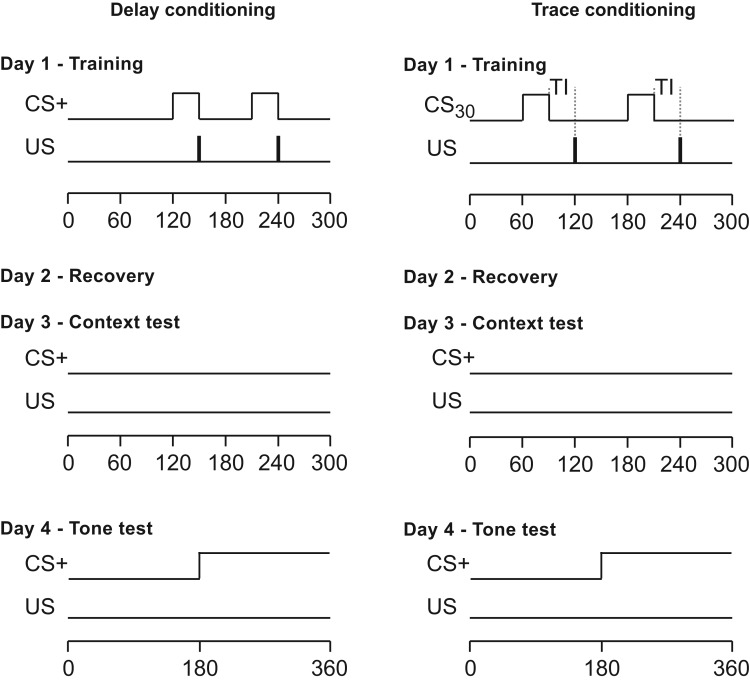
In both paradigms, mice were trained during a single session (day 1) of the experiment (Figure 1). Mice were transported to the chambers in individual containers filled with bedding from the home cage. During training, mice received 2 pairings between a tone (CS: 80 dB; pulse, 6 clicks per second; duration, 30 s) and a 0.45-mA foot shock (US; duration, 2 s). In delay conditioning, the shock coterminated with the tone, whereas in trace conditioning, a 30-s trace interval separated the termination of CS from the onset of foot shock (Figure 1). The 2 CS–US pairings were separated by 60-s intervals. The training session ended with a 60-s posttraining period, bringing the session to a total of 300 s in both paradigms. After a day of recovery (day 2), the mice were tested for fear-induced contextual memory by exposing them to the original training context on day 3; the test, carried out in an extinction mode, lasted 300 s. On day 4, the mice were tested for tone-induced fear memory. The tone test was run in a modified chamber, with the floor and the walls replaced by plastic inserts (opaque white for the floor and semitransparent white at the front and opaque black at the back for the walls), changing the geometry of the chamber by eliminating corners and yielding a total floor area of the modified chamber of about 671 cm2. These modifications did not change the light intensity in the chamber. A culture dish containing a drop of mint extract (McCormick, Sparks, MD) was placed underneath the floor of each chamber to provide a distinct novel odor. During the test, the mice were allowed to explore the modified environment for the first 180 s; during the second 180 s, a tone (CS) with the same characteristics as that used during training was delivered (Figure 1). The mice were retested for remote contextual and tone fear memories after 20 d (days 24 and 25, respectively).
Figure 1.
Schematic representation of the stimuli parameters used in delay and trace conditioning paradigms of the study. On day 1, mice were exposed to 2 pairings between an 30-s auditory CS+ and a brief (2 s) foot shock (US). During delay training, the presentations of CS+ and US overlap and coterminated, whereas during trace training, the CS30 and US were separated by a 30-s trace interval (TI). After 1 d of recovery (day 2), the memory of the association between the training context and US was evaluated on day 3, and the memory of the association between the tone CS and US was assessed on day 4. The tests were repeated on days 24 and 25, respectively. Both tests were performed in the extinction mode, with no foot shock administered during testing.
Statistical analyses.
A general linear model of factorial ANOVA (SPSS version 20, Chicago, IL), with type of conditioning (delay compared with trace), strain (B6, 129, FVB), sex (males compared with females) as between subject, and memory retention (recent compared with remote) as within-subject factors was used to analyze the data. Partial eta squared (ηp2) is reported as the estimate of the degree of association between main factors and conditioned context and tone memories.47 Simple effects were evaluated by using one-way ANOVA. Bonferroni adjustment of α level (MODLSD Bonferroni t tests, SPSS version 20) was applied in multiple planned comparisons. Comparisons between 2 independent groups were done by using Student t tests. The critical α level was set to 0.05, and all values in the text and figures represent means ± SEM. Due to limitations in space and because of the considerable number of analyses, only significant results are reported.
Results
Training.
Exploration of training chamber.
All 3 strains showed comparable exploration of the training chamber in both paradigms during the time preceding the first tone presentation (Figure 2 A and B).
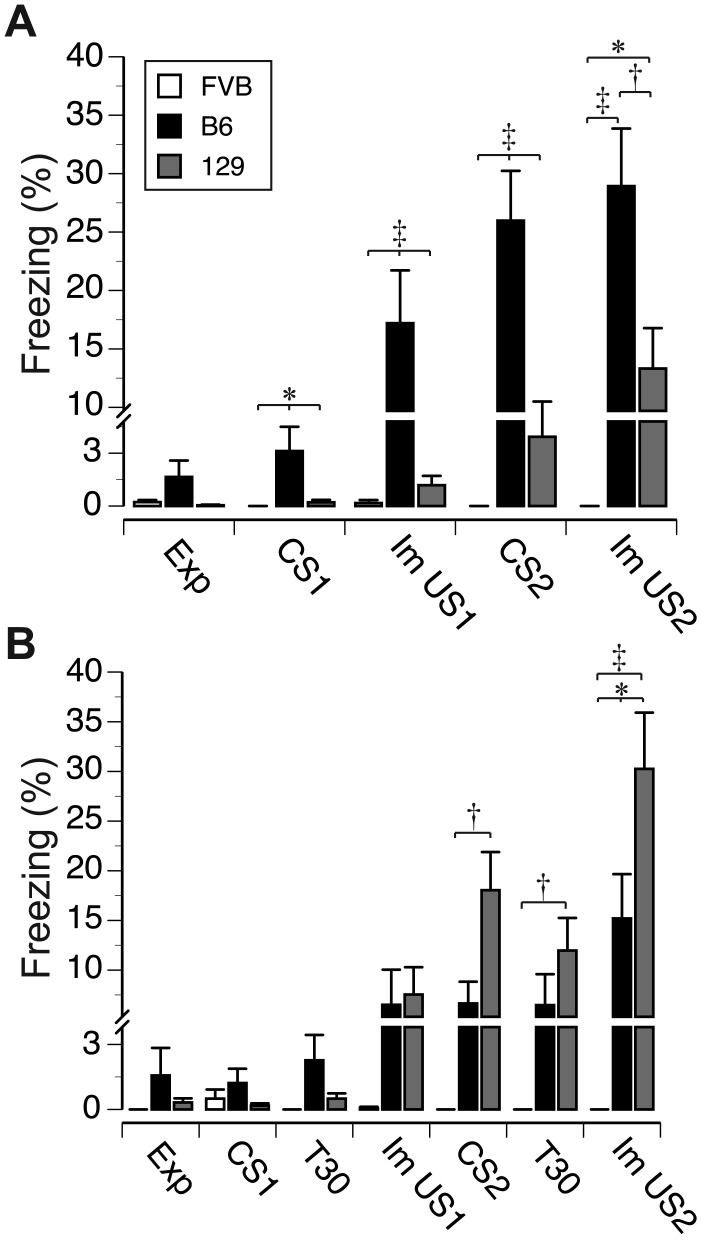
Figure 2.
Mean percentage of freezing by FVB, B6, and 129 mice during training in (A) delay and (B) trace fear conditioning paradigms. Exp, exploration of the training chamber before the first onset of tone; CS1 and CS2, 30-s tone administration; Im US1 and Im US2, immediate freezing during the 60-s interval after foot shock administration; T30, 30-s trace interval separating CS and US during trace conditioning paradigm. Vertical bars represent SEM; *, P < 0.05; †, P < 0.01; ‡, P < 0.001.
Freezing to tone and foot shock.
The strains differed in their freezing to foot shock (F2,84 = 18.9, P < 0.001), and there was a difference in freezing response between the 2 tone presentations (F1,84 = 67.7, P < 0.001). Overall, B6 and 129 mice showed comparable duration of pauses in exploration during the first tone presentation (CS1), but they both paused longer than did FVB mice (P < 0.001 for both comparisons, Bonferroni t tests). All mice froze longer during the second tone presentation (CS2) after the first CS–US pairing (CS1, 0.9% ± 0.3%; CS2, 9.1% ± 1.4%). There were also significant interactions between tone presentations and strain (F2,84 = 18.9, P < 0.001), tone presentation and type of conditioning (F2,84 = 17.9, P < 0.001), and tone presentations, strain, and type of conditioning (F2,84 = 20.8, P < 0.001). B6 mice paused longer than did the FVB and 129 mice during the CS1 presentation in delay conditioning (P < 0.05 for both comparisons, Bonferroni t tests; Figure 2 A) but not in trace conditioning (Figure 2 B). During the CS2 presentation, there was a significant main effect of strain (F2,84 = 19.7, P < 0.001) and a significant interaction between strain and type of conditioning (F2,84 = 20.1, P < 0.001). B6 mice froze longer than did 129 and FVB mice during delay conditioning (P < 0.001 for both comparisons, Figure 2 A), but during trace conditioning, 129 mice froze significantly longer than did other strains (P < 0.001 for both comparisons, Figure 2 B). There were significant main effects of strain (F2,84 = 24.7, P < 0.001) and shock presentation (F1,84 = 30.9, P < 0.001) on immediate freezing during the 60-s postUS periods. B6 and 129 mice froze longer than did FVB (P < 0.001 for both comparisons), and freezing after US1 was significantly shorter than was that after US2 presentation (US1, 5.4% ± 1.2%; US2, 14.6 ± 2.0%). There were also significant interactions between strain and shock presentation (F2,84 = 9.5, P < 0.001), and between strain and type of conditioning (F2,84 = 11.2, P < 0.001). During delay conditioning, B6 mice froze the longest after US1 (P < 0.001 for all comparisons) and US2 (B6 compared with 129, P < 0.02; B6 compared with FVB, P < 0.001), and 129 mice froze longer (P < 0.05) than did FVB mice (Figure 2 A). In trace conditioning, the main effect of strain on freezing after US1 was not significant (F2,42 = 2.5, P = 0.1, Figure 2 B). The strains differed in freezing after the US2 presentation (F2,84 = 13.1, P < 0.001), with 129 mice freezing the longest (compared with B6, P < 0.05; compared with FVB, P < 0.001) and B6 freezing longer (P < 0.05) than FVB mice (Figure 2 B). The strains also showed different freezing behavior during trace intervals (F2,42 = 5.3, P < 0.01), with longer freezing during the second interval (F1,42 = 14.0, P < 0.001, Figure 2 B).
In summary, B6, 129, and FVB strains showed comparable levels of exploration of the novel environment of the training chamber; however, they differed in their immediate response to tone and foot shock in each of the training paradigms. Whereas B6 mice froze the longest during tone presentations and immediately after the shock delivery during the delay training, 129 mice showed the longest freezing after both stimuli during trace conditioning. FVB mice showed almost no freezing during conditioning sessions in both paradigms.
Contextual memory.
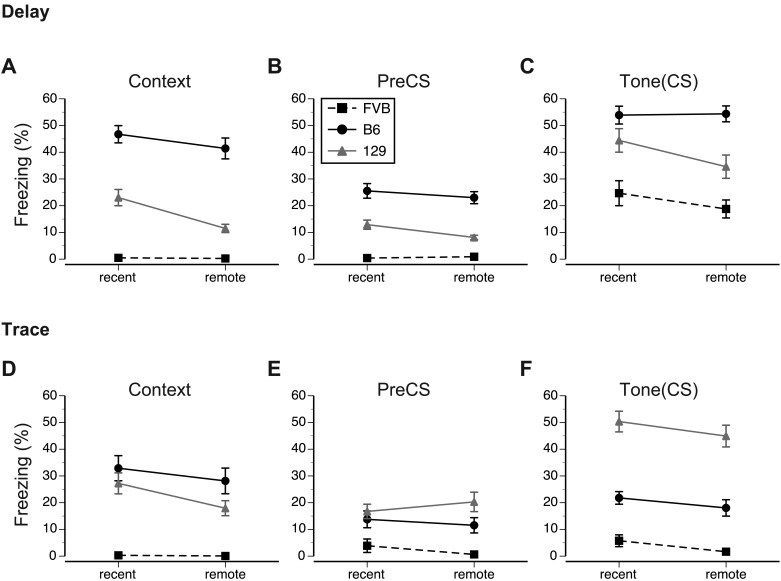
The 3 strains differed in contextual memory (F2,84 = 93.8, P < 0.001, ηp2 = 0.69) and its retention (F1,84 = 24.9, P < 0.001, ηp2 = 0.30). Regarding overall rank order of freezing during context testing, B6 had the highest freezing percentage, followed by 129 and then FVB (all pairwise comparisons were significant at α = 0.001, Bonferroni t tests), and overall freezing decreased during remote memory tests. There were significant interactions between strain and type of conditioning (F2,84 = 6.4, P < 0.01, ηp2 = 0.13), strain and memory retention (F2,84 = 7.9, P < 0.01, ηp2 = 0.16), and between strain and sex (F2,84) = 6.6, P < 0.01, ηp2 = 0.14). The analysis of simple effects revealed significant effects of strain (F2,42 = 106.4, P < 0.001, ηp2 = 0.84) and memory retention (F1,42 = 14.9, P < 0.001, ηp2 = 0.26) after delay conditioning. After scores were pooled across memory tests, B6 mice froze longer (P < 0.001) than did 129 mice, whereas FVB mice showed virtually no freezing during in the training context (Figure 3 A). The decrease in remote memory was significant in 129 (t15 = 4.7, P < 0.001) but not in B6 mice. Male and female mice of all strains showed comparable recent and remote memories after delay conditioning (Figure 4 A). Simple-effects analyses after trace conditioning revealed significant main effects of strain (F2,42 = 24.6, P < 0.001, ηp2 = 0.54) and memory retention (F1,42 =10.2, P < 0.01, ηp2 = 0.20). Freezing in B6 and 129 mice was comparable but longer than that in FVB mice (P < 0.001 for all comparisons, Figure 3 D). The interaction between strain and memory retention approached significance (F2,42 = 3.1, P = 0.06, ηp2 = 0.13), with only 129 mice showing weaker remote memory (t15 = 2.8, P < 0.02, Figure 3 D). Male and female 129 mice differed in memory retention (F2,42 = 5.2, P < 0.01, ηp2 = 0.20, interaction effect), mainly due to the weaker remote memory retention of 129 male mice (t7 = 3.7, P < 0.01, Figure 4 D).
Figure 3.
Mean percentage of freezing exhibited by mice during recent (day 3 or 4) and remote (day 24 or 25) memory tests after (A–C) delay and (D–F) trace conditioning paradigms. For delay conditioning, (A) the context test was performed in the original training chamber. Conditioned tone fear memory was evaluated in the modified training chamber. (B) Freezing behavior of mice during 3-min exploration of the modified chamber (preCS), which preceded (C) the 3-min phase tone (CS) presentation. In the case of trace conditioning, the procedures for conducting (D) context and (E, F) tone tests were identical. Both memory retention tests were performed in the extinction mode, without shock administration. Vertical bars represent SEM. See the Results section for details regarding the statistical analyses pertaining to each test.
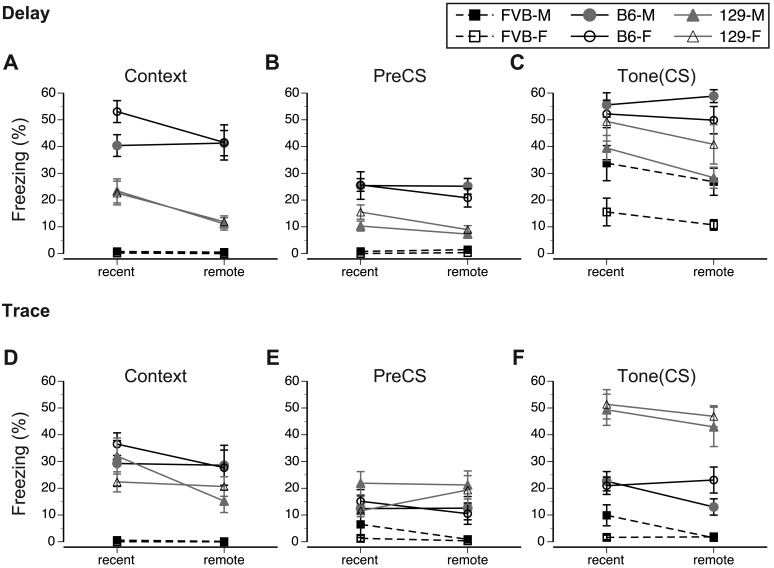
Figure 4.
Mean percentage of freezing shown by male (M) and female (F) mice of FVB, B6, and 129 strains during recent (day 3 or 4) and remote (day 24 or 25) memory test after (A–C) delay and (D–F) trace conditioning paradigms. Vertical bars represent SEM. See the legend to Figure 3 for details related to the experimental designs of tests.
In summary, B6 mice showed strong and stable contextual memory after both delay and trace conditioning. In contrast, 129 mice showed significant decreases in the retention of remote context memory, whereas FVB mice showed virtually no freezing during tests in both paradigms. Overall, the context memory of B6 and 129 strains was stronger in delay than in trace conditioning. Although our study revealed no sex-associated differences in remote and recent contextual memory during delay conditioning, in trace conditioning male 129 mice showed a significant decrease in remote tone memory.
Tone memory.
Freezing during the exploration of altered training chambers (preCS phase) and during presentation of tone (CS phase of the test) is presented in Figure 3 B and C for delay conditioning and in Figure 3 E and F for trace conditioning. Overall analysis revealed significant main effects of strain (F2,84 = 67.9, P < 0.001, ηp2 = 0.62), type of conditioning (F1,84 = 19.8, P < 0.001, ηp2 = 0.19), memory retention (F1,84) = 10.5, P < 0.01, ηp2 = 0.11), and phase of the test (F1,84 = 379.4, P < 0.001, ηp2 = 0.82)—but not sex—on freezing. Mice froze longer during tone presentation than during the preCS exploration of the modified chambers. In addition, freezing to tone was longer after delay than trace conditioning, and mice froze longer during recent than remote memory tests (Figure 3 C and F). Both B6 and 129 mice showed longer freezing during tone tests than did FVB mice. The analysis also revealed significant 2- and 3-way interactions between type of conditioning and phase of the test (F1,84 = 48.5, P < 0.001); strain and phase (F2,84 = 26.3, P < 0.001); phase and memory retention (F1,84 = 5.4, P < 0.001); phase, type of conditioning, and strain (F1,84 = 12.5, P < 0.001); phase, type of conditioning, and sex (F1,84 = 4.1, P < 0.05), and phase, strain, and sex (F2,84 = 6.0, P < 0.01).
To evaluate further the strength of tone fear memory, we analyzed the increase in freezing between the preCS and CS phases of the test within each training paradigm. In delay conditioning, there was a significant overall effect of strain (F2,42 = 25.4, P < 0.001, ηp2 = 0.58), with B6 mice freezing longer than both 129 (P < 0.01) and FVB (P < 0.001) and 129 mice freezing longer (P < 0.01) than FVB mice (Figure 3 C). Posthoc analysis revealed that mice in each strain froze longer during tone presentation than during preCS phase of the exploration in the modified training chamber (B6: F1,14 = 187.2, P < 0.001, ηp2 = 0.93; 129: F(1,14) = 65.2, P < 0.001, ηp2 = 0.82; FVB: F1,14 = 46.8, P < 0.001, ηp2 = 0.77), indicating selective memory of the association between tone and foot shock. Whereas the retention of memory, determined by comparing remote and recent memory scores, was not affected in B6 and FVB mice (Figure 3 C), 129 mice showed significant decreases in remote memory scores (F1,14 = 11.6, P < 0.01, ηp2 = 0.45; Figure 3 C). Although tone memory was not sexually differentiated in B6 and 129 mice, FVB male mice showed stronger (F1,14 = 8.4, P < 0.05, ηp2 = 0.36) memory than did FVB female mice (Figure 4 C).
The response to tone also differed between strains after trace conditioning (F1,42 = 80.0, P < 0.001, ηp2 = 0.79). The rank order of the overall freezing shown by strains was 129 followed by B6 and then FVB (P < 0.001 for all pairwise comparisons, Figure 3 F). The analysis revealed that among the 3 strains, B6 mice froze longer during tone presentation (F1,14 = 11.1, P < 0.01, ηp2 = 0.41), but their response depended on sex, phase, and memory retention test (F1,14 = 5.5, P < 0.05, ηp2 = 0.28, interaction effect). Although no sex-associated differences occurred during the preCS phase, male B6 mice tended to freeze less during the remote memory tone test than did their female counterparts (Figure 4 F). In addition, among the 3 strains, 129 mice froze longer during tone presentation (F1,14 = 205.4, P < 0.001, ηp2 = 0.94), but their freezing was affected significantly by phase and memory retention test (F1,14 = 5.7, P < 0.05, ηp2 = 0.29, interaction effect). The interaction reflected the opposite direction of the change in freezing during the preCS and CS phases of the remote compared with recent memory tests (Figure 3 E and F). Overall, FVB mice did not significantly increase freezing to tone presentation (F1,14 = 4.1, P = 0.06, ηp2 = 0.23, phase effect, Figure 3 E and F).
In summary, B6 and 129 strains showed selective increases in freezing to tone after both delay and trace training paradigms. In contrast, FVB mice showed significant increases in freezing to tone only after delay conditioning. The strength of tone fear memory showed by the B6 and 129 strains depended on the training paradigm. Whereas B6 mice showed the strongest memory after delay conditioning, 129 mice showed the strongest memory after trace conditioning. In addition, 129 mice showed decreased freezing during the remote memory test after delay—but not trace—conditioning. Male FVB mice exhibited greater tone memory in delay conditioning than did their female counterparts. The FVB mice showed no evidence of fear memory in trace conditioning.
Discussion
In the present study, we compared the contextual and tone fear conditioned memories of B6, 129 and FVB inbred mouse strains obtained from Charles River Laboratories (Wilmington, MA) by using both delay and trace FC training paradigms. The experimental parameters we used here (CS, 80-dB pulsating tone; US, 0.45-mA foot shock) have previously been used in FC paradigms with mice, with no reports of ceiling or floor effects in the freezing response in most mouse inbred strains,33,59 and the altered olfactory characteristics of the modified training chamber increased the salience of the CS during the tone test.70 Our overall results revealed that context and tone fear memories were stronger after delay than trace conditioning, confirming previously published reports.55 However, the strength of memory depended on the strain, conditioning paradigm, and memory test. In agreement with published reports, B6 mice manifested the strongest contextual and tone fear memory in delay conditioning.5,6,59,61,72 In contrast, in trace conditioning, 129 mice showed the strongest conditioned tone—but not context—fear memory. The strain factor explained most of the variance in context memory in both FC paradigms (delay conditioning, ηp2 = 0.84; trace conditioning, ηp2 = 0.54), whereas the contribution of other factors was less prominent. In contrast, FVB mice showed virtually no freezing in the original training or modified contexts in both paradigms, results that might reflect their poor performance in visually guided behavioral tests. This poor performance of FVB mice has been attributed to progressive impairment in their visual acuity52,63,73,76 due to the presence of PDE6B, which has been implicated in retinal degeneration.25,31,40,69 Our findings contrast with previous studies investigating FC memory in FVB mice. For example, one group59 demonstrated low (13%) and another12 strong (50%) freezing in FVB mice in context tests after delay training, and a third group28 reported freezing of about 25% by FVB mice in trace conditioning. Given that FVB/NJ mice were used in the cited studies whereas we tested FVB/CrL mice, the supplier or commercial source of the strains used in FC experiments might present an important factor of experimental design. In agreement with our results, FVB/NTac mice have been reported to show impaired context memory14 as well as—contrary to our findings—impairment in tone memory13,14 after delay training. It is also likely that subtle differences in training protocols, especially those that involve 3 CS–US pairings12 or 3 pairings with stronger (0.7 mA) US,28 independently contribute to the observed differences in the development of conditioned fear memory in FVB mice.
Retesting our mice at 25 d after CS–US pairings allowed us to evaluate the stability of remote memory and, because both recent and remote memory tests were carried out in extinction mode (that is, no US was administered during the test), the retest evaluated the resistance of the developed memories to extinction. In light of the evidence indicating that extinction usually occurs after frequently administered retests, without presentation of US, and short intervals between retests,56,64 the effect of extinction on memory in our paradigm, which included only 2 retests, might not be evident, but it cannot be excluded. Our design did not include an independent group focusing exclusively on evaluations of remote memory. Notwithstanding, our results show that a relatively mild foot shock, administered during 2 CS–US pairings, resulted in relatively strong remote context and tone memories, which in most cases did not decrease as compared with recent memories evaluated 3 to 4 d after training. We conclude that the evaluation of remote fear memories after 25 d presents a feasible additional testing time point in experiments investigating drug efficacy on memory retention in preclinical trials using mouse models of neurodegeneration.36,38,71
Furthermore, our study revealed that sex affected the development of conditioned memory within strains, but compared with strain, sex explained less of the variance in data (when sex was a factor, ηp2 varied between 0.20 and 0.35 on average). The differences between sexes were most pronounced in amygdala-dependent tone fear memory in delay conditioning. Overall, male B6 and FVB mice showed stronger tone memory than did their female counterparts, however the opposite sex-associated effect occurred in the 129 strain. The sex-associated difference in tone memory after delay conditioning was especially pronounced in FVB mice, with male mice showing significantly higher freezing rates than those of female mice. This relatively small effect of sex on conditioned memory might justify male-only experiments, with the caveat that the exclusion of female mice might prevent the evaluation of hormonal effects on the formation of fear memory or may impede the identification of the molecular mechanisms that are specific to a particular sex.53,54 Interestingly, sex-related differences in conditioned memory were less pronounced after trace conditioning. At present, we cannot easily reconcile these differences, because—despite considerable literature describing fear conditioning in mice—the effect of sex on conditioned memory has not been reported frequently, even in studies in which both male and female were used. The inclusion of both sexes and the choice of a delay FC paradigm might be beneficial in the characterization of mouse models of human diseases, including Alzheimer disease and schizophrenia, in which sex-associated differences in the time of onset or severity are reported.24,34,57
In conclusion, the present study characterized 3 inbred strains obtained from Charles River Laboratories and commonly used in behavioral research—B6, 129, and FVB—in delay and trace FC paradigms. We demonstrated that B6 mice manifest robust context and tone fear memory after delay training that is in agreement with good performance of B6 mice obtained from other suppliers. In addition, our 129 mice showed strong and long-lasting tone fear memory, especially after trace conditioning. 129 substrains obtained from the Jackson Laboratory have been reported to show good performance in delay FC,20 although some 129 substrains show considerable variability in the acquisition of extinction.16 The demonstration of strong and stable tone fear memory in the 129S2/SvPasCrl strain after trace conditioning adds new information about the development of fear memory in this substrain, especially given that 129S1/SvImJ mice failed to condition to the trace CS.70 Furthermore, we found that male—but not female—FVB mice showed reliable tone fear memory only after delay conditioning. This result is in agreement with recently published evidence of the good tone memory in trace fear conditioning of male FVB mice obtained from the Jackson Laboratory.28 However, although a previous study28 demonstrated good performance of FVB/NJ male mice in context tests (female mice were not tested), neither male nor female FVB/NCrl mice showed any freezing behavior in the current study. Our findings confirm the importance of the choice of the optimal experimental FC protocols70 but also indicate that the substrains obtained from various suppliers might provide additional variability, and initial prescreening might be required before using them in models with cognitive experimental endpoints.
Acknowledgments
We thank Drs Valerie Bolivar and Douglas Wahlsten for helpful discussions and comments on the manuscript. We also thank the staff of Animal Care Services of the University of Florida for excellent husbandry care of the mice.
References
- 1.Adachi M, Autry AE, Covington HE, 3rd, Monteggia LM. 2009. MeCP2-mediated transcription repression in the basolateral amygdala may underlie heightened anxiety in a mouse model of Rett syndrome. J Neurosci 29:4218–4227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ammassari-Teule M, Restivo L, Pietteur V, Passino E. 2001. Learning about the context in genetically defined mice. Behav Brain Res 125:195–204 [DOI] [PubMed] [Google Scholar]
- 3.Arai JA, Li S, Hartley DM, Feig LA. 2009. Transgenerational rescue of a genetic defect in long-term potentiation and memory formation by juvenile enrichment. J Neurosci 29:1496–1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bailey CH, Bartsch D, Kandel ER. 1996. Toward a molecular definition of long-term memory storage. Proc Natl Acad Sci USA 93:13445–13452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balogh SA, Radcliffe RA, Logue SF, Wehner JM. 2002. Contextual and cued fear conditioning in C57BL/6J and DBA/2J mice: context discrimination and the effects of retention interval. Behav Neurosci 116:947–957 [DOI] [PubMed] [Google Scholar]
- 6.Balogh SA, Wehner JM. 2003. Inbred mouse strain differences in the establishment of long-term fear memory. Behav Brain Res 140:97–106 [DOI] [PubMed] [Google Scholar]
- 7.Barondes SH, Cohen HD. 1966. Puromycin effect on successive phases of memory storage. Science 151:594–595 [DOI] [PubMed] [Google Scholar]
- 8.Blair HT, Sotres-Bayon F, Moita MA, Ledoux JE. 2005. The lateral amygdala processes the value of conditioned and unconditioned aversive stimuli. Neuroscience 133:561–569 [DOI] [PubMed] [Google Scholar]
- 9.Blanchard RJ, Blanchard CD, Rodgers J, Weiss SM. 1990. The characterization and modelling of antipredetor defensive behavior. Neurosci Biobehav Rev 14:463–472 [DOI] [PubMed] [Google Scholar]
- 10.Blanchard RJ, Blanchard DC. 1969. Crouching as an index of fear. J Comp Physiol Psychol 67:370–375 [DOI] [PubMed] [Google Scholar]
- 11.Bogue M. 2003. Mouse Phenome Project: understanding human biology through mouse genetics and genomics. J Appl Physiol 95:1335–1337 [DOI] [PubMed] [Google Scholar]
- 12.Bolivar VJ, Pooler O, Flaherty L. 2001. Inbred strain variation in contextual and cued fear conditioning behavior. Mamm Genome 12:651–656 [DOI] [PubMed] [Google Scholar]
- 13.Bothe GW, Bolivar VJ, Vedder MJ, Geistfeld JG. 2004. Genetic and behavioral differences among 5 inbred mouse strains commonly used in the production of transgenic and knockout mice. Genes Brain Behav 3:149–157 [DOI] [PubMed] [Google Scholar]
- 14.Bothe GW, Bolivar VJ, Vedder MJ, Geistfeld JG. 2005. Behavioral differences among 14 inbred mouse strains commonly used as disease models. Comp Med 55:326–334 [PubMed] [Google Scholar]
- 15.Calandreau L, Trifilieff P, Mons N, Costes L, Marien M, Marighetto A, Micheau J, Jaffard R, Desmedt A. 2006. Extracellular hippocampal acetylcholine level controls amygdala function and promotes adaptive conditioned emotional response. J Neurosci 26:13556–13566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Camp M, Norcross M, Whittle N, Feyder M, D'Hanis W, Yilmazer-Hanke D, Singewald N, Holmes A. 2009. Impaired Pavlovian fear extinction is a common phenotype across genetic lineages of the 129 inbred mouse strain. Genes Brain Behav 8:744–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cannon A, Yang B, Knight J, Farnham IM, Zhang Y, Wuertzer CA, D'Alton S, Lin WL, Castanedes-Casey M, Rousseau L, Scott B, Jurasic M, Howard J, Yu X, Bailey R, Sarkisian MR, Dickson DW, Petrucelli L, Lewis J. 2012. Neuronal sensitivity to TDP43 overexpression is dependent on timing of induction. Acta Neuropathol 123:807–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corcoran KA, Lu Y, Turner RS, Maren S. 2002. Overexpression of hAPPswe impairs rewarded alternation and contextual fear conditioning in a transgenic mouse model of Alzheimer's disease. Learn Mem 9:243–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crawley JN. 2007. What's wrong with my mouse? Behavioural phenotypying of transgenic and knockout mice. New Jersey (NJ): John Wiley and Sons [Google Scholar]
- 20.Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, Hitzemann RJ, Maxson SC, Miner LL, Silva AJ, Wehner JM, Wynshaw-Boris A, Paylor R. 1997. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology (Berl) 132:107–124 [DOI] [PubMed] [Google Scholar]
- 21.D'Adamo P, Wolfer DP, Kopp C, Tobler I, Toniolo D, Lipp HP. 2004. Mice deficient for the synaptic vesicle protein Rab3a show impaired spatial reversal learning and increased explorative activity but none of the behavioral changes shown by mice deficient for the Rab3a regulator Gdi1. Eur J Neurosci 19:1895–1905 [DOI] [PubMed] [Google Scholar]
- 22.Debiec J, Diaz-Mataix L, Bush DE, Doyere V, Ledoux JE. 2010. The amygdala encodes specific sensory features of an aversive reinforcer. Nat Neurosci 13:536–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Delgado MR, Olsson A, Phelps EA. 2006. Extending animal models of fear conditioning to humans. Biol Psychol 73:39–48 [DOI] [PubMed] [Google Scholar]
- 24.Einstein G. 1999. To each her own: sexual dimorphisms in Alzheimer's disease. Neurobiol Aging 20:439–440 [DOI] [PubMed] [Google Scholar]
- 25.Errijgers V, Van Dam D, Gantois I, Van Ginneken CJ, Grossman AW, D'Hooge R, De Deyn PP, Kooy RF. 2007. FVB.129P2-Pde6b+ Tyrc-ch/Ant, a sighted variant of the FVB/N mouse strain suitable for behavioral analysis. Genes Brain Behav 6:552–557 [DOI] [PubMed] [Google Scholar]
- 26.Fanselow MS. 1990. Factors governing one-trial contextual conditioning. Anim Learn Behav 18:264–270 [Google Scholar]
- 27.Fanselow MS, Decola JP, Young SL. 1993. Mechanisms responsible for reduced contextual conditioning with massed unsignaled unconditional stimuli. J Exp Psychol Anim Behav Process 19:121–137 [PubMed] [Google Scholar]
- 28.Farley SJ, McKay BM, Disterhoft JF, Weiss C. 2011. Reevaluating hippocampus-dependent learning in FVB/N mice. Behav Neurosci 125:871–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frankland PW, Josselyn SA, Anagnostaras SG, Kogan JH, Takahashi E, Silva AJ. 2004. Consolidation of CS and US representations in associative fear conditioning. Hippocampus 14:557–569 [DOI] [PubMed] [Google Scholar]
- 30.Gerlai R. 1996. Gene-targeting studies of mammalian behavior: is it the mutation or the background genotype? Trends Neurosci 19:177–181 [DOI] [PubMed] [Google Scholar]
- 31.Gimenez E, Montoliu L. 2001. A simple polymerase chain reaction assay for genotyping the retinal degeneration mutation (Pdebrd1) in FVB/N-derived transgenic mice. Lab Anim 35:153–156 [DOI] [PubMed] [Google Scholar]
- 32.Glazner KA, Odero GL, Anema E, Motnenko A, Schapansky J, Grossman D, Oliver DR, Glazner GW, Albensi BC. 2010. Strain-specific differences in memory and neuropathology in a mouse model of Alzheimer's disease. Life Sci 86:942–950 [DOI] [PubMed] [Google Scholar]
- 33.Gould TJ, Feiro O, Moore D. 2004. Nicotine enhances trace cued fear conditioning but not delay cued fear conditioning in C57BL/6 mice. Behav Brain Res 155:167–173 [DOI] [PubMed] [Google Scholar]
- 34.Hafner H. 2003. Gender differences in schizophrenia. Psychoneuroendocrinology 28 Suppl 2:17–54 [DOI] [PubMed] [Google Scholar]
- 35.Hanna A, Iremonger K, Das P, Dickson D, Golde T, Janus C. 2012. Age-related increase in amyloid plaque burden is associated with impairment in conditioned fear memory in CRND8 mouse model of amyloidosis. Alzheimers Res Ther 4:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Howlett DR. 2011. APP transgenic mice and their application to drug discovery. Histol Histopathol 26:1611–1632 [DOI] [PubMed] [Google Scholar]
- 37.Hurst JL, West RS. 2010. Taming anxiety in laboratory mice. Nat Methods 7:825–826 [DOI] [PubMed] [Google Scholar]
- 38.Janus C. 2008. Conditionally inducible tau mice—designing a better mouse model of neurodegenerative diseases. Genes Brain Behav 7 Suppl 1:12–27 [DOI] [PubMed] [Google Scholar]
- 39.Janus C, Welzl H. 2010. Mouse models of neurodegenerative diseases: criteria and general methodology. Methods Mol Biol 602:323–345 [DOI] [PubMed] [Google Scholar]
- 40.Jimenez AJ, Garcia-Fernandez JM, Gonzalez B, Foster RG. 1996. The spatiotemporal pattern of photoreceptor degeneration in the aged rd/rd mouse retina. Cell Tissue Res 284:193–202 [DOI] [PubMed] [Google Scholar]
- 41.Kim J, Miller VM, Levites Y, West KJ, Zwizinski CW, Moore BD, Troendle FJ, Bann M, Verbeeck C, Price RW, Smithson L, Sonoda L, Wagg K, Rangachari V, Zou F, Younkin SG, Graff-Radford N, Dickson D, Rosenberry T, Golde TE. 2008. BRI2 (ITM2b) inhibits Aβ deposition in vivo. J Neurosci 28:6030–6036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim JJ, Rison RA, Fanselow MS. 1993. Effects of amygdala, hippocampus, and periaqueductal gray lesions on short-term and long-term contextual fear. Behav Neurosci 107:1093–1098 [DOI] [PubMed] [Google Scholar]
- 43.Kishimoto Y, Nakazawa K, Tonegawa S, Kirino Y, Kano M. 2006. Hippocampal CA3 NMDA receptors are crucial for adaptive timing of trace eyeblink conditioned response. J Neurosci 26:1562–1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.LeDoux JE. 2000. Emotion circuits in the brain. Annu Rev Neurosci 23:155–184 [DOI] [PubMed] [Google Scholar]
- 45.LeDoux JE, Cicchetti P, Xagoraris A, Romanski LM. 1990. The lateral amygdaloid nucleus: sensory interface of the amygdala in fear conditioning. J Neurosci 10:1062–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lehman EJ, Kulnane LS, Gao Y, Petriello MC, Pimpis KM, Younkin L, Dolios G, Wang R, Younkin SG, Lamb BT. 2003. Genetic background regulates β-amyloid precursor protein processing and β-amyloid deposition in the mouse. Hum Mol Genet 12:2949–2956 [DOI] [PubMed] [Google Scholar]
- 47.Levine TR, Hullett CR. 2002. Eta squared, partial eta squared, and misreporting of effect size in communication research. Hum Commun Res 28:612–625 [Google Scholar]
- 48.Levites Y, Jansen K, Smithson LA, Dakin R, Holloway VM, Das P, Golde TE. 2006. Intracranial adeno-associated virus-mediated delivery of antipan amyloid β, amyloid β40, and amyloid β42 single-chain variable fragments attenuates plaque pathology in amyloid precursor protein mice. J Neurosci 26:11923–11928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li Y, Liu W, Oo TF, Wang L, Tang Y, Jackson-Lewis V, Zhou C, Geghman K, Bogdanov M, Przedborski S, Beal MF, Burke RE, Li C. 2009. Mutant LRRK2(R1441G) BAC transgenic mice recapitulate cardinal features of Parkinson's disease. Nat Neurosci 12:826–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Magara F, Muller U, Li ZW, Lipp HP, Weissmann C, Stagljar M, Wolfer DP. 1999. Genetic background changes the pattern of forebrain commissure defects in transgenic mice underexpressing the β-amyloid-precursor protein. Proc Natl Acad Sci USA 96:4656–4661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maynard CJ, Cappai R, Volitakis I, Cherny RA, Masters CL, Li QX, Bush AI. 2006. Gender and genetic background effects on brain metal levels in APP transgenic and normal mice: implications for Alzheimer β-amyloid pathology. J Inorg Biochem 100:952–962 [DOI] [PubMed] [Google Scholar]
- 52.Mineur YS, Crusio WE. 2002. Behavioral and neuroanatomical characterization of FVB/N inbred mice. Brain Res Bull 57:41–47 [DOI] [PubMed] [Google Scholar]
- 53.Mizuno K, Giese KP. 2010. Towards a molecular understanding of sex differences in memory formation. Trends Neurosci 33:285–291 [DOI] [PubMed] [Google Scholar]
- 54.Mizuno K, Ris L, Sanchez-Capelo A, Godaux E, Giese KP. 2006. Ca2+/calmodulin kinase kinase α is dispensable for brain development but is required for distinct memories in male, though not in female, mice. Mol Cell Biol 26:9094–9104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moore MD, Cushman J, Chandra D, Homanics GE, Olsen RW, Fanselow MS. 2010. Trace and contextual fear conditioning is enhanced in mice lacking the α4 subunit of the GABA(A) receptor. Neurobiol Learn Mem 93:383–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Myers KM, Davis M. 2002. Behavioral and neural analysis of extinction. Neuron 36:567–584 [DOI] [PubMed] [Google Scholar]
- 57.Ott BR. 1999. Cognition and behavior in patients with Alzheimer's disease. J Gend Specif Med 2:63–69 [PubMed] [Google Scholar]
- 58.Owen EH, Christensen SC, Paylor R, Wehner JM. 1997. Identification of quantitative trait loci involved in contextual and auditory-cued fear conditioning in BXD recombinant inbred strains. Behav Neurosci 111:292–300 [DOI] [PubMed] [Google Scholar]
- 59.Owen EH, Logue SF, Rasmussen DL, Wehner JM. 1997. Assessment of learning by the Morris water task and fear conditioning in inbred mouse strains and F1 hybrids: implications of genetic background for single-gene mutations and quantitative trait loci analyses. Neuroscience 80:1087–1099 [DOI] [PubMed] [Google Scholar]
- 60.Paylor R, Crawley JN. 1997. Inbred strain differences in prepulse inhibition of the mouse startle response. Psychopharmacology (Berl) 132:169–180 [DOI] [PubMed] [Google Scholar]
- 61.Paylor R, Tracy R, Wehner J, Rudy JW. 1994. DBA/2 and C57BL/6 mice differ in contextual fear but not auditory fear conditioning. Behav Neurosci 108:810–817 [DOI] [PubMed] [Google Scholar]
- 62.Phillips RG, LeDoux JE. 1994. Lesions of the dorsal hippocampal formation interfere with background but not foreground contextual fear conditioning. Learn Mem 1:34–44 [PubMed] [Google Scholar]
- 63.Pugh PL, Ahmed SF, Smith MI, Upton N, Hunter AJ. 2004. A behavioural characterisation of the FVB/N mouse strain. Behav Brain Res 155:283–289 [DOI] [PubMed] [Google Scholar]
- 64.Quirk GJ. 2002. Memory for extinction of conditioned fear is long-lasting and persists following spontaneous recovery. Learn Mem 9:402–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Radwanska K, Medvedev NI, Pereira GS, Engmann O, Thiede N, Moraes MF, Villers A, Irvine EE, Maunganidze NS, Pyza EM, Ris L, Szymanska M, Lipinski M, Kaczmarek L, Stewart MG, Giese KP. 2011. Mechanism for long-term memory formation when synaptic strengthening is impaired. Proc Natl Acad Sci USA 108:18471–18475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schafe GE, Nader K, Blair HT, LeDoux JE. 2001. Memory consolidation of Pavlovian fear conditioning: a cellular and molecular perspective. Trends Neurosci 24:540–546 [DOI] [PubMed] [Google Scholar]
- 67.Seabrook TJ, Iglesias M, Bloom JK, Spooner ET, Lemere CA. 2004. Differences in the immune response to long-term Aβ vaccination in C57BL/6 and B6D2F1 mice. Vaccine 22:4075–4083 [DOI] [PubMed] [Google Scholar]
- 68.Seong E, Saunders TL, Stewart CL, Burmeister M. 2004. To knockout in 129 or in C57BL/6: that is the question. Trends Genet 20:59–62 [DOI] [PubMed] [Google Scholar]
- 69.Sidman RL, Green MC. 1965. Retinal degeneration in the mouse: location of the rd locus in linkage group Xvii. J Hered 56:23–29 [DOI] [PubMed] [Google Scholar]
- 70.Smith DR, Gallagher M, Stanton ME. 2007. Genetic background differences and nonassociative effects in mouse trace fear conditioning. Learn Mem 14:597–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Van Dam D, De Deyn PP. 2006. Drug discovery in dementia: the role of rodent models. Nat Rev Drug Discov 5:956–970 [DOI] [PubMed] [Google Scholar]
- 72.Waddell J, Dunnett C, Falls WA. 2004. C57BL/6J and DBA/2J mice differ in extinction and renewal of extinguished conditioned fear. Behav Brain Res 154:567–576 [DOI] [PubMed] [Google Scholar]
- 73.Wahlsten D. 1973. Contributions of the genes albinism (c) and retinal degeneration (rd) to a strain-by-training procedure interaction in avoidance learning. Behav Genet 3:303–316 [DOI] [PubMed] [Google Scholar]
- 74.Wahlsten D, Metten P, Phillips TJ, Boehm SL, 2nd, Burkhart-Kasch S, Dorow J, Doerksen S, Downing C, Fogarty J, Rodd-Henricks K, Hen R, McKinnon CS, Merrill CM, Nolte C, Schalomon M, Schlumbohm JP, Sibert JR, Wenger CD, Dudek BC, Crabbe JC. 2003. Different data from different labs: lessons from studies of gene-environment interaction. J Neurobiol 54:283–311 [DOI] [PubMed] [Google Scholar]
- 75.Wolfer DP, Stagljar-Bozicevic M, Errington ML, Lipp HP. 1998. Spatial memory and learning in transgenic mice: fact or artifact? News Physiol Sci 13:118–123 [DOI] [PubMed] [Google Scholar]
- 76.Wong AA, Brown RE. 2006. Visual detection, pattern discrimination, and visual acuity in 14 strains of mice. Genes Brain Behav 5:389–403 [DOI] [PubMed] [Google Scholar]