Abstract
Many people in our society experience curtailment and disruption of sleep due to work responsibilities, care-giving, or life style choice. Delineating the health effect of acute and chronic disruptions in sleep is essential to raising awareness of and creating interventions to manage these prevalent concerns. To provide a platform for studying the health impact and underlying pathophysiologic mechanisms associated with inadequate sleep, we developed and characterized an approach to creating chronic disruption of sleep in laboratory mice. We used this method to evaluate how 3 durations of sleep fragmentation (SF) affect sleep recuperation and blood and lung analyte concentrations in male C57BL/6J mice. Mice housed in environmentally controlled chambers were exposed to automated SF for periods of 6, 12, or 24 h or for 12 h daily during the light (somnolent) phase for 4 sequential days. Sleep time, slow-wave amplitude, or bout lengths were significantly higher when uninterrupted sleep was permitted after each of the 3 SF durations. However, mice did not recover all of the lost slow-wave sleep during the subsequent 12- to 24-h period and maintained a net loss of sleep. Light-phase SF was associated with significant changes in serum and lung levels of some inflammatory substances, but these changes were not consistent or sustained. The data indicate that acute light-phase SF can result in a sustained sleep debt in mice and may disrupt the inflammatory steady-state in serum and lung.
Abbreviations: DWA, δ wave amplitude; DE, disk environment; E, time of euthanasia; G-CSF, granulocyte colony-stimulating factor; HC, home cage; HPA, hypothalamic–pituitary–adrenal; IP10, interferon-γ-induced protein 10 (CXCL10); KC, keratinocyte-derived chemokine (CXCL1); LCN2, lipocalin 2; MCP1, monocyte chemotactic protein 1 (CCL2); M-CSF, macrophage colony-stimulating factor; MIP1α, macrophage inflammatory protein; NREMS, non-rapid-eye-movement sleep; REMS, rapid-eye-movement sleep; SF, sleep fragmentation; SMET, simple main-effects test; SWS, slow-wave sleep; tPAI1, total plasminogen activator inhibitor 1
Many people in our society experience frequent curtailment and disruption of sleep due to work responsibilities, care-giving, or life style choice.4 In 1960, most Americans reported sleeping for 8.0 to 8.9 h per night, whereas in more recent surveys, many people report less than 5 h of sleep per night, with a mode of around 7 h.38,45,54 Evidence is accumulating to show that many common disease conditions develop in association with disrupted sleep.57 For example, abundant evidence indicates that disturbed or inadequate sleep is associated with increased risk for obesity, glucose intolerance, and type 2 diabetes (reviewed in reference 1). In addition, many individuals who suffer from acute and chronic disease conditions experience illness-related disruption of sleep.57 For example, as many as 75% of asthmatic persons are awakened by symptoms at least once a week, with about 40% experiencing symptoms on a nightly basis.65 In general, epidemiologic studies have shown that relatively long (8 h or longer) and short (less than 7 h) durations of nightly sleep are associated with greater risk of mortality from all causes.2,11,12,37,66
Societal and individual efforts to control sleep loss generally are based on recognition of the negative effects of sleep loss and sleepiness on performance and health. However, attempts to manage these problems may be offset by a personal desire to limit sleep time or the need to extend waking hours. Management of sleep duration is complicated further in that people often subjectively underestimate their sleepiness in comparison with objective measures of sleepiness, alertness, and performance.3,27,46,74 In addition, circadian disruption, interrupted sleep, and delayed sleep may lead to different risks or adverse effects. For example, chronic shift work, which disrupts both sleep and circadian coordination, may increase the risk of weight gain or type 2 diabetes to a greater degree than do sleep interruptions or delays that do not disrupt diurnal synchronization.47,52 Delineating the health effects of acute and chronic disruptions in sleep is essential to raising awareness of and creating interventions to manage these prevalent concerns.
The well-known homeostatic and circadian modulation of sleep and sleep propensity create potentially competing consequences with regard to reactions to loss of sleep during normal diurnal periods of sleep. In that situation, homeostatic drives to recover lost sleep may be countered by circadian drives that promote waking. Depending on the balance of these factors, the individual could either fully or partially recover the lost sleep or develop and maintain a sleep debt. These considerations led us to compare the effect of 3 durations of sleep fragmentation (SF) in laboratory mice: the initial 6 h of the light (somnolent) phase, mimicking a delay in the normal circadian onset of the somnolent phase; the entire duration of the light (somnolent) phase, mimicking some aspects of shift work; and an entire 24-h day (mimicking an ‘all-nighter’). The approach we use to cause SF has previously been reported to produce significant sleep disruption without concomitant increases in serum glucocorticoids or reductions in body weight.62 Our data document both the effects of varying durations of acute SF on subsequent recuperative sleep and the influence of exposure to SF on lung and serum markers of inflammation.
Materials and Methods
Experimental animals.
Male C57BL/6J mice were purchased from the Jackson Laboratory (Bar Harbor, ME) at 6 wk of age. Prior to experimental use, mice were housed in groups of 5 in an environmentally controlled chamber that was maintained under a 12:12-h light:dark cycle, a temperature of 25 °C, and relative humidity of 40% to 60%. Food (LabDiet 5001, PMI Nutrition International, St Louis, MO) and tap water were always available ad libitum. Cages were solid-bottom and open-top and contained woodchip bedding (Beta Chip, Northeastern Products, Warrensburg, NY). Mice were maintained by using conventional husbandry practices, with cages changed weekly. All mice were housed individually after assignment to a study group. All procedures involving animals were approved in advance by the Laboratory Animal Care and Use Committee of the Southern Illinois University School of Medicine (protocol number 168-07-007). The animal facility at Southern Illinois University School of Medicine has maintained full AAALAC accreditation since 1982. All mice were free of known infections with common rodent microbial and parasitic agents, as monitored by using monthly testing of sentinel mice housed in the same room.
Surgery.
Mice were surgically implanted with electrodes to permit monitoring of the EEG and EMG. Anesthesia was induced by subcutaneous injection of a mixture of ketamine (50 mg/kg) and xylazine (50 mg/kg) and was supplemented with additional anesthetic during surgery when needed. All surgery was conducted by using standard aseptic techniques. EEG electrodes consisted of 4 insulated stainless steel wires (Plastics One, Roanoke, VA) that were positioned visually parallel to and under the skull in bilateral frontal (approximately 1 mm anterior to bregma and 2 mm to the left and right of midline) and parietotemporal (approximately 3 to 4 mm posterior to bregma and 2 mm to the left and right of midline) positions. All electrodes were inserted into a pedestal that was secured to the skull with dental acrylic. One of the electrodes was made continuous with cable shielding and served as a ground; this electrode was not used for data acquisition. Two of the other 3 electrodes were referenced against each other in the combination that provided the best visual differentiation of 3 vigilance states (wakefulness, slow-wave sleep [SWS], and rapid-eye-movement sleep [REMS]) when signals were displayed on a polygraph. EMG electrodes (Plastics One) were placed subcutaneously overlying nuchal muscles of the left and right sides of the body and were referenced against each other.
After surgery, mice were housed in individual cages in a sound shielded chamber under a 12:12-h light:dark cycle at 25 ± 1 °C. Ibuprofen (1 mg/mL) was added to the drinking water from 1 d before through 4 d after surgery, to provide analgesia.28 Other husbandry and housing conditions were the same as those described previously, with the exception that individually housed mice received a small weigh boat as an enrichment device. A minimum of 2 wk was permitted for recovery from surgery prior to the start of data collection.
Sleep fragmentation.
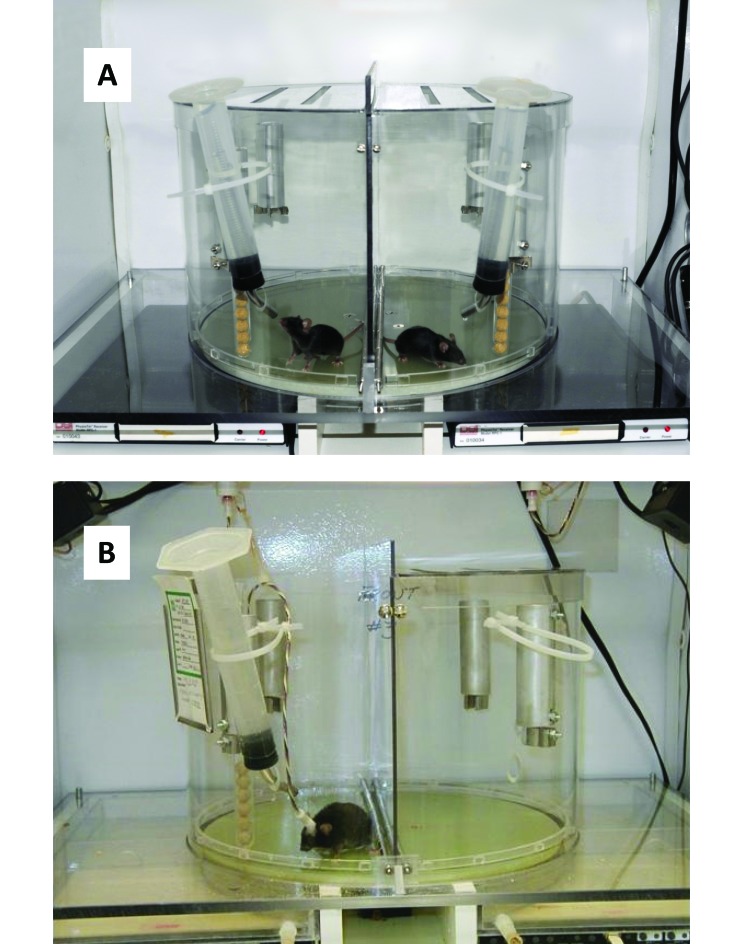
For induction of SF, mice were placed on a disk that rotates slowly in a random direction for 8 s of every 30-s interval; food and water were available (Figure 1). The disk diameter was 15 in., and the wall height was 10 in. A perforated fitted cover was placed over this cylinder to prevent the mice from escaping. The disk rotated at a speed of 20 to 25 s per full rotation. Disk rotation was not associated with noise audible to humans. Additional details of construction are available on request (see also reference 62). Bedding was not used in the device to minimize the likelihood of interference with disk rotation and potential entrapment of the mice.
Figure 1.
Apparatus for producing sleep fragmentation in mice. (A) A clear wall separates the 2 mice housed in the sleep fragmentation apparatus. (B) Here a mouse is housed on the unit and is tethered for sleep recording. Receivers positioned under the disk can be used for concurrent measurement of core temperature and locomotor activity. Food and water are available ad libitum.
The 8-s period of rotation occurred at a random time within each 30-s interval during exposure to SF. An 8-s period of disk rotation was chosen because at the speed used, 8 s allowed the disk to rotate 180°, thus ensuring that the mouse would contact the wall during each rotational period. Mice that are asleep when rotation occurs awaken when they contact the wall that bisects the cylinder. Because the timing of disk rotation was random within the 30-s period and because each rotation required 8 s, disk rotations in successive 30-s intervals could be separated by as much as 44 s and were limited by the hardware to a minimum of 3 s between rotations. Therefore, if the disk rotated during the first 8 s of one 30-s interval and during the last 8 s of the next interval, the second rotation followed the first by 44 s. If a rotation occurred during the last 30 s of an interval, another rotation could not begin within the next 3 s. During SF, mice experienced 120 8-s epochs of disc rotation each hour. When mice were housed on the disk, it was cleaned daily immediately after light onset. This cleaning took only a few minutes and was an inherent part of the paradigm.
Each disk held 2 mice that were separated by a clear acrylic wall that bisected the disk. Mice were assigned randomly to receive exposure to SF for 6, 12, or 24 h, respectively designated as SF(0–6), SF(0–12) and SF(0–24), with diurnal times 0 or 24 denoting the time of light onset and time 12 denoting the time of dark onset (Figure 2). Some mice were exposed to SF(0–12) for 4 sequential days, designated as SF(0–12)×4. SF always began at light onset (circadian time 0). Mice were housed on the SF disk and connected to the tether at 48 h prior to the beginning of data collection. During this adaptation period, the disk rotated for 8 s of every 30 min to accustom the mice to this process. Data collected on the second day of adaptation were used as baselines for each mouse (that is, the disk environment [DE] condition). The mice remained on the disk for the duration of the study.
Figure 2.
Experimental design. Open box, light phase of the diurnal cycle; filled box, dark phase; X, time of euthanasia of mice for collection of serum and lung tissue; HC, home cage; DE, disk environment; SF, sleep fragmentation.
Sleep measurement.
For collection of EEG and EMG data, mice were tethered to a 6-channel electrical swivel by using a lightweight cable (Plastics One) that permitted unrestricted movement. Throughout all recording sessions, the mice could move about freely in their cages and had continuous access to food and water. Analog signals from the EEG electrodes were amplified and then filtered into δ (1–4 Hz) and θ (5–8 Hz) components by using band-pass filters (Coulbourn Electronics, LeHigh Valley, PA). Filtered data were stored after analog-to-digital conversion (Cambridge Electronic Design, Cambridge, UK) as average values for each 10-s interval of the recording period. EMG analog signals were processed similarly, without filtering.
Sleep data were scored by assigning a specific vigilance state (SWS, REMS, or waking) to each 10-s epoch of the recording period by using a computer-assisted scoring method and custom software (Quality Software, Springfield, IL). For each mouse, EEG and EMG tracings were initially examined visually to set threshold values for δ wave amplitude (DWA) that occurred in association with SWS, EMG amplitude associated with periods of movement, and θ-to-δ ratios associated with REMS. The computer algorithm used these thresholds to score each animal's vigilance states over the entire recording period. An interval was scored as SWS whenever the DWA exceeded the SWS threshold concurrent with a low-amplitude EMG signal. REMS was identified by low-amplitude EEG and EMG signals that occurred together with a high θ-to-δ ratio in the EEG. At all other times, mice were considered to be awake. Transitional epochs received vigilance state assignments according to the average DWA and EMG signals during the epoch. All computer-scored data were verified visually prior to final analysis. This visual assessment also documented that EEG-based arousal occurred in association with disk rotation. Sleep values were summarized into 6-h time blocks over the entire duration of the study. Parameters analyzed were percentage time in SWS, percentage time in REMS, DWA during SWS, the lengths of uninterrupted epochs (bouts) of SWS and REMS, and the number of bouts of SWS and REMS.
Cytokine, chemokine, insulin and adipokine measurements.
For collection of serum and lung samples, separate groups of mice that did not undergo surgery were exposed to SF(0–6), SF(0–12), or SF(0–24) . In all cases, euthanasia was performed immediately after the period of SF. Control mice were housed individually in a standard home cage (HC) without manipulation and underwent euthanasia at the same time points as did the SF mice. An additional group of mice (n = 8) were exposed to the DE (that is, placement on the disk with rotation occurring for 8 s of every 30 min) prior to euthanasia. The durations of SF, the time points for euthanasia (E), and the total numbers of mice in each treatment group were as follows: HC(E6), n = 12; DE(E6), n = 8; SF(0–6)E6, n = 12; HC(E12), n = 8; DE(E12), n = 8; SF(0–12)E12, n = 8; HC(E24), n = 8; DE(E24), n = 10; SF(0–24)E24, n = 8. Euthanasia was performed by cardiac exsanguination under isoflurane anesthesia. Serum and lung were collected and frozen at –80 °C for subsequent analysis.
Cytokines, chemokines, insulin, and adipokines were measured by using multiplex bead-based assays as described by the manufacturer (MCytoMag 70K and MadKMag 71K, Millipore, Billerica, MA) and were analyzed (model 100IS, Luminex, Austin, TX) by using BioPlex Manager 5.0 software (BioRad, Hercules, CA). Minimal detectable concentrations (pg/mL) for individual analytes were 10.3 for IL1α, 5.4 for IL1β, 1.0 for IL2, 1.1 for IL6, 2.0 for IL10, 0.7 for IL17, 6.7 for monocyte chemotactic protein 1 (MCP1), 1.7 for granulocyte colony-stimulating factor (G-CSF), 1.1 for IFNγ, 0.8 for IFNγ-induced protein (IP10), 2.3 for keratinocyte-derived chemokine (KC), 7.7 for macrophage inflammatory protein 1α (MIP1α), 4.2 for leptin, 1.1 for resistin, 4.0 for total plasminogen activator inhibitor 1 (tPAI1), and 13.0 for insulin. For adiponectin, serum samples were measured by using the Quantikine ELISA kit (R and D Systems, Minneapolis, MN) according to the manufacturers’ instructions. Samples were assayed in duplicate and reported in pg/mL for cytokines and adipokines and ng/mL for adiponectin.
Statistics.
Sleep data were evaluated by using repeated-measures ANOVA (general linear measures procedure) with the SAS statistics package. Baseline and postSF values then were compared at individual time points by using a simple main-effects test (SMET).35 Although comparisons were preplanned, we nonetheless assessed the results of the SMET by using a conservative P value (that is, 0.02) to mitigate potential erroneous interpretations that may have resulted from performing multiple comparisons.
The SPSS statistics package (version 20, IBM, New York, NY) was used for analysis of cytokine data. Statistical evaluation of cytokine concentrations was performed by using log-transformed values, due to the nonnormal distribution of values.24,55 SF, DE, and HC conditions were compared by using one-way ANOVA for each duration of exposure (for example, SF(0–6)E6 compared with HC(E6) compared with DE(E6)), with Tukey follow-up. Cytokine concentrations that were below the assay limits of detection were assigned the minimal detectable concentration for purposes of statistical analysis. Descriptive statistics are expressed throughout as mean ± SEM. A P value of less than 0.05 was considered to indicate statistically significant effects.
Results
Separate groups of mice were used for measurements of sleep parameters and tissue analytes. The experimental design is illustrated in Figure 2.
Behavior.
SF(0–6).
In the first study, sleep was monitored in C57BL/6J mice (n = 7) for 24 h before, during, and for 18 h after 6 h of SF that began immediately after light onset. Sleep data were analyzed by using repeated-measures ANOVA for treatment (DE [that is, baseline] compared with SF) as a function of time (Table 1). All sleep parameters showed significant (P < 0.05) effects of treatment or time or both, with significant interactions. Follow-up assessment by using SMET indicated that as compared with the baseline period, the 6-h period of SF was associated with significant (P < 0.02) reductions in the amount of time spent in SWS and REMS (Figures 3 A and 4 A), the length of SWS and REMS bouts (Figures 3 D and 4 D), and the number of SWS and REMS episodes (Figure 4 G). After the end of the SF period, mice showed longer bouts of SWS and REMS during the remaining 6 h of the light phase (Figures 3 D and 4 D) as compared with baseline values; no additional significant effects were detected during the 18- h period after SF when data were analyzed over 6-h intervals (Figures 3 and 4). However, when data were evaluated over shorter (2-h) intervals, as is often done in short-term sleep loss studies, mice showed increases in some measures, as follows, based on paired t tests : percentage time in SWS during hours 8 to 10 (53% ± 6% during baseline compared with 61% ± 5% after SF, P = 0.032); DWA during SWS during hours 6 through 8 (97 ± 1% during baseline compared with 102% ± 1% after SF, P = 0.003); percentage time in REMS during hours 12 through 14 (0.5% ± 0.2% during baseline compared with 2.2% ± 0.8% after SF, P = 0.040); REMS bout length during hours 10 through 12 (0.27 ± 0.08 min during baseline compared with 0.85 ± 0.18 min after SF, P = 0.028) and 14 to 16 h (0.54 ± 0.12 min during baseline compared with 0.99 ± 0.06 min after SF, P = 0.029), number of REMS bouts during hours 12 through 14 (1.1 ± 0.6 during baseline compared with 4.3 ± 1.4 after SF, P = 0.025). Taken together, the REMS rebound was sufficient for recovery of lost REMS within the subsequent 18-h period, but mice did not recover lost SWS and maintained a significant SWS debt (Figure 5).
Table 1.
ANOVA Pvalues
| SF(0–6), n = 7 |
SF(0–12)×4, n = 7 |
SF(0–24), n = 6 |
|||||||
| Treatment | Time | Interaction | Treatment | Time | Interaction | Treatment | Time | Interaction | |
| % time in SWS | 0.0021 | 0.0001 | <0.0001 | 0.0337 | <0.0001 | <0.0001 | 0.0098 | 0.0003 | <0.0001 |
| SWS bout length | 0.0321 | 0.0001 | <0.0001 | 0.0046 | <0.0001 | <0.0001 | 0.0443 | <0.0001 | <0.0001 |
| No. of SWS bouts | NS | 0.0053 | 0.0039 | 0.0028 | <0.0001 | <0.0001 | 0.0044 | <0.0001 | <0.0001 |
| DWA during SWS | NS | NS | 0.0215 | NS | <0.0001 | 0.0114 | NS | 0.0024 | NS |
| % time in REMS | NS | 0.0165 | 0.0020 | NS | <0.0001 | <0.0001 | NS | 0.0002 | <0.0001 |
| REMS bout length | NS | 0.0006 | 0.0006 | 0.0466 | <0.0001 | <0.0001 | 0.0060 | <0.0001 | <0.0001 |
| No. of REMS bouts | NS | 0.0050 | 0.0012 | NS | 0.0053 | <0.0001 | NS | 0.0170 | 0.0011 |
NS, not significant (P > 0.05).
Figure 3.
SWS in C57BL/6J mice before, during and after exposure to sleep fragmentation. Data are given as mean ± SEM of values collected during the preceding 6 h, with n = 7 for SF(0–6) and SF(0–12)×4 d but n = 6 for SF(0–24). Baseline values were collected on the disk environment with minimal rotation and are repeat-plotted over the SF days to facilitate visual comparison. The dashed horizontal line indicates the mean value during the 24-h baseline period. DWA values are normalized to the 24-h average baseline values for each mouse and are presented as percentages. Solid bars on the x axis indicate the dark phase; cross-hatched bars indicate the period of SF (hours 0 through 6 in left panels, 0 through 12 for 4 consecutive days in middle panels, and 0 through 24 in right panels). *, P < 0.05 (paired t test).
Figure 4.
REMS in C57BL/6J mice before, during and after exposure to sleep fragmentation. Data are given as mean ± SEM of values collected during the preceding 6 h. n = 7 for SF(0–6) and SF(0–12)x4 d, and n = 6 for SF(0–24). Baseline values were collected on the disk environment with minimal rotation and are repeat-plotted over the SF days to facilitate visual comparison. The dashed horizontal line indicates the mean value during the 24-h baseline period. Solid bars on the x axis indicate the dark phase; cross-hatched bars indicate the period of SF (hours 0 through 6 in left panels, 0 through 12 for 4 consecutive days in middle panels, and 0 through 24 in right panels). *, P < 0.05 (paired t test).
Figure 5.
Sleep debt during and after exposure to SF. Data for individual mice were converted into the difference between the value during SF and that measured during the comparable time point during the baseline (DE) period. Bars represent the mean ± SEM values for all mice at each time point, with n = 7 for SF(0–6) and SF(0–12)×4 but n = 6 for SF(0–24). *, P < 0.05 (paired t test).
SF(0–12)×4.
In a second study, sleep was monitored in C57BL/6J mice (n = 7) for 48 h before and throughout 4 subsequent sequential days on which SF occurred during the 12-h light (somnolent) phase of the diurnal cycle but not during the dark (active) phase. For this group of mice, data from the 2 baseline (DE) days were averaged and are shown. All sleep parameters showed significant (P < 0.02) effects of treatment or time or both, with significant interactions (Table 1). Follow-up assessment using SMET indicated that during the light-phase exposure to SF, as compared with the baseline period, mice spent significantly less time in SWS and REMS (Figures 3 B and 4 B) and had shorter bouts of SWS and REMS (Figures 3 E and 4 E) and a greater number of SWS bouts (Figure 3 H; P < 0.02 for all comparisons). During the subsequent dark phases, mice showed only sporadic significant changes in measures of SWS (Figure 3) but developed significant and sustained increases in the time spent in REMS (P < 0.02; Figure 4 B). During the initial 12-h SF and recovery periods, mice recovered lost REMS but retained a significant SWS debt (Figure 5).
SF(0–24).
Sleep was monitored in C57BL/6J mice (n = 6) during and for 24 h before and after exposure to SF for 24 h. Most of the sleep parameters showed significant (P < 0.02) effects of treatment and/or time, with some significant interactions (Table 1). Follow-up assessment using SMET indicated that during the 24-h period of SF, as compared with the baseline (DE) period, mice spent significantly less time in both SWS and REMS (Figures 3 C and 4 C), showed shorter bouts of SWS and REMS (Figures 3 F and 4 O) and a greater number of SWS and REMS bouts at some time points (Figures 3 I and 4 I; P < 0.02 for all comparisons). During the subsequent 24-h period, mice showed significant (P < 0.02) increases in both SWS bout length (Figure 3 F) and DWA during SWS (Figure 3 L), indicating consolidation and increased depth of sleep. During the day after SF, mice largely had recovered lost REMS but retained a significant SWS debt (P < 0.02; Figure 5).
Serum cytokines, chemokines, adipokines and insulin.
Insulin and a panel of cytokines, chemokines and adipokines were measured in serum collected from mice that were euthanized either immediately after placement on the disk with minimal rotation (8 s every 30 min for 6, 12, or 24 h; DE) or exposure to SF (disk rotation for 8 s of every 30 s for 6, 12, or 24 h) or at the same time points for mice maintained under HC conditions (n = 8 to 12 per group).
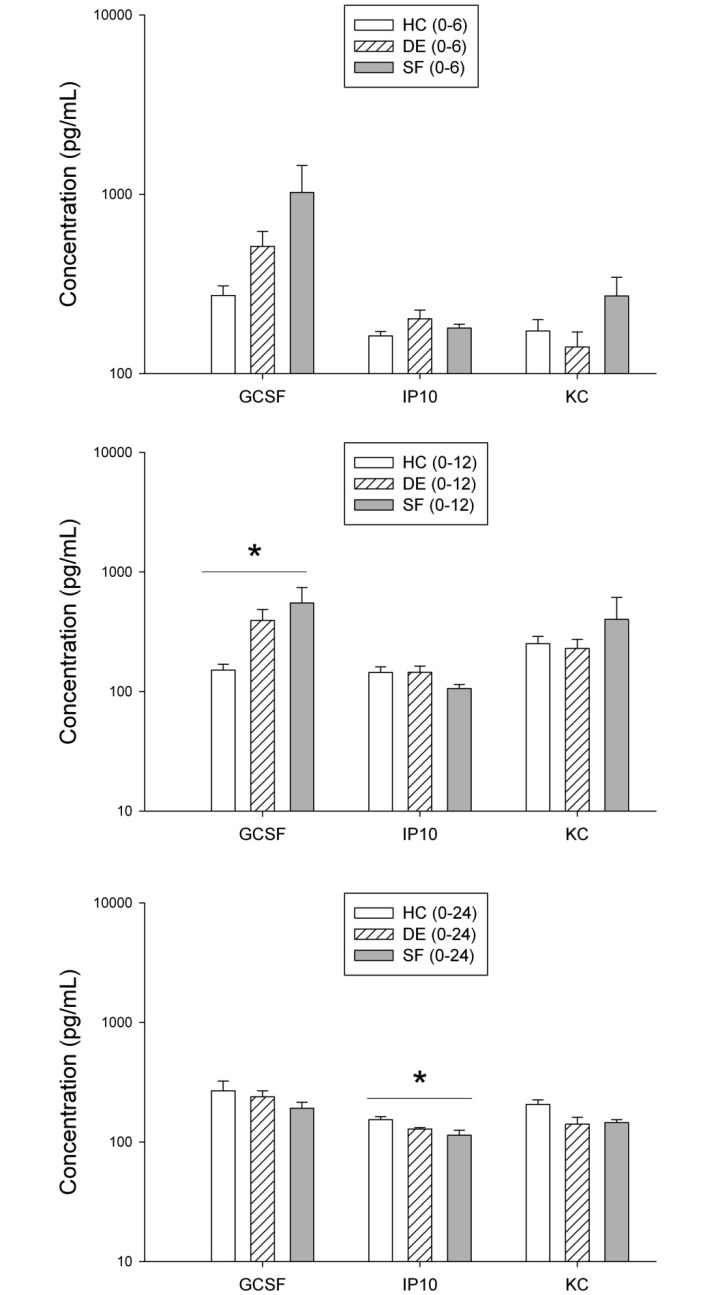
In most samples, concentrations of IL1β, IL5, IL6, IL10, MCP1, and MIP1α were below the limits of assay detection, and therefore these analytes could not be assessed. Concentrations of G-CSF, IP10, and KC were measurable within the limits of the assay. Exposure to SF for 6 h was not associated with significant changes in any of these 3 analytes. Mice exposed to SF(0–12) and SF(0–24) showed significant changes in serum G-CSF and IP10, respectively (P < 0.05, Figure 6).
Figure 6.
Cytokines and chemokines in serum of mice exposed to HC, DE, or SF. All concentrations are expressed as pg/mL. Data are plotted against a log scale and were analyzed based on log-transformed data. Data are given as mean ± SEM. HC(E6), n = 10; DE(E6), n = 8; SF(0–6)E6, n = 12; HC(E12), n = 7; DE(E12), n = 8; SF(0–12)E12, n = 8; HC(E24), n = 8; DE(E24), n = 10; SF(0–24)E24, n = 8. *, P < 0.05 (ANOVA with Tukey follow-up).
SF(0–6) was associated with significantly (P < 0.05, Tukey) lower serum leptin, as compared with HC; leptin concentrations were 66 ± 8, 43 ± 14 and 25 ± 6 pg/mL for HC, DE, and SF mice, respectively (n = 8 per group). Resistin was significantly (P < 0.05, Tukey) higher in mice exposed to DE as compared with HC; resistin concentrations were 100 ± 6, 137 ± 13, and 110 ± 5 pg/mL for HC, DE, and SF, respectively (n = 8 per group). Values for insulin, tPAI1, and adiponectin were not significantly different across treatment groups
Lung cytokines and chemokines.
A panel of cytokines and chemokines were measured in lung collected from mice that were euthanized either immediately after DE or SF for 6, 12, or 24 h or at the same time points for HC mice (n = 8 to 12 per group; Figure 7). Exposure to SF for 6 or 12 h was associated with sporadic significant (P < 0.05, Tukey) changes in several proinflammatory mediators, but these effects were not consistent between the 2 durations (Figure 7). Compared with those exposed to SF for 6 or 12 h, mice exposed to SF(0–24) showed significant changes (P < 0.05, Tukey) in a greater number of mediators (Figure 7). Notably, exposure to SF for 24 h was associated with significantly lower concentrations of IL1α, IL2, IL10, IL17, G-CSF, IFNγ, and KC as compared with values from HC or DE mice or both. MIP1α was below limit of detection in all lung samples.
Figure 7.
Cytokines and chemokines in lung of mice exposed to HC, DE, or SF. All concentrations are expressed as pg/mg protein. Data are plotted against a log scale and were analyzed based on log-transformed data. Data are given as mean ± SEM. HC(E6), n = 9; DE(E6), n = 8; SF(0–6)E6, n = 12; HC(E12), n = 9; DE(E12), n = 8; SF(0–12)E12, n = 10; HC(E24), n = 8; DE(E24), n = 10; SF(0–24)E24, n = 10. * denotes P < 0.05 by ANOVA with Tukey follow-up.
Discussion
The data presented here characterize sleep disruptions created in mice exposed to SF for 3 different durations within a 24-h cycle: the initial 6 h of the light (somnolent) phase (SF(0–6)); the entire duration of the light (somnolent) phase (SF(0–12)); and the entire 24-h day (SF(0–24)). SF(0–6) causes a delay in the normal time of sleep onset and may be relevant to extended wakefulness or difficulty in initiating sleep in people. SF(0–12) may mimic some aspects of human shift work, given that sleep in this model is perturbed during the diurnal phase normally associated with sleep but can occur freely during the normal active phase. SF(0–24) causes abnormal arousal during an anticipated sleep period, with no opportunity for consolidated sleep until the next anticipated sleep period.
In our system, exposure to SF(0–6) creates disturbed sleep in a manner that is temporally analogous to that produced by the so-called ‘gentle handling’ method of sleep deprivation that is used in many studies of sleep loss in rodents. However, an important distinction is that SF does not cause complete loss of sleep. Nonetheless, as in studies that used gentle handling to cause total sleep deprivation (for example, references 5, 21, 29, 30, and 69), we found that SF(0–6) was associated with transient increases in the proportion of time spent in SWS and REMS, the number and length of REMS bouts, and DWA during SWS during some 2-h intervals within the 18-h period after SF as compared with the same interval on the baseline (DE) day. Mice exposed to SF(0–6) in our study completely recovered lost REMS during the subsequent 18-h period in which uninterrupted sleep was permitted yet did not recover lost SWS and retained a significant SWS debt at the end of the recording period. Mice exposed to SF(0–12) or SF(0–24) also maintained a SWS debt after a recovery period equivalent to the period of SF but did recover lost REMS. Furthermore, mice exposed to SF(0–12) did not appear to recover lost SWS time during the dark phase even after repeated exposure for up to 4 sequential days. Therefore, our model produces a combination of SF, sleep loss, and accrued SWS debt. In contrast to SWS, mice did appear to recover lost REMS during the recovery period after all SF regimens.
As in our findings, the literature also contains reports of limited recovery of lost sleep in rodents. A recent study that used the same SF apparatus as that we used here reported that exposure of C57BL/6J mice to light-phase SF for 9 consecutive days resulted in severe fragmentation of NREMS and almost total absence of REMS throughout 9 sequential 12-h light phases.62 During the dark period, when ad libitum sleep was permitted, the amounts of time spent in NREMS and REMS were not different from control values, indicating that the mice recovered little of the lost sleep.62 Analysis of the EEG revealed a trend for increased power in the peak frequency of the NREMS EEG spectra during the dark period, but this effect did not achieve statistical significance.62 Therefore, these previous findings62 are quite similar to those we report here. However, another group produced SF in C57BL/6J mice by applying an automated tactile stimulus every 2 min for 6 h after light onset or for 15 consecutive days during the entire 12-h light phase.53,59,60 After the 6-h exposure, mice spent more time in SWS and REMS during the subsequent dark period, with no change in total SWS time over the 24-h period, and DWA was elevated during the first 2 h after SF ended.59 Mice that similarly underwent 15 d of SF during the 12-h light phase preserved their sleep duration, sleep state distribution, and cumulative δ frequency power.53,60 Another group housed C57BL/6 mice on a rotating disk similar to our system, with the disk programmed to produce 60 arousals per hour during an entire 24-h day, resulting in a total of 8 h of rotation daily.7 In contrast, mice in our study experienced 120 arousals per hour (1 rotation every 30 s, or 120 per hour), for a total of 16, 32, and 64 min of rotation during SF for 6, 12, or 24 h. General effects on SWS and REMS time, bout length, and number of bouts were similar between the studies in reference 7 and the current study.
An automated, 2-compartment rotational device similar to the one described here for mice has been used to produce SF in rats and can also be used to produce total sleep deprivation by increasing the duration, directional variability, and speed of disk rotation.41,42 Treadmill systems have also been used to evaluate the effect of SF in rats.48,49 Rats exposed to 24 h of treadmill-induced SF showed a large reduction in total REMS time and shorter NREMS episode duration but near-normal levels of total NREMS time per 24 h.49 EEG measures during the recovery phase after either 6 or 24 h of SF showed increases in the average NREMS episode duration and NREMS δ power during sleep.49 In addition, extracellular adenosine in the basal forebrain, which is postulated to reflect sleep drive, was significantly elevated during SF.49
In other studies, rats that were permitted to sleep after long-term total sleep deprivation showed a large REMS rebound during the first 24 h after sleep is permitted, with little or no rebound in NREMS.17,61 Similar patterns of recovery have been reported in rats that experienced multiple cycles of restricted and ad libitum sleep.18 Another group found that rats exposed to cycles of 3 h of SD and 1 h of sleep opportunity continuously for 4 d showed a 60% reduction in total sleep time over 24 h, an increase in REMS and NREMS time during the sleep opportunities, an initial increase but subsequent gradual attenuation in NREMS EEG δ power within and across days, and modest or negative compensatory sleep during the 2-d recovery period, such that most of the lost NREMS, REMS, and EEG δ power was not recovered in that time.13 In a similar study, rats were subjected to 18 or 20 h of sleep deprivation followed by a 6-h or 4-h sleep opportunity, respectively, for 5 consecutive days.33,34 In response to the first sleep deprivation block on day 1, rats responded during the sleep opportunity with enhanced NREMS δ power and increased REMS as compared with baseline.33,34 However, after the blocks of sleep deprivation on days 2 to 5, rats did not show enhanced NREMS δ power during the sleep opportunities, did not increase NREMS and REMS duration despite accumulating a growing sleep debt on each consecutive day, and—despite significant loss of sleep over 5 d—regained virtually none of their lost sleep during a full 3-d recovery period.33,34
In a human study, healthy middle-aged men experienced a night of normal sleep followed by 1 night of sleep deprivation or 5 consecutive nights with 4 h of sleep per night (repeated sleep restriction), in either case followed by a recovery night.58 Objective and subjective sleepiness increased immediately in response to sleep restriction, and sleep latencies after the second and third sleep restriction periods were equivalent to those observed after sleep deprivation.58 A statistical model revealed that perceived sleepiness and performance lapses did not progressively worsen across days of sleep restriction.58 Thus, adaptation to chronic sleep restriction appeared to develop beyond 3 d of restriction in human males.58 These findings in rats and people thus appear to be consistent with our current findings and those of others in mice with regard to limited or absent resolution of sleep debt after cessation of a period of disrupted sleep. However, one group concluded that slow-wave amplitude was homeostatically conserved in rats during and after period of sleep loss;40 This group studied rats that were permitted to sleep only during the initial 4 h during the light phase for 5 d. During the daily period in which sleep was prevented, the number of short (less than 20 s) sleep bouts increased, and slow-wave (1 to 6 Hz) power in the EEG was higher during both waking and short sleep bouts, most prominently in the occipital cortex.40 During the 4-h sleep periods and the recovery period after the sleep restriction phase of the study, slow-wave amplitude was higher than baseline levels, particularly in the frontal cortex.40 Other work has generally not used a similar approach to data analysis. Furthermore, the ability to recover lost slow-wave amplitude may not be equivalent to recovery of sleep. Therefore, these issues will clearly require additional study.
The timing of the cessation of sleep deprivation or SF likely contributes to the subsequent recuperative response, given that circadian modulation of the propensity for wakefulness may mitigate a homeostatic need for sleep when sleep is permitted only at the ‘wrong’ diurnal time. Others13,31,36,39,61,68 previously have demonstrated circadian influences on the homeostatic response to sleep loss in a number of species, albeit not (to our knowledge) in mice. In our study, the duration and termination of SF are confounded with respect to evaluation of circadian compared with homeostatic influences, but the model nonetheless offers an approach to studying these interactions. Such information would have implications for individuals who engage in chronic or irregular shift work, as behavioral accommodation to the sleep loss may take several days to develop or may never occur to a degree sufficient to be restorative. Our mouse data suggest that spontaneous adaptation to misalignment of circadian cues for sleep and the opportunity to engage in sleep does not occur in a manner sufficient to restore lost sleep. However, in comparison with mice, this accommodation may be accelerated in shift workers because they can anticipate the occurrence of opportunities to sleep.
Our study also evaluated the effect of SF on serum concentrations of insulin and panels of cytokines, chemokines, and adipokines. Inadequate sleep is increasingly being associated with impaired glucose regulation in both people and animals (reviewed in references 70 and 75). In addition to adipokines produced by adipocytes, several cytokines and chemokines, including MCP1 (CCL2) and IL6, are produced by macrophages in adipose tissue and influence glucose homeostasis.56,64 Our study found no significant effect of acute SF for as long as 24 h on serum concentrations of insulin, tPAI1, or adiponectin, and effects on leptin and resistin were transient. However, in longer studies, other groups have reported significant changes in these measures. For example, one study showed that mice exposed to light-phase SF for 10 d developed impaired glucose tolerance.7 In rats, exposure to repeated long periods of disrupted sleep or control conditions over 10 wk led to low plasma concentrations of leptin,18 and rats subjected to 20 h of SD per day for 8 d with a daily 4-h period of unrestricted rest per day showed reduced plasma glucose, insulin, and leptin levels.6 In a crossover study of healthy human men, a single night of SF reduced REMS but not SWS and was associated with a shift in normal circadian patterns of insulin concentrations.23
In our study, serum concentrations of several markers of inflammation (G-CSF, IP10, and KC) were likewise minimally affected by SF for periods of as long as 24 h. In contrast, a considerable number of analytes were altered in lung by exposure to SF(0–24). A significant reduction on concentrations relative to HC or DE conditions or both occurred for several analytes (IL1α, IL2, IL10, IL17, G-CSF, IFNγ, and KC). The development of immune suppression after sleep disruption has previously been reported by others.14-16,63,76
The induction of SF and SD in rodents often generates concern about the creation of nonspecific stress, which is relevant to both animal wellbeing and data interpretation. Production of sleep loss by either gentle handling or automated methods generally elicits only modest, if any, activation of the hypothalamic–pituitary–adrenal (HPA) axis in mice or rats (for example, see references 9, 19, 22, 25, 26, 51, and 67). We did not measure sympathoadrenal or HPA axis activation in mice in our study. However, a recent study that used the same SF apparatus and schedule as we used here reported that mice exposed to light-phase SF for 9 consecutive days had no significant increases in serum glucocorticoids or reductions in body weight.62 Some degree of nonspecific stress is likely to be inherent to states of sleep loss, even in people. In both humans and rodents, sleep deprivation and restriction are often associated with modest activation of both the autonomic sympathoadrenal system and the HPA axis, and this activated state could in turn influence the reactions of these systems to subsequent homeostatic challenges.50 Theoretically, HPA activation or diurnal disruption could become progressively more severe as the duration of sleep disruption continues, or, alternatively, animals may adapt to this perturbation, such that HPA activation abates with time. Illustrating this adaptation, one study of C57BL/6J mice found that gentle handling of mice daily for 6 d was associated with a time-dependent elevation in serum corticosterone concentrations,44 whereas a subsequent similar study reported no change after 6 d.71 Mild nonspecific stress and activation of stress-responsive neuroendocrine systems may be inherent to states of sleep loss, and this altered physiologic steady-state may reflect, incorporate, or exacerbate, as compared with confound or cause, both the loss of sleep and its consequences. Furthermore, activation of the HPA axis or administration of glucocorticoids promotes arousal and sleeplessness, and in humans, insomnia is associated with a 24-h increase of ACTH and cortisol secretion, consistent with hyperarousal.10,72,73
The approach to SF that we have described here has some limitations that merit consideration. Both the social environment and other aspects of the environment are well known to influence both sleep and the response to sleep loss.8,20,30,32,43 During SF as produced by our device, the mice must be housed in the unusual environment of a rotating disk that allows visual and likely olfactory but not physical contact with another mouse. This environment could cause nonspecific efects that might influence sleep responses, covariate measures, or both. However, all perturbations designed to completely or partially diminish sleep in animals likely generate some nonspecific stimuli that are difficult to control. Gentle handling, for example, introduces novel sensory and perhaps motor and psychologic stimuli that could also influence sleep and various other covariate measures, and these perturbations are not applied uniformly to all subjects, as is the case with disk rotation. Perhaps some strategies could be adopted to mitigate the unusual nature of the disk environment in our device. For example, although we did not include bedding in the device in our study, this modification is possible and would perhaps make the environment more similar to the home cage. In addition, mice could be group-housed on the disk. This modification would perhaps alleviate effects due to physical isolation from other mice, although it might preclude obtaining sleep recordings from individual mice. Furthermore, group housing could potentially result in fighting in this setting, as often occurs in standard caging, particularly for male mice.
In summary, the current study characterizes sleep disruptions and recovery in mice exposed to 3 different durations of SF. Sleep amount, depth, and consolidation were significantly higher during the ad-libitum sleep phase that occurred after each of the 3 SF regimens, yet in response to SF for 12 or 24 h, mice did not recover the lost SWS time during the subsequent 12- or 24-h period, respectively, thus accruing a SWS debt. In contrast, mice did recover lost REMS. SF(0–24) was associated with lower concentrations of some inflammatory mediators in lung, whereas effects on serum analytes were minimal. Therefore, in this model, acute SF can create a sustained sleep debt and a modified inflammatory environment in lung. We speculate that these changes could contribute to greater risk of lung disease secondary to more prolonged sleep perturbation.
Acknowledgments
We thank Michelle Randle and Sarah Barrett for technical support, Tom Handy for photography, Dr Tom Gardiner for equipment fabrication and programming, and Dr Steve Verhulst for consultation on the statistical analysis. This work was supported in part by NIH grant AI080576 and by the Southern Illinois University School of Medicine.
References
- 1.Aldabal L, Bahammam AS. 2011. Metabolic, endocrine, and immune consequences of sleep deprivation. Open Respir Med J 5:31–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ayas NT, White DP, Al-Delaimy WK, Manson JE, Stampfer MJ, Speizer FE, Patel S, Hu FB. 2003. A prospective study of self-reported sleep duration and incident diabetes in women. Diabetes Care 26:380–384 [DOI] [PubMed] [Google Scholar]
- 3.Balkin TJ, Rupp T, Picchioni D, Wesensten NJ. 2008. Sleep loss and sleepiness: current issues. Chest 134:653–660 [DOI] [PubMed] [Google Scholar]
- 4.Banks S, Dinges DF. 2007. Behavioral and physiological consequences of sleep restriction. J Clin Sleep Med 3:519–528 [PMC free article] [PubMed] [Google Scholar]
- 5.Baracchi F, Opp MR. 2008. Sleep–wake behavior and responses to sleep deprivation of mice lacking both interleukin 1β receptor 1 and tumor necrosis factor α receptor 1. Brain Behav Immun 22:982–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barf RP, Desprez T, Meerlo P, Scheurink AJ. 2012. Increased food intake and changes in metabolic hormones in response to chronic sleep restriction alternated with short periods of sleep allowance. Am J Physiol Regul Integr Comp Physiol 302:R112–R117 [DOI] [PubMed] [Google Scholar]
- 7.Baud MO, Magistretti PJ, Petit JM. 2013. Sustained sleep fragmentation affects brain temperature, food intake, and glucose tolerance in mice. J Sleep Res 22:3–12 [DOI] [PubMed] [Google Scholar]
- 8.Bittman EL, Kilduff TS, Kriegsfeld LJ, Szymuziak R, Toth LA, Turek FW. 2013. Animal care practices in experiments on biological rhythms and sleep: Report of the Joint Task Force of the Society for Research on Biological Rhythms and the Sleep Research Society. J Am Assoc Lab Anim Sci 52:437–443 [PMC free article] [PubMed] [Google Scholar]
- 9.Bodosi B, Gardi J, Hajdu I, Szentirmai E, Obal F, Krueger JM. 2004. Rhythms of ghrelin, leptin, and sleep in rats: effects of the normal diurnal cycle, restricted feeding, and sleep deprivation. Am J Physiol Regul Integr Comp Physiol 287:R1071–R1079 [DOI] [PubMed] [Google Scholar]
- 10.Bonnet MH, Arand DL. 2010. Hyperarousal and insomnia: state of the science. Sleep Med Rev 14:9–15 [DOI] [PubMed] [Google Scholar]
- 11.Cappuccio FP, D'Elia L, Strazzulo P, Miller MA. 2010. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep 33:585–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chien KL, Chen PC, Hsu HC, Su TC, Sung FC, Chen MF. 2010. Habitual sleep duration and insomnia and the risk of cardiovascular events and all-cause death: report from a community-based cohort. Sleep 33:177–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deurveilher S, Rusak B, Semba K. 2012. Time-of-day modulation of homeostatic and allostatic sleep responses to chronic sleep restriction in rats. Am J Physiol Regul Integr Comp Physiol 302:R1411–R1425 [DOI] [PubMed] [Google Scholar]
- 14.Dinges DF, Douglas SD, Hamaran S, Zaugg L, Kapoor S. 1995. Sleep deprivation and human immune function. Adv Neuroimmunol 5:97–110 [DOI] [PubMed] [Google Scholar]
- 15.Dinges DF, Douglas SD, Zaugg L, Campbell DE, McMann JM, Whitehouse WG, Orne EC, Kapoor SC, Icaza E, Orne MT. 1994. Leukocytosis and natural killer cell function parallel neurobehavioral fatigue induced by 64 hours of sleep deprivation. J Clin Invest 93:1930–1939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Everson CA. 2005. Clinical assessment of blood leukocytes, serum cytokines, and serum immunoglobulins as responses to sleep deprivation in laboratory rats. Am J Physiol Regul Integr Comp Physiol 289:R1054–R1063 [DOI] [PubMed] [Google Scholar]
- 17.Everson CA, Gilliland MA, Kushida CA, Pilcher JJ, Fang VS, Refetoff S, Bergmann BM, Rechtschaffen A. 1989. Sleep deprivation in the rat. IX. Recovery. Sleep 12:60–67 [PubMed] [Google Scholar]
- 18.Everson CA, Szabo A. 2011. Repeated exposure to severely limited sleep results in distinctive and persistent physiological imbalances in rats. PLoS ONE 6:e22987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Everson CA, Thalacker CD, Hogg N. 2008. Phagocyte migration and cellular stress induced in liver, lung, and intestine during sleep loss and sleep recovery. Am J Physiol Regul Integr Comp Physiol 295:R2067–R2074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Febinger HY, George A, Priestley J, Toth LA, Opp MR. 2013. Effects of housing condition and cage change on characteristics of sleep in mice. J Am Assoc Lab Anim Sci 53:29–37 [PMC free article] [PubMed] [Google Scholar]
- 21.Franken P, Malafosse A, Tafti M. 1999. Genetic determinants of sleep regulation in inbred mice. Sleep 22:155–169 [PubMed] [Google Scholar]
- 22.Gip P, Hagiwara G, Sapolsky RM, Cao VH, Heller HC, Ruby NF. 2004. Glucocorticoids influence brain glycogen levels during sleep deprivation. Am J Physiol Regul Integr Comp Physiol 286:R1057–R1062 [DOI] [PubMed] [Google Scholar]
- 23.Gonnissen HK, Hursel R, Rutters F, Martens EA, Westerterp-Plantenga MS. 2012. Effects of sleep fragmentation on appetite and related hormone concentrations over 24 h in healthy men. Br J Nutr 8:1–9 [DOI] [PubMed] [Google Scholar]
- 24.Haack M, Sanchez E, Mullington JM. 2007. Elevated inflammatory markers in response to prolonged sleep restriction are associated with increased pain experience in healthy volunteers. Sleep 30:1145–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hagewoud R, Havekes R, Novati A, Keijser JN, Van der Zee EA, Meerlo P. 2010. Sleep deprivation impairs spatial working memory and reduces hippocampal AMPA receptor phosphorylation. J Sleep Res 19:280–288 [DOI] [PubMed] [Google Scholar]
- 26.Hagewoud R, Havekes R, Tiba PA, Novati A, Hogenelst K, Weinreder P, Van der Zee EA, Meerlo P. 2010. Coping with sleep deprivation: shifts in regional brain activity and learning strategy. Sleep 33:1465–1473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harvey AG, Tang NK. 2012. (Mis)perception of sleep in insomnia: a puzzle and a resolution. Psychol Bull 138:77–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hayes KE, Raucci JR, Gades NM, Toth LA. 2000. An evaluation of analgesic regimens for abdominal surgery in mice. Contemp Top Lab Anim Sci 39:18–23 [PubMed] [Google Scholar]
- 29.Huber R, Deboer T, Tobler I. 2000. Effects of sleep deprivation on sleep and sleep EEG in 3 mouse strains: empirical data and simulations. Brain Res 857:8–19 [DOI] [PubMed] [Google Scholar]
- 30.Jhaveri KA, Trammell RA, Toth LA. 2007. Effect of environmental temperature on sleep, locomotor activity, core body temperature, and immune responses of C57BL/6J mice. Brain Behav Immun 21:975–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kas MJH, Edgar DM. 1999. Circadian-timed wakefulness at dawn opposes compensatory sleep responses after sleep deprivation in Octodon Degus. Sleep 22:1045–1053 [DOI] [PubMed] [Google Scholar]
- 32.Kaushal N, Nair D, Gozal D, Ramesh V. 2012. Socially isolated mice exhibit a blunted homeostatic sleep response to acute sleep deprivation compared to socially paired mice. Brain Res 1454:65–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim Y, Bolortuya Y, Chen L, Basheer R, McCarley RW, Strecker RE. 2012. Decoupling of sleepiness from sleep time and intensity during chronic sleep restriction: evidence for a role of the adenosine system. Sleep 35:861–869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim Y, Laposky AD, Bergmann BM, Turek FW. 2007. Repeated sleep restriction in rats leads to homeostatic and allostatic responses during recovery sleep. Proc Natl Acad Sci USA 104:10697–10702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kirk RE. 1982. Experimental design: procedures for the behavioral sciences (psychology). Stamford (CT): Wadsworth Publishing [Google Scholar]
- 36.Klerman EB, Boulos Z, Edgar DM, Mistlberger RE, Moore-Ede MC. 1999. Circadian and homeostatic influences on sleep in the squirrel monkey: sleep after sleep deprivation. Sleep 22:45–59 [DOI] [PubMed] [Google Scholar]
- 37.Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler MR. 2002. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry 59:131–136 [DOI] [PubMed] [Google Scholar]
- 38.Kripke DF, Simons RN, Garfinkel L, Hammond EC. 1979. Short and long sleep and sleeping pills. Is increased mortality associated? Arch Gen Psychiatry 36:103–116 [DOI] [PubMed] [Google Scholar]
- 39.Lancel M, van Riezen H, Glatt A. 1991. Effects of circadian phase and duration of sleep deprivation on sleep and EEG power spectra in the cat. Brain Res 548:206–214 [DOI] [PubMed] [Google Scholar]
- 40.Leemburg S, Vyazovskiy VV, Olcese U, Bassett CL, Tononi G, Cirelli C. 2010. Sleep homeostasis in the rat is preserved during chronic sleep restriction. Proc Natl Acad Sci USA 107:15939–15944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leenaars CH, Dematteis M, Joosten RN, Eggels L, Sandberg H, Schirris M, Feenstra MG, van Someren EJ. 2011. A new automated method for rat sleep deprivation with minimal confounding effects on corticosterone and locomotor activity. J Neurosci Methods 196:107–117 [DOI] [PubMed] [Google Scholar]
- 42.Leenaars CH, Joosten RN, Zwart A, Sandberg H, Ruimschotel E, Hanegraaf MA, Dematteis M, Feenstra MG, van Someren EJ. 2012. Switch-task performance in rats is disturbed by 12 h of sleep deprivation but not by 12 h of sleep fragmentation. Sleep 35:211–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leys LJ, McGaraughty S, Radek RJ. 2012. Rats housed on corncob bedding show less slow-wave sleep. J Am Assoc Lab Anim Sci 51:764–768 [PMC free article] [PubMed] [Google Scholar]
- 44.Longordo F, Fan J, Steimer T, Kopp C, Luthi A. 2011. Do mice habituate to ‘gentle handling’? A comparison of resting behavior, corticosterone levels and synaptic function in handled and undisturbed C57BL/6J mice. Sleep 34:679–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luckhaupt SE, Tak S-W, Calvert GM. 2010. The prevalence of short sleep duration by industry and occupation in the National Health Interwive Survey. Sleep 33:149–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Majer M, Jones JF, Unger ER, Yougnblood LS, Decker MJ, Gurbaxani B, Heim C, Reeves WC. 2007. Perception versus polysomnographic assessment of sleep in CFS and nonfatigued control subjects: results from a population-based study. BMC Neurol 7:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maury E, Ramsey KM, Bass J. 2010. Circadian rhythms and metabolic syndrome: from experimental genetics to human disease. Circ Res 106:447–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McCoy JG, Tartar JL, Bebis AC, Ward CP, McKenna JT, Baxter MG, McGaughy J, McCarley RW, Strecker RE. 2007. Experimental sleep fragmentation impairs attentional set-shifting in rats. Sleep 30:52–60 [DOI] [PubMed] [Google Scholar]
- 49.McKenna JT, Tartar JL, Ward CP, Thakkar MM, Cordeira JW, McCarley RW, Strecker RE. 2007. Sleep fragmentation elevates behavioral, electrographic and neurochemical measures of sleepiness. Neuroscience 146:1462–1473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meerlo P, Sgoifo A, Suchecki D. 2008. Restricted and disrupted sleep: effects on autonomic function, neuroendocrine stress systems, and stress responsivity. Sleep Med Rev 12:197–210 [DOI] [PubMed] [Google Scholar]
- 51.Mongrain V, Hernandez SA, Pradervand S, Dorsaz S, Curie T, Hagiwara G, Gip P, Heller HC, Franken P. 2010. Separating the contribution of glucocorticoids and wakefulness to the molecular and electrophysiological correlates of sleep homeostasis. Sleep 33:1147–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morselli L, Leproult R, Balbo M, Spiegel K. 2010. Role of sleep duration in the regulation of glucose metabolism and appetite. Best Pract Res Clin Endocrinol Metab 24:687–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nair D, Zhang SX, Ramesh V, Hakim F, Kaushal N, Wang Y, Gozal D. 2011. Sleep fragmentation induces cognitive deficits via nicotinamide adenine dinucleotide phosphate oxidase-dependent pathways in mouse. Am J Respir Crit Care Med 184:1305–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.National Center for Health Statistics 2005. Percentage of adults who reported an average of <6 hours of sleep per 24-hour period, by sex and age group—United States, 1985 and 2004. MMWR Morb Mortal Wkly Rep 54:933 [Google Scholar]
- 55.Olivier J, Johnson JWD, Marshall GD. 2008. The logarithmic transformation and the geometric mean in reporting experimental IgE results: what are they and when and why to use them? Ann Allergy Asthma Immunol 100:333–337 [DOI] [PubMed] [Google Scholar]
- 56.Ouchi N, Parker JL, Lugus JJ, Walsh K. 2011. Adipokines in inflammation and metabolic disease. Nat Rev Immunol 11:85–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Parish JM. 2009. Sleep-related problems in common medical conditions. Chest 135:563–572 [DOI] [PubMed] [Google Scholar]
- 58.Philip P, Sagaspe P, Prague M, Tassi P, Capelli A, Bioulac B, Commenges D, Taillard J. 2012. Acute versus chronic partial sleep deprivation in middle-aged people: differential effect on performance and sleepiness. Sleep 35:997–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ramesh V, Kaushal N, Gozal D. 2009. Sleep fragmentation differentially modifies EEG delta power during slow-wave sleep in socially isolated and paired mice. Sleep Science 2:64–75 [Google Scholar]
- 60.Ramesh V, Nair D, Zhang SX, Hakim F, Kaushal N, Kayali F, Wang Y, Li RC, Carreras A, Gozal D. 2012. Disrupted sleep without sleep curtailment induces sleepiness and cognitive dysfunction via the tumor necrosis factor α pathway. J Neuroinflammation 9:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rechtschaffen A, Bergmann BM, Gilliland MA, Bauer K. 1999. Effects of method, duration, and sleep stage on rebounds from sleep deprivation in the rat. Sleep 22:11–31 [DOI] [PubMed] [Google Scholar]
- 62.Ringgold KM, Barf RP, George A, Sutton BC, Opp MR. 2013. Prolonged sleep fragmentation of mice exacerbates febrile responses to lipopolysaccharide. J Neurosci Methods 219:104–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rogers NL, Sziba MP, Staab JP, Evans DL, Dinges DF. 2001. Neuroimmunologic aspects of sleep and sleep loss. Semin Clin Neuropsychiatry 6:295–307 [DOI] [PubMed] [Google Scholar]
- 64.Schipper HS, de Jager W, van Dijk MEA, Meerding J, Zelissen PMJ, Adan RA, Prakken BJ, Kalkhoven E. 2010. A multiplex immunoassay for human adipokine profiling. Clin Chem 56:1320–1328 [DOI] [PubMed] [Google Scholar]
- 65.Sutherland ER. 2005. Nocturnal asthma. J Allergy Clin Immunol 116:1179–1186 [DOI] [PubMed] [Google Scholar]
- 66.Tamakoshi A, Ohno Y. 2004. Self-reported sleep duration as a predictor of all-cause mortality: results from the JACC study, Japan. Sleep 27:51–54 [PubMed] [Google Scholar]
- 67.Tartar JL, Ward CP, Cordeira JW, Legare SL, Blanchette AJ, McCarley RW, Strecker RE. 2009. Experimental sleep fragmentation and sleep deprivation in rats increases exploration in an open-field test of anxiety while increasing plasma corticosterone levels. Behav Brain Res 197:450–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Trachel L, Tobler I, Achermann P, Borbely AA. 1991. Sleep continuity and the REM–nonREM cycle in the rat under baseline conditions and after sleep deprivation. Physiol Behav 49:575–580 [DOI] [PubMed] [Google Scholar]
- 69.Turner J, Hughes LF, Toth LA. 2010. Sleep, activity, temperature, and arousal responses of mice deficient for muscarinic receptor M2 or M4. Life Sci 86:158–169 [DOI] [PubMed] [Google Scholar]
- 70.Van Cauter E. 2011. Sleep disturbances and insulin resistance. Diabet Med 28:1455–1462 [DOI] [PubMed] [Google Scholar]
- 71.Vecsey CG, Wimmer ME, Havekes R, Park AJ, Perron IJ, Meerlo P, Abel T. 2013. Daily acclimation handling does not affect hippocampal long-term potentiation or cause chronic sleep deprivation in mice. Sleep 36:601–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vgontzas AN, Bixler EO, Wittman AM, Zachman K, Lin H-M, Vela-Bueno A, Kales A, Chrousos GP. 2001. Middle-aged men show higher sensitivity of sleep to the arousing effects of corticotropin-releasing hormone than young men: clinical implications. J Clin Endocrinol Metab 86:1489–1495 [DOI] [PubMed] [Google Scholar]
- 73.Vgontzas AN, Chrousos GP. 2002. Sleep, the hypothalamic–pituitary–adrenal axis, and cytokines: multiples interactions and disturbances in sleep disorders. Endocrinol Metab Clin North Am 31:15–36 [DOI] [PubMed] [Google Scholar]
- 74.Wells ME, Vaughn BV. 2012. Poor sleep challenging the health of a nation. Neurodiagn J 52:233–249 [PubMed] [Google Scholar]
- 75.Wolk R, Somers VK. 2007. Sleep and the metabolic syndrome. Exp Physiol 92:67–78 [DOI] [PubMed] [Google Scholar]
- 76.Zager A, Andersen ML, Ruiz FS, Antunes IB, Tufik S. 2007. Effects of acute and chronic sleep loss on immune modulation of rats. Am J Physiol Regul Integr Comp Physiol 293:R504–R509 [DOI] [PubMed] [Google Scholar]