Abstract
Significance: Nervous system injuries, both in the peripheral nervous system (PNS) and central nervous system are a major cause for pain, loss-of-function, and impairment of daily life. As nervous system injuries commonly heal slowly or incompletely, new therapeutic approaches may be required.
Recent Advances: The observation that cultured neurons are able to respond to exogenous electric fields (EFs) by sprouting more neurites and directing growth along the field, along with the presence of endogenous EFs in the developing vertebrate nervous system have led to the suggestion of the use of EFs in a regenerative therapeutic setting. This review discusses the effects of EFs on nervous cells, and their use in the treatment of nervous injuries in the eye, limb nerves, and the spinal cord. Exogenous EFs have been shown to be neuroprotective in various injury models of the eye, including traumatic injury, congenital degenerative retinopathy, and glaucoma. In the PNS, EFs are able to stimulate regrowth and functional recovery in damaged limb nerves. In the spinal cord, axonal regeneration and improved quality of life may be achieved using EF stimulation.
Critical Issues: The optimal paradigm for electrical stimulation has not been determined, and the mechanisms behind the effect of EF are still largely unknown.
Future Directions: Although the therapeutic use of EFs in the nervous system is still in its infancy, it is a promising therapeutic avenue for otherwise hard to treat injuries. The cellular/molecular mechanisms of such regulation need to be fully investigated, and the efficiency of applied EFs during wound healing needs to be optimized in a systematic approach in both animal models and future clinical trials.

Bing Song, MD, PhD
Scope and Significance
Nervous system injuries can be divided into two categories: central nervous system (CNS) insult and peripheral nervous system (PNS) injury. CNS and PNS injuries commonly have poor clinical outcome, and cause heavy financial burdens to the society, as well as emotional and physical challenges to the patients and their families. In the United States alone ∼450,000 people have sustained traumatic spinal cord injuries (SCIs), with more than 10,000 new cases emerging every year. In China, the incidence of SCI is ∼60,000 per year. The average annual medical cost ranges from $15,000 to $30,000 per year per SCI patient, and the estimated lifetime cost ranges between $500,000 and more than $3 million, depending on injury severity.1 Despite various efforts in treatment of nervous system injuries; the clinical outcomes were not satisfactory largely due to the limited regeneration capacity of the nervous system. Many new therapeutic approaches, including chemical, physical, and electrical means have been suggested. This work discusses the application of exogenous electric fields (EFs) in the treatment of CNS and PNS injuries, including in the eye, limb nerves, and spinal cord. It does not address the clinical use of exogenous EFs for pain control.
Translational Relevance
Previous studies have demonstrated endogenous EFs are formed immediately at wound formation.2 EFs regulate the wound healing3 and promote the nervous reinnervation during wound healing.4 Much preclinical work in animal models suggests exogenous EFs are beneficial in nervous system injury. The stimulation paradigms and surgical procedures used in animal studies would be potentially suitable for clinical trials, as proved by previous pre-clinical studies.
Clinical Relevance
Nervous system injuries heal slowly and often incompletely. Several trials using electric stimulation have been performed in both PNS and CNS injury repairs (see details below). Although the clinical outcome has been encouraging in some cases, there is an apparent lack of systematic characterization and optimization of using EFs in such treatments, including the EF pattern and voltage, as well as potential interaction and combined application with other guidance factors (e.g., chemical gradients, physical cues, extracellular matrix, and others). Nevertheless, EFs still hold great potential and may represent a new therapeutic avenue in treating nervous system injuries.
Clinical problems addressed
The sensitive nature and limited regenerative capacity of the nervous system makes it exquisitely sensitive to injury. Traumatic injury can cause severe damage to nerve tracts throughout the PNS or CNS. Neuropathy of the PNS is nontraumatic damage caused by various factors among prolonged diabetes mellitus, infection, inflammation, compression, or congenital disorders. What is common in these assaults to the nervous system is that regeneration can be limited, either due to the inherent limits of the cells involved or due to local inhibitory factors generated from cellular debris or inflammation.
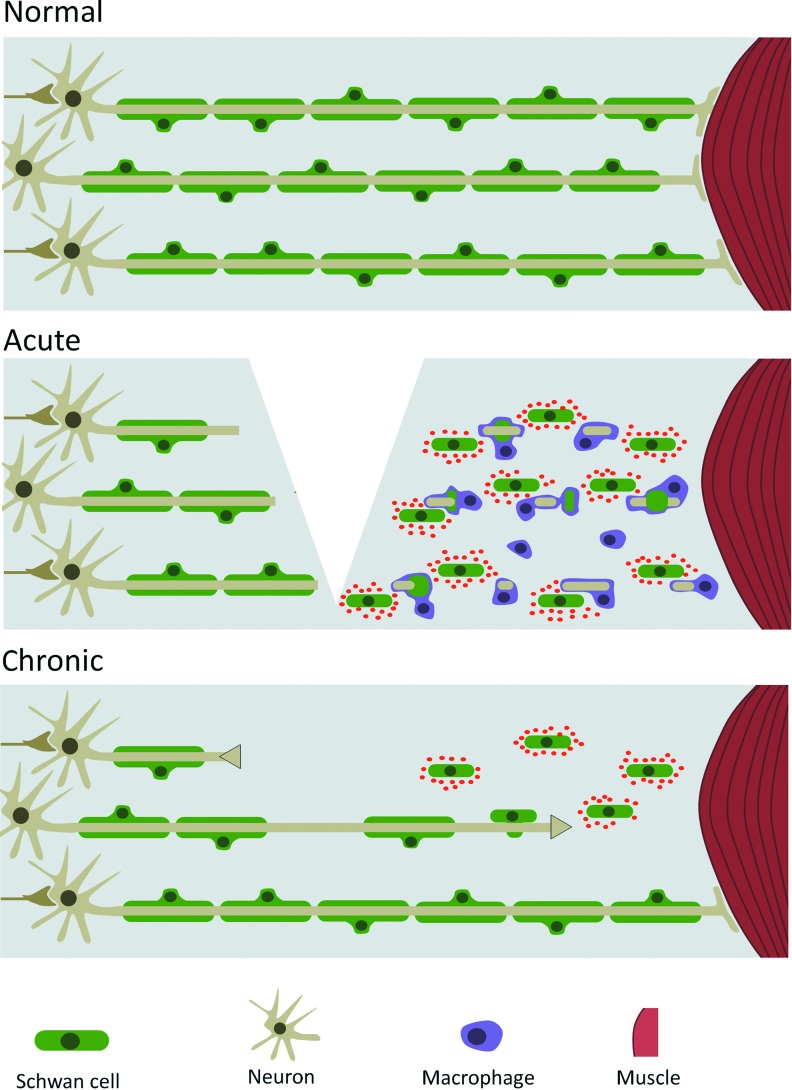
In both the PNS and CNS, after injury, axons severed from the cell body will undergo Wallerian degeneration and fragment and disintegrate over the course of several days.5 After injury, immune cell infiltration leads to clearing of the debris. In the PNS, the ensheathing Schwann cells de-differentiate and express appropriate chemoattractants and repellents to stimulate and guide axonal regrowth and reinnervation (Fig. 1).6 However, for large-scale injuries, or those with large amounts of cellular debris and inflammation, these mechanisms are inadequate, and consequently regeneration is incomplete.
Figure 1.
Effects of injury in the peripheral nervous system. In the normal situation, peripheral axons are enveloped by supportive Schwann cells. After an injury, damaged axons retract from the site of injury, and infiltrating macrophages clear axonal and cellular debris. Intact Schwann cells dedifferentiate and start expressing chemoattractants and neurotrophic factors. After the initial inflammatory phase, axons that have a short distance to cross will be attracted by the Schwann cells, and eventually reinnervate their muscle target and will once again be enveloped by differentiated Schwann cells. However, when the distance is too large, no reinnervation will take place, and the axonal stump will retract and the neuron will likely undergo apoptosis. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
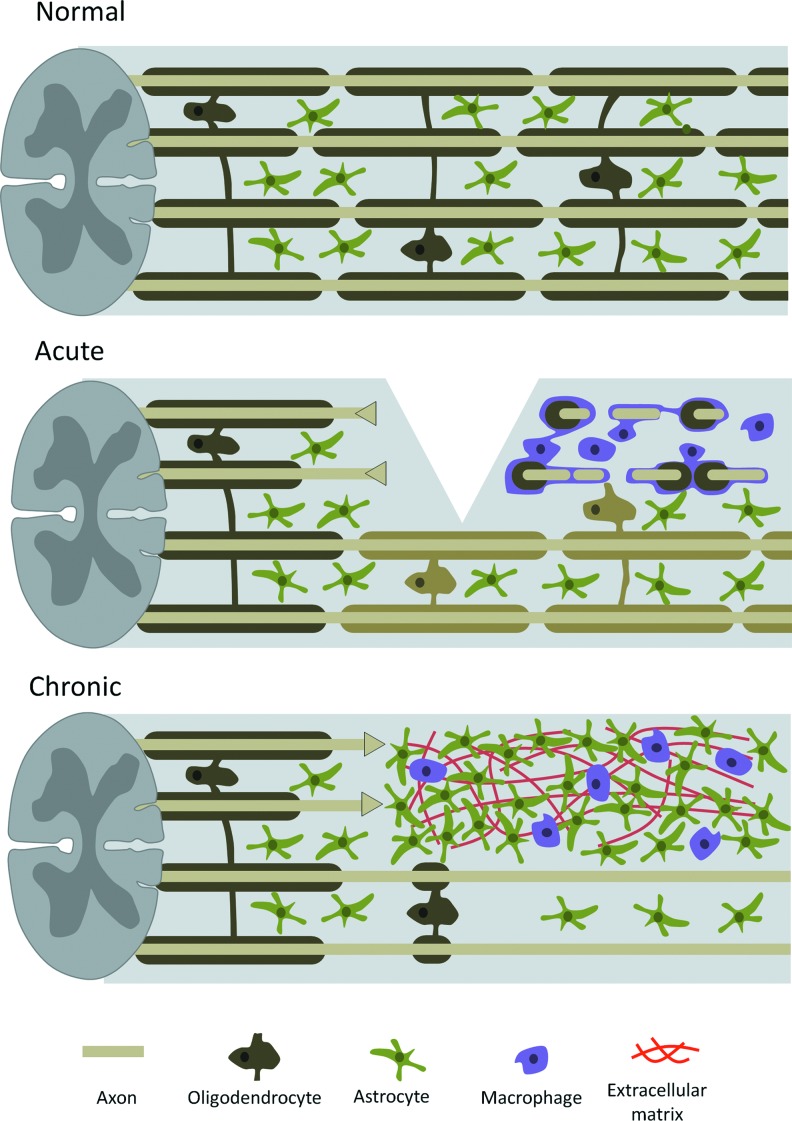
In contrast, such a mechanism does not operate in the CNS. The equivalent to Schwann cells—the oligodendrocytes—do not stimulate neural regrowth, and in combination with the hypertrophy of supporting astrocytes and immune cells, the wound environment remains un-supportive for regrowth. For an overview of the cellular response to spinal cord damage, see Fig. 2. In these situations where regrowth and reinnervation is absent or limited, the use of exogenous EFs may offer a new therapeutic option.
Figure 2.
Cellular response in spinal cord injury. In the healthy spinal cord, intact nerve bundles are surrounded by oligodendrocytes and supported by astrocytes. In the acute phase of injury, severed axons retract toward the soma, while their distal stumps and remaining myelin debris are phagocytosed by microglia and infiltrating macrophages. Damaged oligodendrocytes lead to demyelination of nearby intact axons. In the chronic phase, when the acute inflammatory response has been resolved, reactive astrocytes have proliferated and formed a dense glial scar, which includes trapped immune cells and dense networks of extracellular matrix, which is inhibitory to axons attempting reinnervation to the distal end of the injury. Remyelination may take place to once again envelop naked axons. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Relevant basic science context
Endogenous and exogenous EFs in the nervous system
EFs in the nervous system have been used clinically in several settings. Their use was pioneered in the field of pain control. Chronic pain may be controlled by EF stimulation of the affected area, the spinal cord, or brain. This is based on the “gating theory” of pain, where the exogenous EF signals override the pain signals coming from the periphery. Direct deep brain stimulation has also been beneficial in improving quality of life in Parkinson's disease patients, by ameliorating symptoms through stimulation of basal ganglia.7 The activation of output signals and the consequent pattern changes in neuronal activity throughout the basal ganglia motor circuit is the mechanism responsible for improvement of symptoms. However, one of the major disadvantages is the diminished response over time to deep brain stimulation, limiting its long-term usefulness. From a regenerative point of view, EFs are still a largely untried therapeutic avenue.
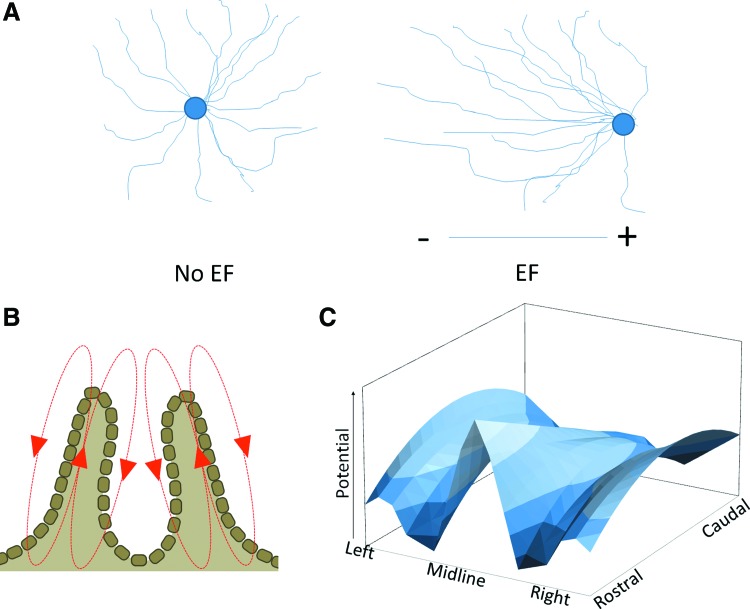
A number of effects of exogenous EFs on nervous cells in vitro and in vivo have been described in the past decades. Initial studies in cultured Xenopus embryonic neurons showed that applied EFs induced neurite sprouting and promoted the turning of the growth cones of extending neurons (Fig. 3A).8 The presence of an exogenous EF is able to increase the number of filopodia on the growth cones, even when these were first collapsed pharmacologically,9 promoting growth cone motility.
Figure 3.
Electric fields (EFs) during neural development. (A) Effects of applied EFs on cultured neurons. In the absence of EF, neurite outgrowth has no directionality, whereas in an applied field, outgrowth is strongly directed toward the anode. (B) Direction and location of the EF before neural tube closure. (C) During vertebrate development, EF strength along the neural tube varies by anatomical location, with both rostal-caudal and lateral-midline gradients. Plot represents the fields in a stage 15 axolotl embryo. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
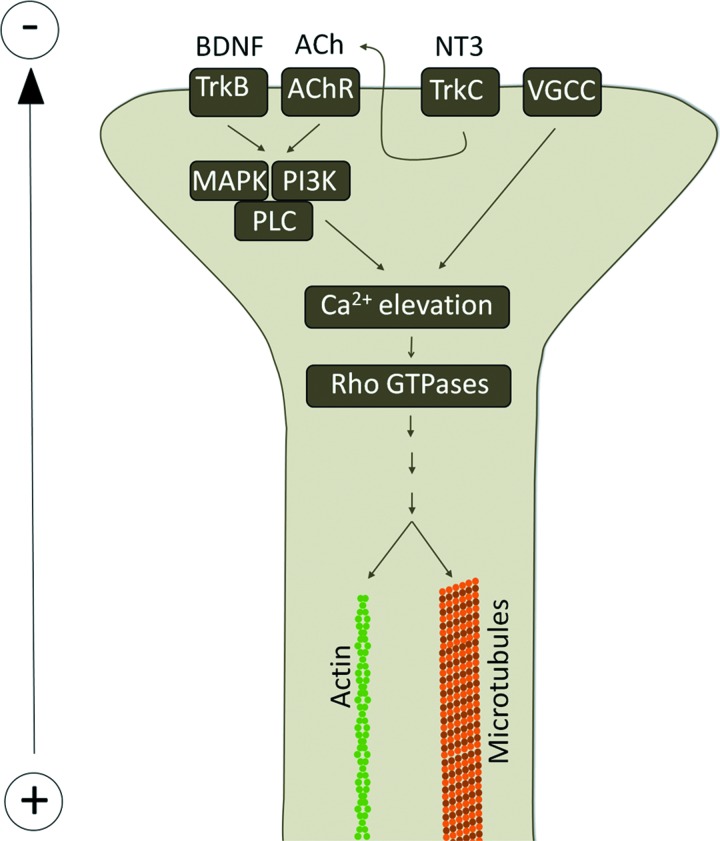
The effects of EFs on nerve growth have also been noted during normal physiological development. In the early embryonic development, endogenous EFs exist during the neural tube formation in Xenopus10 and chicken.11 Blocking the electric current or reversing its polarity in developing Xenopus embryos hinders closure of the neural tube,12 and when the field is disrupted following closure of the neural tube internal tissues disaggregate.13 The role of the endogenous EF in patterning the neural tube is illustrated by the complex three-dimensional pattern of the field, with both rostral-to-caudal and left-to-right gradients (Fig. 3B, C).14 The response of neuronal outgrowth to EF has been shown to be dependent on dynamic microtubules and small GTPases Cdc42 and Rac.15 The effects of EFs can be further modulated by neurotransmitters or neurotrophic factors.16,17 For an overview of these mechanisms, see Fig. 4. The observed effects of EFs both in vivo and in vitro have led to the suggestions of their use in a number of pathological situations affecting the PNS or CNS. The application of exogenous EFs has been used in various injury models, including in the eye, PNS, and in the spinal cord.
Figure 4.
Mechanisms of electric growth cone guidance. Electrotaxis of growth cones is at least partially mediated by elevation of intracellular calcium though activation of voltage gated calcium channels (VGCC). The effect is increased in the presence of neutotrophins, such as BDNF or NT3 and neurotransmitters, such as acetylcholine (ACh), via activation of PLC through MAPK or PI3K. The elevated calcium levels activate a number of Rho GTPases, leading to actin and microtubule modification and growth cone mobility. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Discussion of Findings and Relevant Literature
EFs in the eye
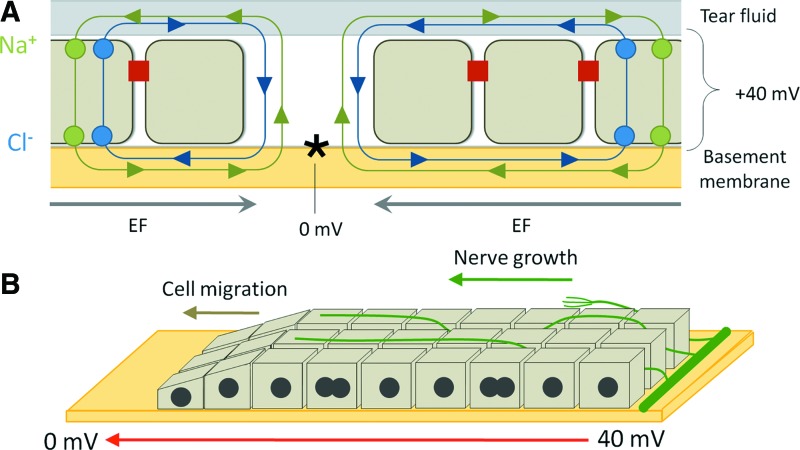
Damage to a number of components of the eye has been shown to respond favorably to the presence of both endogenous and exogenous EFs. The cornea normally maintains a transcorneal potential difference (TCPD) of approximately +40 mV, positive internally, by active pumping of Cl− outward and Na+/K+ inward.18–20 In the event of an injury, the TCPD collapses. However, at ∼1 mm from the injury site, normal TCPD is maintained, leading to the establishment of an EF parallel to the cornea, between injured and uninjured epithelium, of at least 40 mV/mm (Fig. 5A).21 This field has been observed to stimulate wound closure by promoting cell division and migration in corneal epithelial cells.4,21,22 However, apart from the effect on epithelial cells, the endogenous EFs also promote nervous repair in the injured cornea. In the damaged cornea, new nerve sprouting occurs rapidly, perpendicular to the wound edge (Fig. 5B).23 By manipulating the endogenous EF in ex vivo wounded corneas, Song et al. showed EFs are important guiding factors for directionality of nerve regrowth.4 The perpendicular direction of new sprouting is enhanced by pharmacologically enhancing EFs, and directionality is lost when EFs are diminished. The effect of the EF is strong enough to induce turning in newly sprouted nerve that did not have an initially perpendicular direction. Apart from determining nerve sprouting direction, the presence of EFs also increases the number of newly sprouting nerves. In the stronger field close to the wound edge, sprouting rate is higher, and this can be further modified by pharmacological manipulation of the field.
Figure 5.
The EF in the damaged cornea. (A) The transcorneal potential is normally maintained by the inward flow of sodium and the outward flow of chloride, with tight junctions between epithelial cells prohibiting free return of ions. At a wound (asterisk), this ionic barrier is broken, leading to short-circuit of the potential and the establishing of a lateral EF between intact epithelium and wound site. (B) The transcorneal potential is disrupted at the site of injury, leading to a net EF, along which cell division, cell migration, and nerve growth takes place. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Further into the eye, retinal damage is a prime cause for loss of vision. In a number of different injury situations, the beneficial effect of exogenous EFs on retinal damage has been described. In these studies, transcorneal electrical stimulation (TES) was used, with two concentric ring electrodes are placed on the surface of the eye to deliver current. In an experimental setup modeling glaucoma, ischemic damage to the retina was caused by raising intraocular pressure. EF application via TES had a neuroprotective effect in the model, preserving both the morphology and electrophysiological properties of the retinal ganglion cells.24 TES also has a neuroprotective effect following optic nerve transsection or crush, rescuing de-innervated ganglion cells from apoptosis, possibly in an insulin-like growth factor 1 dependant mechanism.25,26 Retinal degeneration following exposure to intense light is also ameliorated by both pre- and post-exposure TES, which is accompanied by increased expression of growth factors ciliary neurotrophic factor and brain-derived neurotrophic factor (BDNF) and of the anti-apoptotic factor B-cell lymphoma 2.27 In a rat model of degenerative retinitis pigmentosa, TES protected photoreceptor cells from degeneration.28 Although EF treatment of the eye has so far not been used in a clinical setting, the ease of application means this will likely translate to the clinic soon.
EFs in the PNS
PNS injuries are common as a result of trauma and other conditions, such as diabetic neuropathy. Although in contrast to the CNS, extensive regeneration of the PNS is possible, it is often incomplete. PNS damage leads to loss of function, often accompanied by atrophy in the affected muscles, and either loss of sensation or persistent neuropathic pain. While chronic neuropathic pain has been controlled using EFs, stimulation may prove to be useful in regenerative medicine too. In general, a positive effect of EF stimulation on regrowth is seen in the PNS, although some studies show contradicting results.
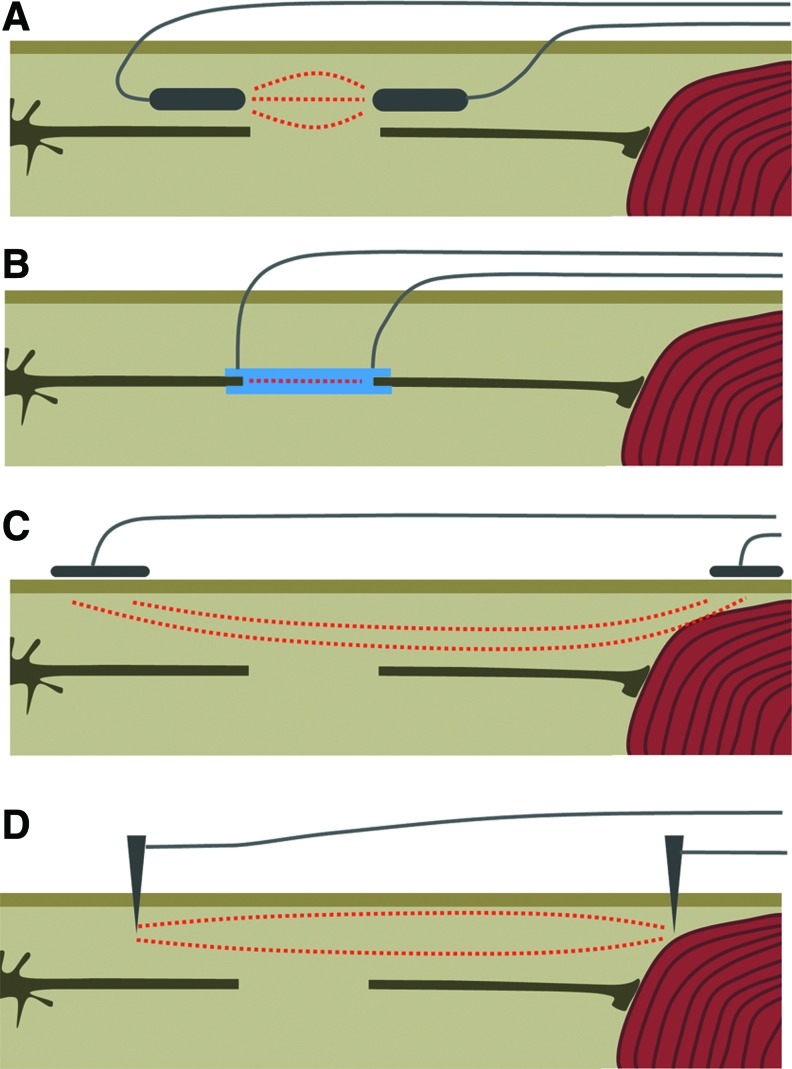
The most direct method to stimulate nerve regrowth is to implant electrodes directly over the injured nerve. Early work using short-term or long-term alternating current (AC) stimulation following a femoral nerve transsection or sciatic nerve crush significantly sped up reinnervation.29,30 Application of a direct current (DC) EF following a sciatic nerve crush injury resulted in a functional improvement, along with an increased vascularisation and higher nerve fiber density.31 The alternative approach to direct electrode implantation is to implant an electrically conductive nerve conduit across both ends of the damaged nerve. After implantation, an AC was delivered through the nerve conduit, which led to improvement in both motor and sensory function, along with increased axonal regeneration, myelination, and increased expression of BNDF.32 Human trials have been limited, but in patients with degenerated median nerves due to severe carpal tunnel syndrome, high-frequency AC EF stimulation through implanted electrodes promoted axonal regrowth and led to improved electrophysiological parameters but did not significantly improve functional measurements.33
Apart from stimulation on the damaged nerve, several groups have studied the effects of using EFs on target muscles deinnervated due to nerve damage. Using long-duration (28 day) transcutaneous AC EFs on the tibialis anterior muscles following a sciatic nerve crush injury was found to not to have an effect on reinnervation of the muscle, and increased muscle atrophy during the stimulation period.34 In a resection of the sciatic nerve, AC EF stimulation through intramuscular needle electrodes resulted in improved electrophysiological parameters and increased vascularisation, but only at moderate currents (≤2 mA) whereas high currents (4 mA) had a detrimental effect. This effect was independent of nerve fiber number or size, as these parameters remained unchanged.35 Effects of stimulation on the muscle is inconclusive, due to the limited data available. For an overview of the stimulation methods used, see Fig. 6.
Figure 6.
The different approaches for electric stimulation in peripheral nerve damage. (A) Implantation of electrodes over the severed end of the axons. (B) Implantation of an electrically conductive nerve conduit over the nerve stumps. (C) Noninvasive stimulation by skin patch electrodes over the target muscle and the proximal end of the damage nerve. (D) Needle electrodes on target muscle and proximal end of the damaged nerve. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
It has been suggested that the beneficial effect of AC EFs on regrowth in the PNS is growth factor mediated. EF stimulation has been shown to increase BDNF expression in injured nervous tissue. The EF driven BDNF expression may then increase the expression the human natural killer-1 carbohydrate motif, which is contained in a number of cell surface adhesion molecules, such as neural cell adhesion molecule.36 Based on the literature, it appears a short stimulation period (<1 day) is sufficient to stimulate significant regrowth, making it likely that a growth factor mediated mechanism is involved in the effect.
EFs in the SCI
Traumatic SCI is one of the main reasons for lower body paralysis. In humans, functional recovery after SCI is limited, and treatment options are few. The only currently accepted therapeutic option is the early administration of large doses of steroids to limit tissue damage due to acute inflammatory responses. However, this is not without controversy. The main limiting factor in functional recovery is the inability of severed or damaged axons to reconnect with their corresponding targets on the other side of the lesion. The axons of damaged spinal neurons will retract and cell bodies degenerate if not reinnervated swiftly. However, local inflammatory responses and glial hypertrophy form a glial scar which is not conductive to the passage of growing axons. The implantation of electrodes over the injury site has been suggested as a means to promote and guide regrowth of the axons. Early work using a hemisection of the spinal cord of the guinea pig with DC stimulation over 4 weeks or more showed enhanced axonal growth, although not directly though the lesion,37 and showed functional recovery in some of the treated animals.37,38 In a rat spinal compression injury model, functional improvement following DC stimulation was seen, although no noticeable histological improvement was observed.39
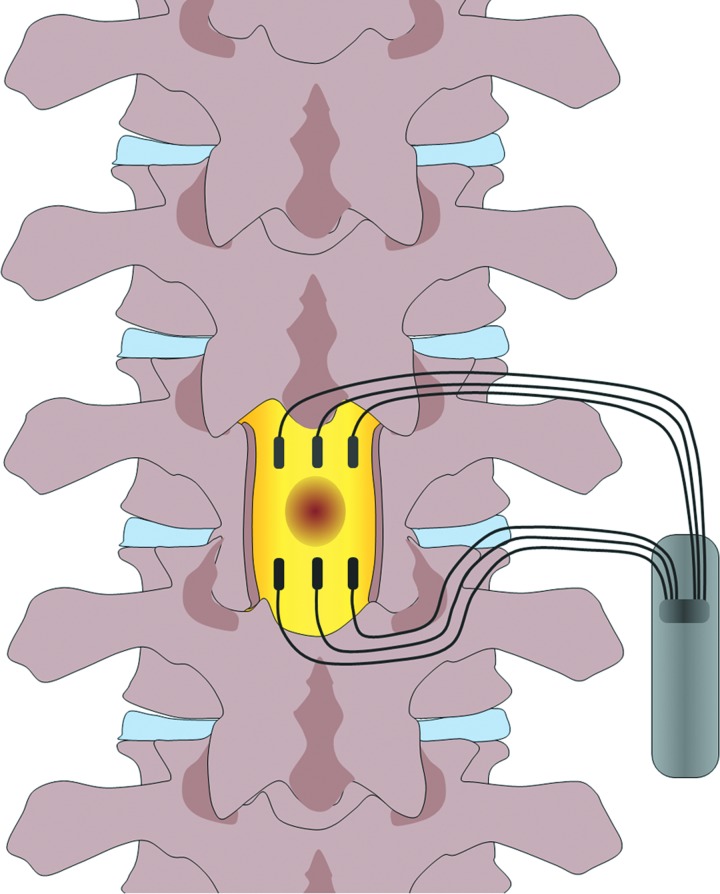
It has been noted that the direction of the DC field is crucial for the beneficial effect, in a crush spinal injury, with the cathode caudal of the injury site, improvement was seen in histological, electrophysiological, and functional parameters, while no such enhancement was seen with the opposite polarity.40 As a mono-polar field would stimulate axonal growth in one direction, but repress growth in the opposite, a steady field would only promote either efferent motor neurons or afferent sensory neurons, but not both. To circumvent this problem, an oscillating field has been used, which changes direction every 15 min, which was hypothesized to be sufficient time to promote growth, but not to induce regression in the opposite direction. This oscillating field stimulator (OFS) has been tested in two studies in dogs with sub-acute spinal injuries, with beneficial outcomes.41,42 These studies led to a phase 1 trial with an OFS in humans with acute traumatic SCI.43 The stimulator (Fig. 7) was implanted within 3 weeks after injury, and remained in place for 15 weeks. The stimulation provided significant improvement in two measures of sensory sensitivity and in seven out of nine patients improved motor scores were observed, compared to historical data for untreated patients. Although an erratum was later published concerning some discrepancies in functional scoring,44 the fundamental conclusions of the trial are still compelling.
Figure 7.
The design of oscillating field stimulators as used in a human phase 1 trial. The injury site is flanked by two sets of three electrodes, with the polarity of the field changing every 15 min. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
As reactive astrocytes are an important component of the inhibitory scar formed at the site of injury, the effect of EFs on the glial scar have also been investigated. An OFS stimulation on rats spinal cords with a puncture wound showed that the number of reactive astrocytes were reduced and astrocytes outside the injury site rearranged to align to an angle close to the EF.45 Interestingly, the other major component of the inhibitory glial scar, macrophages, do not seem to be affected by exogenous EFs.46
Apart from enhancing endogenous repair mechanisms, EFs may also play a role in cell transplantation therapies. In recent years, neural stem cell transplantation therapy has been investigated for its potential in the treatment of CNS damage. The mechanisms of the beneficial effects of neural stem cell implantation are under active debate. They may be due to functional reconnection by the neurons differentiated from the implanted cells, or alternatively due to neuroprotection via neurotrophic factors released by implanted cells. Regardless of the actual mechanism, the positive supporting role of stem cells replacement has been acknowledged.47 EFs may be used to enhance the effectiveness of stem cell implantation. Interestingly, Cao and colleagues showed in a recent study that endogenous electric signals could regulate the directional migration of neuroblast cells toward the rostral migratory stream in vivo.48 This suggests exogenous EFs may modulate neural stem cell behavior. Indeed, we and other labs proved that neural stem cells can be controlled to recruit directionally in an applied physiological level of EF,49,50 and our lab has shown is it possible to regulate such cellular response in a three-dimensional organotypic spinal cord slice culture model with electric stimulation,50 which suggests great potential for the use of exogenous EFs after grafting in vivo, as an innovative approach to optimize the stem cell based therapy in treating nervous system injuries.
Concluding Remarks
Although the use of EFs for nervous system injuries in a therapeutic setting is still limited, pre-clinical work suggests a potential role for otherwise hard to treat injuries. It is unlikely that the use of EFs alone will turn out to be the silver bullet for nervous system injuries, but it may certainly be a useful addition to the clinic. The exact mechanisms by which EFs promote nervous regrowth are still unclear, and may be dependent on the choice of stimulation; AC or DC. It is likely that these mediate beneficial effects through different mechanisms. In AC stimulation, the likely effect is the stimulation of release and/or production of various neurotrophic factors, providing a nonspecific supportive environment for the regeneration of nervous cells. In contrast, DC is able to provide directional attractive cues for regeneration, potentially speeding up reinnervation, likely in addition to altering gene expression. Further fundamental research to elucidate the exact mechanisms of the beneficial effects of various stimulation paradigms will be required to validate their use in the clinic. The observations that the effect of EFs may be enhanced by certain neurotransmitters or neurotrophic factors opens up an avenue for treatment by combining these factors with EF to perhaps synergistically act on regrowing axons. Important future research direction will be to optimize the stimulation protocol for any given application, and to gain a more fundamental understanding of the mechanisms of EF-mediated cell behavior. Although the field of therapeutic EFs is still in its infancy, it is nonetheless a promising avenue for both PNS and CNS injuries.
Take-Home Messages.
• Endogenous EFs play an important role in the development of the nervous system.
• Applied EFs stimulate neuronal outgrowth.
• In the eye, EFs are neuroprotective and may stimulate regrowth of axons into damaged areas.
• In peripheral nerve injuries, applying an EF over the site of nerve damage may promote regrowth and reinnervation, although the exact stimulation protocol is important, as it may also be detrimental to healing when not applied correctly.
• In the spinal cord, EFs can stimulate axonal growth over the injury site, and cause functional recovery. A human trial of EF stimulation in SCI has shown promising results.
• The sensitivity of neurons and neural stem cells EFs can potentially be used in clinical practice.
Abbreviations and Acronyms
- AC
alternating current
- BDNF
brain-derived neurotrophic factor
- Cdc42
cell division cycle 42
- CNS
central nervous system
- DC
direct current
- EF
electric field
- OFS
oscillating field stimulator
- PNS
peripheral nervous system
- SCI
spinal cord injury
- TCPD
transcorneal potential difference
- TES
transcorneal electrical stimulation
Acknowledgments and Funding Sources
This work was funded by a European Research Council StG grant 243261, and Royal Society URF award UF051616, UF090098 to B.S.
Author Disclosure and Ghostwriting
No competing financial interests exist. The content of this article was expressly written by the authors listed. No ghostwriters were used to write this article.
About the Authors
Niels Haan is a postdoctoral researcher in the Song lab, working on cell based therapies for SCIs. Bing Song is a Professor of Cell Biology and Regenerative Dentistry in Cardiff University School of Dentistry. Song was awarded a Royal Society University Research Fellowship (2005–2013) to investigate the role of electric signals in the regulation of neural stem cell behavior. Professor Song's research interests are in electric signal regulated cell migration, division, and differentiation during wound healing and tissue regeneration. One of his major research areas focuses on stem cell based therapies for treating SCI.
References
- 1.Centers for Disease Control and Prevention: Spinal cord injury: fact sheet. Available online at www.cdc.gov/traumaticbraininjury/scifacts.html (accessed July12, 2013)
- 2.Reid B, Song B, McCaig CD, and Zhao M: Wound healing in rat cornea: the role of electric currents. FASEB J 2005; 19:379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao M, Song B, Pu J, Wada T, Reid B, Tai G, Wang F, Guo A, Walczysko P, Gu Y, Sasaki T, Suzuki A, Forrester JV, Bourne HR, Devreotes PN, McCaig CD, and Penninger JM: Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-gamma and PTEN. Nature 2006; 442:457. [DOI] [PubMed] [Google Scholar]
- 4.Song B, Zhao M, Forrester J, and McCaig C: Nerve regeneration and wound healing are stimulated and directed by an endogenous electrical field in vivo. J Cell Sci 2004; 117:4681. [DOI] [PubMed] [Google Scholar]
- 5.Gaudet AD, Popovich PG, and Ramer MS: Wallerian degeneration: gaining perspective on inflammatory events after peripheral nerve injury. J Neuroinflammation 2011; 8:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Webber CA, Christie KJ, Cheng C, Martinez JA, Singh B, Singh V, Thomas D, and Zochodne DW: Schwann cells direct peripheral nerve regeneration through the Netrin-1 receptors, DCC and Unc5H2. Glia 2011; 59:1503. [DOI] [PubMed] [Google Scholar]
- 7.Okun MS: Deep-brain stimulation for Parkinson's disease. N Engl J Med 2012; 367:1529. [DOI] [PubMed] [Google Scholar]
- 8.Hinkle L, McCaig CD, and Robinson KR: The direction of growth of differentiating neurones and myoblasts from frog embryos in an applied electric field. J Physiol 1981; 314:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCaig CD: Nerve growth in the absence of growth cone filopodia and the effects of a small applied electric field. J Cell Sci 1989; 93 (Pt 4):715. [DOI] [PubMed] [Google Scholar]
- 10.Robinson KR. and Stump RF: Self-generated electrical currents through Xenopus neurulae. J Physiol 1984; 352:339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hotary KB. and Robinson KR: Endogenous electrical currents and the resultant voltage gradients in the chick embryo. Dev Biol 1990; 140:149. [DOI] [PubMed] [Google Scholar]
- 12.Hotary KB. and Robinson KR: Endogenous electrical currents and voltage gradients in Xenopus embryos and the consequences of their disruption. Dev Biol 1994; 166:789. [DOI] [PubMed] [Google Scholar]
- 13.Borgens RB. and Shi R: Uncoupling histogenesis from morphogenesis in the vertebrate embryo by collapse of the transneural tube potential. Dev Dyn 1995; 203:456. [DOI] [PubMed] [Google Scholar]
- 14.Shi R. and Borgens RB: Three-dimensional gradients of voltage during development of the nervous system as invisible coordinates for the establishment of embryonic pattern. Dev Dyn 1995; 202:101. [DOI] [PubMed] [Google Scholar]
- 15.Rajnicek AM, Foubister LE, and McCaig CD: Temporally and spatially coordinated roles for Rho, Rac, Cdc42 and their effectors in growth cone guidance by a physiological electric field. J Cell Sci 2006; 119:1723. [DOI] [PubMed] [Google Scholar]
- 16.Erskine L. and McCaig CD: Growth cone neurotransmitter receptor activation modulates electric field-guided nerve growth. Dev Biol 1995; 171:330. [DOI] [PubMed] [Google Scholar]
- 17.McCaig CD, Sangster L, and Stewart R: Neurotrophins enhance electric field-directed growth cone guidance and directed nerve branching. Dev Dyn 2000; 217:299. [DOI] [PubMed] [Google Scholar]
- 18.Candia OA: Short-circuit current related to active transport of chloride in frog cornea: effects of furosemide and ethacrynic acid. Biochim Biophys Acta 1973; 298:1011. [DOI] [PubMed] [Google Scholar]
- 19.Klyce SD: Transport of Na, Cl, and water by the rabbit corneal epithelium at resting potential. Am J Physiol 1975; 228:1446. [DOI] [PubMed] [Google Scholar]
- 20.Candia OA. and Cook P: Na+-K+ pump stoichiometry and basolateral membrane permeability of frog corneal epithelium. Am J Physiol 1986; 250:F850. [DOI] [PubMed] [Google Scholar]
- 21.Chiang M, Robinson KR, and Vanable JW, Jr.: Electrical fields in the vicinity of epithelial wounds in the isolated bovine eye. Exp Eye Res 1992; 54:999. [DOI] [PubMed] [Google Scholar]
- 22.Sta Iglesia DD. and Vanable JW, Jr.: Endogenous lateral electric fields around bovine corneal lesions are necessary for and can enhance normal rates of wound healing. Wound Repair Regen 1998; 6:531. [DOI] [PubMed] [Google Scholar]
- 23.Rozsa AJ, Guss RB, and Beuerman RW: Neural remodeling following experimental surgery of the rabbit cornea. Invest Ophthalmol Vis Sci 1983; 24:1033. [PubMed] [Google Scholar]
- 24.Wang X, Mo X, Li D, Wang Y, Fang Y, Rong X, Miao H, and Shou T: Neuroprotective effect of transcorneal electrical stimulation on ischemic damage in the rat retina. Exp Eye Res 2011; 93:753. [DOI] [PubMed] [Google Scholar]
- 25.Morimoto T, Miyoshi T, Matsuda S, Tano Y, Fujikado T, and Fukuda Y: Transcorneal electrical stimulation rescues axotomized retinal ganglion cells by activating endogenous retinal IGF-1 system. Invest Ophthalmol Vis Sci 2005; 46:2147. [DOI] [PubMed] [Google Scholar]
- 26.Miyake K, Yoshida M, Inoue Y, and Hata Y: Neuroprotective effect of transcorneal electrical stimulation on the acute phase of optic nerve injury. Invest Ophthalmol Vis Sci 2007; 48:2356. [DOI] [PubMed] [Google Scholar]
- 27.Ni YQ, Gan DK, Xu HD, Xu GZ, and Da CD: Neuroprotective effect of transcorneal electrical stimulation on light-induced photoreceptor degeneration. Exp Neurol 2009; 219:439. [DOI] [PubMed] [Google Scholar]
- 28.Morimoto T, Fujikado T, Choi JS, Kanda H, Miyoshi T, Fukuda Y, and Tano Y: Transcorneal electrical stimulation promotes the survival of photoreceptors and preserves retinal function in royal college of surgeons rats. Invest Ophthalmol Vis Sci 2007; 48:4725. [DOI] [PubMed] [Google Scholar]
- 29.Al-Majed AA, Brushart TM, and Gordon T: Electrical stimulation accelerates and increases expression of BDNF and trkB mRNA in regenerating rat femoral motoneurons. Eur J Neurosci 2000; 12:4381. [PubMed] [Google Scholar]
- 30.Brushart TM, Hoffman PN, Royall RM, Murinson BB, Witzel C, and Gordon T: Electrical stimulation promotes motoneuron regeneration without increasing its speed or conditioning the neuron. J Neurosci 2002; 22:6631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mendonca AC, Barbieri CH, and Mazzer N: Directly applied low intensity direct electric current enhances peripheral nerve regeneration in rats. J Neurosci Methods 2003; 129:183. [DOI] [PubMed] [Google Scholar]
- 32.Huang J, Lu L, Zhang J, Hu X, Zhang Y, Liang W, Wu S, and Luo Z: Electrical stimulation to conductive scaffold promotes axonal regeneration and remyelination in a rat model of large nerve defect. PLoS One 2012; 7:e39526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gordon T, Chan KM, Sulaiman OA, Udina E, Amirjani N, and Brushart TM: Accelerating axon growth to overcome limitations in functional recovery after peripheral nerve injury. Neurosurgery 2009; 65:A132. [DOI] [PubMed] [Google Scholar]
- 34.Gigo-Benato D, Russo TL, Geuna S, Domingues NR, Salvini TF, and Parizotto NA: Electrical stimulation impairs early functional recovery and accentuates skeletal muscle atrophy after sciatic nerve crush injury in rats. Muscle Nerve 2010; 41:685. [DOI] [PubMed] [Google Scholar]
- 35.Lu MC, Tsai CC, Chen SC, Tsai FJ, Yao CH, and Chen YS: Use of electrical stimulation at different current levels to promote recovery after peripheral nerve injury in rats. J Trauma 2009; 67:1066. [DOI] [PubMed] [Google Scholar]
- 36.Eberhardt KA, Irintchev A, Al-Majed AA, Simova O, Brushart TM, Gordon T, and Schachner M: BDNF/TrkB signaling regulates HNK-1 carbohydrate expression in regenerating motor nerves and promotes functional recovery after peripheral nerve repair. Exp Neurol 2006; 198:500. [DOI] [PubMed] [Google Scholar]
- 37.Borgens RB, Blight AR, and McGinnis ME: Behavioral recovery induced by applied electric fields after spinal cord hemisection in guinea pig. Science 1987; 238:366. [DOI] [PubMed] [Google Scholar]
- 38.Borgens RB, Blight AR, and McGinnis ME: Functional recovery after spinal cord hemisection in guinea pigs: the effects of applied electric fields. J Comp Neurol 1990; 296:634. [DOI] [PubMed] [Google Scholar]
- 39.Wallace MC, Tator CH, and Piper I: Recovery of spinal cord function induced by direct current stimulation of the injured rat spinal cord. Neurosurgery 1987; 20:878. [DOI] [PubMed] [Google Scholar]
- 40.Fehlings MG. and Tator CH: The effect of direct current field polarity on recovery after acute experimental spinal cord injury. Brain Res 1992; 579:32. [DOI] [PubMed] [Google Scholar]
- 41.Borgens RB, Toombs JP, Blight AR, McGinnis ME, Bauer MS, Widmer WR, and Cook JR, Jr.: Effects of applied electric fields on clinical cases of complete paraplegia in dogs. Restor Neurol Neurosci 1993; 5:305. [DOI] [PubMed] [Google Scholar]
- 42.Borgens RB, Toombs JP, Breur G, Widmer WR, Waters D, Harbath AM, March P, and Adams LG: An imposed oscillating electrical field improves the recovery of function in neurologically complete paraplegic dogs. J Neurotrauma 1999; 16:639. [DOI] [PubMed] [Google Scholar]
- 43.Shapiro S, Borgens R, Pascuzzi R, Roos K, Groff M, Purvines S, Rodgers RB, Hagy S, and Nelson P: Oscillating field stimulation for complete spinal cord injury in humans: a phase 1 trial. J Neurosurg Spine 2005; 2:3. [DOI] [PubMed] [Google Scholar]
- 44.Shapiro S: Erratum. J Neurosurg Spine 2008; 8:604 [Google Scholar]
- 45.Moriarty LJ. and Borgens RB: An oscillating extracellular voltage gradient reduces the density and influences the orientation of astrocytes in injured mammalian spinal cord. J Neurocytol 2001; 30:45. [DOI] [PubMed] [Google Scholar]
- 46.Moriarty LJ. and Borgens RB: The effect of an applied electric field on macrophage accumulation within the subacute spinal injury. Restor Neurol Neurosci 1999; 14:53. [PubMed] [Google Scholar]
- 47.De Feo D, Merlini A, Laterza C, and Martino G: Neural stem cell transplantation in central nervous system disorders: from cell replacement to neuroprotection. Curr Opin Neurol 2012; 25:322. [DOI] [PubMed] [Google Scholar]
- 48.Cao L, Wei D, Reid B, Zhao S, Pu J, Pan T, Yamoah EN, and Zhao M: Endogenous electric currents might guide rostral migration of neuroblasts. EMBO Rep 2013; 14:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Feng JF, Liu J, Zhang XZ, Zhang L, Jiang JY, Nolta J, and Zhao M: Guided migration of neural stem cells derived from human embryonic stem cells by an electric field. Stem Cells 2012; 30:349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meng X, Arocena M, Penninger J, Gage FH, Zhao M, and Song B: PI3K mediated electrotaxis of embryonic and adult neural progenitor cells in the presence of growth factors. Exp Neurol 2011; 227:210. [DOI] [PMC free article] [PubMed] [Google Scholar]