Abstract
8-Oxo-7,8-dihydro-2′-deoxyguanosine (dOG), a well-studied oxidation product of 2′-deoxyguanosine (dG), is prone to facile further oxidation forming spiroiminodihydantoin 2′-deoxyribonucleoside (dSp) in the nucleotide pool and in single-stranded oligodeoxynucleotides (ODNs). Many methods for quantification of damaged lesions in the genome rely on digestion of DNA with exonucleases or endonucleases and dephosphorylation followed by LC-MS analysis of the resulting nucleosides. In this study, enzymatic hydrolysis of dSp-containing ODNs was investigated with snake venom phosphodiesterase (SVPD), spleen phosphodiesterase (SPD) and nuclease P1. SVPD led to formation of a dinucleotide, 5′-d(Np[Sp])-3′ (N = any nucleotide) that included the undamaged nucleotide on the 5′ side of dSp as the final product. This dinucleotide was a substrate for both SPD and nuclease P1. A kinetic study of the activity of SPD and nuclease P1 showed a sequence dependence on the nucleotide 5′ to the lesion with rates in the order dG>dA>dT>dC. In addition, the two diastereomers of dSp underwent digestion at significantly different rates with dSp1>dSp2; nuclease P1 hydrolyzed the 5′-d(Np[Sp1])-3′ dinucleotide two- to six-fold faster than the corresponding 5′-d(Np[Sp2])-3′, while for SPD the difference was two-fold. These rates are chemically reasoned based on dSp diastereomer differences in the syn vs. anti glycosidic bond orientation. A method for the complete digestion of dSp-containing ODNs is also outlined based on treatment with nuclease P1 and SVPD. These findings have significant impact on the development of methods to detect dSp levels in cellular DNA.
Introduction
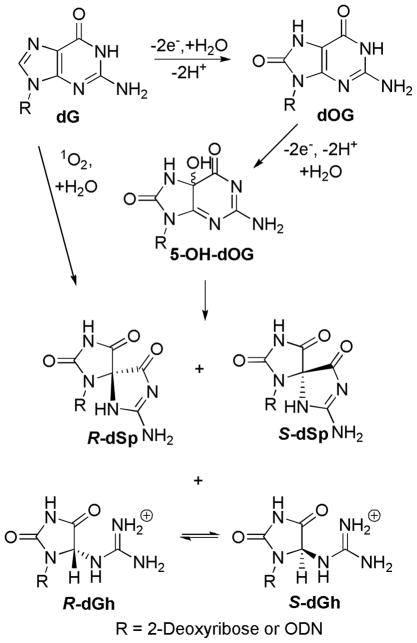
The continual attack of reactive oxygen species (ROS) on the genome results in mutagenesis associated with ageing, cancer and other disorders.1–3 Of the four standard DNA nucleosides, 2′-deoxyguanosine (dG) is the most reactive toward oxidation,4–7 leading to 8-oxo-7,8-dihydro-2′-deoxyguanosine (dOG, Scheme 1) as a common product in DNA. Additionally, dOG is studied as the biomarker of oxidatively damaged DNA in the cell.8–10 Because dOG can base pair with 2′-deoxyadenosine (dA) in addition to 2′-deoxycytidine (dC), unrepaired dOG can lead to dG → dT (thymidine) transversion mutations after synthesis occurs opposite the lesion.11–14 Moreover, dOG is substantially more reactive toward oxidation than dG with most oxidants because of its significantly lower redox potential.6 In this pathway, two oxidation products are formed from the common intermediate 5-hydroxy-dOG (5-HO-dOG), spiroiminodihydantoin 2′-deoxynucleoside (dSp) and 5-guanidinohydantoin 2′-deoxynucleoside (dGh, Scheme 1). These hydantoin products have been characterized in our laboratory and others, and arise from either two-electron oxidation of the initially formed dOG or from direct four-electron oxidation of dG by oxidants such as singlet oxygen.15–24 Yields of dSp increase at high pH (> 7) and in less hindered contexts, such as nucleosides and G-quadruplexes, while dGh dominates at low pH (< 6) and in hindered contexts, such as double-stranded ODNs (dsODN).15, 18, 25–29 Furthermore, the dSp lesion has been detected in Nei-deficient E. coli exposed to chromate,30 and both dGh and dSp have been observed in a mouse model of chronic inflammation.31 dGh and dSp-containing oligodeoxynucleotides (ODNs) have been studied in template DNA in primer extension experiments with the Klenow fragment (exo−) of E. coli polymerase I.32 Both dATP and dGTP are preferentially inserted opposite dGh and dSp, and subsequent primer extension is strongly inhibited. In vivo studies showed both dGh and dSp are highly mutagenic producing dG →dT and dG →dC transversion mutations, in accord with the observations of the polymerase activity.33, 34 dGh and dSp lesions can be repaired by the base excision repair (BER) pathway;35 they are substrates for Fpg36, 37 and Nei in bacteria and viruses,38–40 yOGG1 and yOGG2 in yeast,41 mouse Neil1, Neil2 and Neil330, 42 and human Neil1 and Neil3.43, 44
Scheme 1.
Reaction course for dG oxidation to dOG and the hydantoins, dGh and dSp.
Computational studies reveal that the R and S diastereomers of dSp distort the duplex by perturbing the base stacking and neighboring hydrogen bonding;45 though, NMR and thermal melting studies did not detect a difference between these two diastereomers in a double-stranded ODN (dsODN) construct.46 Differences between the two dSp diastereomers have been observed in mutational spectra in E. coli and in enzymatic excision by the hNeil1 BER glycosylase.33, 43 However, the assignment of the absolute configuration of the two dSp diastereomers has been difficult; studies matching computational results to the observed electronic circular dichroism (ECD) spectra vs. NMR data have led to opposite conclusions.47–49 The dGh diastereomers exist in equilibrium with one another rendering their individual study challenging.18
In order to estimate the amounts of specific oxidized lesions in the genome, many methods rely on DNA digestion by nucleases, dephosphorylation, and LC-MS analysis of the resulting nucleoside mixture.50, 51 Typical nucleases used include the 3′ → 5′ exonuclease snake venom phosphodiesterase (SVPD, also called phosphodiesterase I) that catalyzes Reaction I (N = any nucleotide), the 5′ → ′ exonuclease spleen phosphodiesterase (SPD, also called phosphodiesterase II) that catalyzes Reaction II, and the single-strand specific endonuclease nuclease P1 that catalyzes Reaction I. These nucleases readily digest undamaged ODNs.52–54
| Reaction I |
| Reaction II |
SVPD can effect complete hydrolysis of dOG8 and 5-guanidino-4-nitroimidazole55 lesion-containing ODNs. However, ODNs containing an abasic site when digested by SVPD yielded a dinucleotide, 5′-d(NpX)-3′ (X = abasic site), in which the phosphodiester linkage on the 5′ side of the lesion remained intact.56 In fact, SVPD easily hydrolyzes 5′-d(XpA)-3′ but not 5′-d(ApX)-3′ showing a side preference for digestion. Consequently, SVPD has limited utility in digestion of lesion containing ODNs and DNA if it is the only nuclease selected.
On the other hand, the exonuclease SPD cleaves DNA in a stepwise manner from the 5′-end and accepts lesion-containing substrates such as 5′-d(ApX)-3′, though with a 100-fold slower rate than 5′-d(ApA)-3′.56 Furthermore, SPD digestion efficiency decreases with respect to the nucleotide on the 5′-end of the digestion site in the order dG>dA>dT>dC.57, 58 Studies concerning the kinetics for digestion of the cis-5,6-dihydroxy-5,6-dihydrothymidine (dTg) diastereomers in short ODNs revealed a diastereochemical preference for digestion by SPD in which the 5S,6R isomer was a better substrate than the 5R,6S isomer in the sequence context 5′-d([Tg]pA)-3′.59 In another example, adducts of polycyclic aromatic hydrocarbons at N2 of dG- containing ODNs show resistance to digestion by SPD.60 Thus, SPD has variable activity with lesion-containing substrates that is not readily predictable.
For certain lesions, nuclease P1 also works inefficiently on the phosphodiester bond between the lesion and its 3′ nucleotide (Reaction I); for example, it hydrolyzes 5′-d(ApX)-3′ but not 5′-d(XpA)-3′.56 Nuclease P1 activity is also dependent on the nucleotide identity 5′ to the phosphodiester that is hydrolyzed favoring purines over pyrimidines.61 Furthermore, nuclease P1 also shows a diastereoselective preference for digesting the diastereomers of 5,6-dihydrothymidine (dTh) in which the 5R isomer is hydrolyzed 50-times faster than the 5S isomer.62 The Meunier laboratory was the first to observe the dG oxidation product, 2-iminohydantoin 2′-deoxyribonucleoside (d2-Ih, m/z = dG+34)63 from metal-mediated oxidations, and this heterocycle has been further characterized and observed by our laboratory and others.64–67 Recently, Pratviel and coworkers have shown that nuclease P1 is capable of complete digestion of ODNs containing this lesion in the sequence context 5′-d(Ap[2-Ih])-3′,68 demonstrating again that nuclease P1 activity with lesion-containing substrates is not readily predictable.
In previous studies by Buchko et al., pure dSp-containing ODNs or oxidized calf thymus DNA were digested with SVPD and SPD; in these studies the phosphodiester linkage between the 5′-d(Tp[Sp])-3′ dinucleotide was not hydrolyzed by SVPD and this dinucleotide was slowly cleaved by SPD.69 Later, Joffe et al. digested DNA containing the 5′-d(Cp[Sp])-3′ sequence and found that the phosphodiester bond was resistant to hydrolysis by both SVPD and SPD.21 The Sugden laboratory showed that SVPD and SPD in combination were capable of liberating dSp nucleoside from the sequence 5′-d(Gp[Sp])-3′.70 Box and coworkers treated dSp-containing tetramers and calf thymus DNA with nuclease P1 and obtained the dinucleotide 5′-d([Sp]pN)-3′.71 Lastly, the Tannenbaum laboratory digested calf thymus DNA that contained dSp with a combination of nuclease P1 and SVPD giving a high yield of dSp nucleoside after phosphatase treatment.72, 73 These studies highlight a strong sequence context effect for nuclease digestion of dSp-containing substrates and that nuclease combinations are the most effective for hydrolyzing the phosphodiester bonds flanking a dSp lesion on both 5′ and 3′ ends.
Overall, DNA substrates containing the dSp lesion have been digested via various methods with mixed results. In the present work, dSp-containing ODNs (see Table 1) were hydrolyzed using the most commonly employed enzymes SVPD, SPD and nuclease P1. Digestion rates were determined for ODNs that contained the individual diastereomers of dSp with the three nucleases in which the 5′-base specificity and the diastereoselectivity of digestion were determined. From these data, mechanisms are proposed to explain the results. Moreover, a method for the complete digestion of dSp-containing ODNs was established from the kinetic data to give a high yield of dSp nucleoside from ODNs.
Table 1.
dSp-containing ODN sequences utilized in these nuclease-dependent digestion studies.
| Name | ODN Sequence |
|---|---|
|
| |
| ODN-G | 5′-d(TTT TTT TA G[Sp] CT)-3′ |
| ODN-A | 5′-d(TTT TTT TC A[Sp] GT)-3′ |
| ODN-T | 5′-d(TTT TTT CA T[Sp] GT)-3′ |
| ODN-C | 5′-d(TTT TTT TA C[Sp] GT)-3′ |
Experimental Procedures
Materials
All standard chemicals were purchased from commercial suppliers and used without purification. dOG-containing ODNs were obtained from the DNA/Peptide Core Facility at the University of Utah using commercially available phosphoramidites (Glen Research). SVPD (phosphodiesterase I) and SPD (phosphodiesterase II) were purchased from Worthington Biochemical Corporation, and nuclease P1 and shrimp alkaline phosphatase (SAP) were obtained from US Biochemical.
dSp synthesis in ODNs and nucleosides
To synthesize dSp-containing ODNs, a dOG-containing 12-mer ODN (12 μM, Table 1) was incubated in 10 mM NaPi (pH 7.5) at 65 °C for 10 min, and then Na2IrCl6 was added to a 100–μM concentration in a 500–μL reaction. Next, the reaction was incubated for 45 min at 65 °C and then quenched by the addition of EDTA at a final concentration of 1 mM.32 These conditions provide nearly quantitative conversion to the dSp diastereomers that were purified as described below.
Synthesis of the dSp nucleoside used as a reference material was achieved by dissolving dOG (1 mM) and K2S2O8 (20 mM) in 75 mM KPi (pH 8) at 22 °C, then irradiating the reaction at a distance of 7 cm with 256 nm light for 30 min that gave a ~75% yield, and finally the products were purified as described below.74
Chromatography
Purification of the 12-mer ODNs was accomplished using an anion- exchange HPLC column (Dionex DNAPac PA100, 4 × 250 mm) running a linear gradient of 25% B to 100% B over 30 min in which A = 1:9 CH3CN:ddH2O, and B = 1:9 CH3CN:ddH2O with 1.5 M NaOAc (pH 7) while running a 1 mL/min flow rate and monitoring the absorbance at 260 nm. The ODNs containing dSp diastereomers were collected individually and labeled dSp1 and dSp2 based on their elution order from this column. Next, the samples were desalted by dialyzing against ddH2O in Eppendorf tubes fitted with a 3500 molecular-weight cutoff membrane for 48 h. The diastereotopically pure dinucleotides 5′d(Np[Sp])-3′ were purified utilizing the previous column and mobile phase, but the method was changed to a linear gradient of 0% B to 2.5% B over 10 min, and then from 2.5% B to 40% B over 15 min. Analysis of the nuclease-released nucleosides dA, dG, dT, dC and dOG was performed on a C18 reversed-phase HPLC column (RP-HPLC, Phenomenex 4.6 × 250 mm, 5 μm polar-RP) running an isocratic method of 0.5% B in which A = 0.1% TFA in ddH2O, and B = MeOH, while running a flow rate of 1 mL/min and monitoring the absorbance at 260 nm. dSp nucleosides were analyzed utilizing a Hypercarb column (Thermo Hypersil-Keystone, 3 × 100 mm) running an isocratic gradient of 0.5% B in which A = 0.1% formic acid in ddH2O, and B = 0.1% formic acid in CH3CN, while running a flow rate of 0.6 mL/min and monitoring the absorbance at 240 nm.
Mass spectrometry
ODN and dinucleotide samples were analyzed by negative ion electrospray ionization (ESI) on a Micromass Quattro II tandem mass spectrometer equipped with a Zspray API source. Samples were dissolved in a 1:1 mixture of isopropanol and 1 mM NH4OAc and introduced via infusion at a flow rate of 5 μL/min. The source and desolvation temperatures were 95 °C and 150 °C, respectively. The capillary voltage was set to 2.5 kV, sampling cone voltage to 35 V, and the extractor cone to 3 V. The nucleoside samples were analyzed by positive ion electrospray ionization (ESI). Samples were dissolved in CH3CN and ddH2O (1:1) and introduced via infusion at a flow rate of 7 μL/min. The source and desolvation temperatures were 80 °C and 120 °C, respectively. The capillary voltage was set to 3.5 kV, sampling cone voltage to 35 V, and the extractor cone to 3 V.
Digestion
Three nucleases were used individually (a–c) and in combinations (d) for the digestion studies. (a) SVPD-mediated digestions were carried out in a total volume of 50 μL containing 10 mM Tris-HCl, 4 mM MgCl2 (pH 7.8), 1 nmole purified dSp-containing ODN, 0.5 units of SVPD and 0.2 units of SAP at 37 °C for 18 h. (b) SPD-mediated digestions utilized purified dSp-containing ODNs (1 nmole) hydrolyzed with 1 unit SPD in 10 mM NaPi (pH 6.4) in a total volume of 160 μL at 37 °C. Aliquots (30 μL) were removed at 1, 2, 3 and 4 h from the reaction mixture and placed on dry ice for later analysis. (c) Nuclease P1-mediated digestions were performed in 160-μL solutions containing 10 mM NaOAc (pH 5.3), 1 mM Zn(OAc)2, 1 nmole purified dSp-containing ODN and 1 unit of nuclease P1 at 37 °C. Aliquots (30 μL) were removed at 0.5, 1, 2 and 4 h from the reaction mixture, then quenched with 10 μL of 20 mM EDTA (pH 8.5), and placed on dry ice for later analysis. (d) Combined nuclease digestions were conducted by two methods: (d1) First, each 12-mer ODN (1 nmole) was digested with SVPD (1 unit) and SAP (1 unit) in conditions described in part (a) for 18 h. Then, the reaction was continued by adding 30 μL of 100 mM NaPi (final pH 6.4) and 1 unit SPD. The mixture was then incubated for an additional 18 h at 37 °C. (d2) Initial treatment was carried out as previously described with SVPD and SAP, and then 50 μL of 10 mM NaOAc (pH 5.3), 1 mM Zn(OAc)2 along with 1 unit nuclease P1 was added and the reaction continued for 18 h. Because commercial nucleases contain deaminase impurities, the deaminase inhibitors pentostatin and tetrahydrouridine must be added to reduce the amount of 2′-deoxyinosine and 2′-deoxyuridine in the analysis mixtures.75
ECD measurements
The ECD spectra for diastereomers of the dSp-containing dinucleotides and dSp nucleosides from 200 to 350 nm were recorded in ddH2O at 22 °C.
Results
Preparation of ODNs
The ODN sequences shown in Table 1 were designed to keep the overall base composition the same while varying the identity of the 5′ neighbor to dSp. Importantly, studies were conducted separately with the ODN containing the individual diastereomers of dSp, which are designated here as dSp1 and dSp2 based on their elution order from an anion-exchange HPLC analysis (Dionex DNAPac PA100). Furthermore, it is important to point out that our oxidation conditions yield the dSp1 and dSp2 diastereomers in roughly a 2:3 ratio in ssODN oxidations, and this ratio can be monitored to determine the diastereoselectivity of digestion from ODNs that contain a mixture of the dSp diastereomers. Moreover, it is known that after complete ODN digestion to nucleosides, the dSp1 and dSp2 diastereomers switch order when analyzed by a Hypercarb HPLC column, but they retain the same order as seen on the ion-exchange column when analyzed by a normal phase amino-silica column.48, 49
Hydrolysis of 12-mer ODNs by SVPD
In the first set of studies, SVPD (Reaction I) and SAP (phosphatase) were allowed to react with ODNs that contained a 2:3 mixture of the dSp diastereomers. In each sequence context studied (Table 1), the products were revealed to be a 2:3 ratio of the dSp diastereomer mixture of the dinucleotide 5′-d(Np[Sp])-3′ as observed on an anion-exchange HPLC column. The reaction course was determined to be independent of the dSp stereochemistry, and even at long reaction times (18 h) the products were the two dinucleotides in the same 2:3 ratio (see ESI,† Fig. S1). To further confirm the dinucleotide products, negative-ion ESI-MS was conducted on the 5′-d(Cp[Sp])-3′ mixture of diastereomers, which yielded an m/e peak consistent with the product (see ESI,† Fig. S2). In a final control study, C18 RP-HPLC allowed us to monitor the standard nucleosides dA, dT, dG, and dC, and they were detected in the proper ratio as determined by the ODN sequence.
Hydrolysis of dinucleotides by SPD
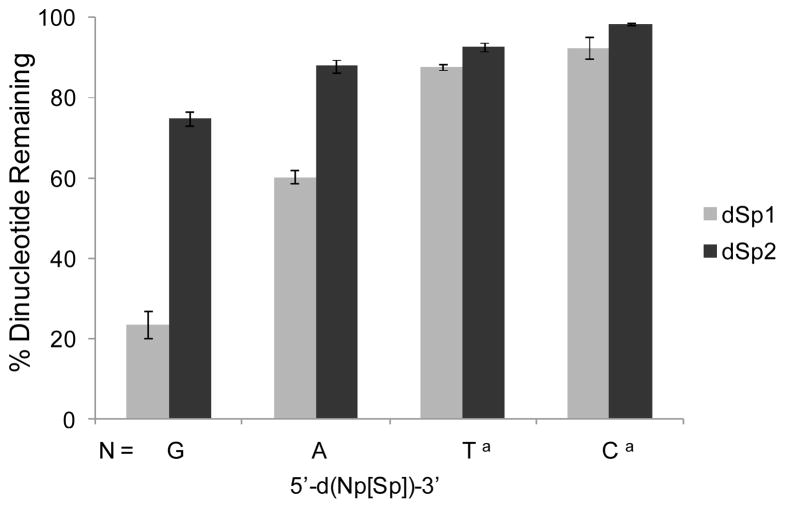
Because the SVPD plus SAP reactions yielded a dinucleotide product with a canonical nucleotide on the 5′ side of the dSp lesion, we elected to study the reaction course for digestion of both dSp diastereomers individually in the dinucleotide substrates with each standard nucleotide 5′ to the lesion with SPD (Reaction II) and SAP. The dinucleotide products were prepared via SVPD and SAP digestion of all 12-mer sequences (Table 1) that contained either dSp1 or dSp2 in each of the 4 sequence contexts. Next, each dinucleotide context was allowed to be hydrolyzed with SPD and SAP, in which the reaction course was monitored by the disappearance of reactant (5′-d(Np[Sp])-3′) on an anion-exchange HPLC column, while observing the appearance of the 5′-dNp product on the same HPLC column and dSp on a Hypercarb column. In Fig. 1 the reaction efficiencies after 4 h are shown for all combinations of dinucleotides. From these data, SPD showed limited hydrolysis of each context that had a 5′ pyrimidine nucleotide adjacent to the dSp lesion (i.e., 5′-d(Cp[Sp])-3′ and 5′-d(Tp[Sp])-3′) in which >85% reactant remained after a 4-h period, and the diastereoselectivity in the extent of reaction was minimal (Fig. 1). In cases with a purine nucleotide located immediately 5′ to the dSp lesion, the dSp1 diastereomer was more efficiently hydrolyzed than the dSp2 substrate. More specifically, when a dG nucleotide was 5′ to the dSp lesion, the dinucleotide with dSp1 was hydrolyzed to an extent three times that of the dinucleotide containing dSp2 in the 4-h reaction period. In cases with a 5′ dA nucleotide next to the dSp lesion, the dinucleotide with dSp1 was digested 1.5-times more than the dinucleotide with the dSp2 isomer. The digestion efficiency can be summarized as the dependence on the identity of the 5′ flanking nucleotide in the order dG>dA≫dT>dC (Fig. 1), and the stereochemical effect on reaction efficiency showed greater digestion of dSp1-containing dinucleotides compared to dSp2-containing dinucleotides, especially in cases where the 5′nucleotide was a purine (Fig. 1).
Fig. 1.
Extent of SPD-catalyzed phosphodiester hydrolysis of the dSp diastereomers in the dinucleotide sequence context 5′-d(Np[Sp])-3′. Digestion efficiencies were determined after 1 nmole of dinucleotide was allowed to react for 4 h at 37 °C in NaOAc buffer at pH 6.4 with 1 unit of SAP present. aThese data were collected with a pH 7 buffer.
Hydrolysis of dinucleotides by nuclease P1
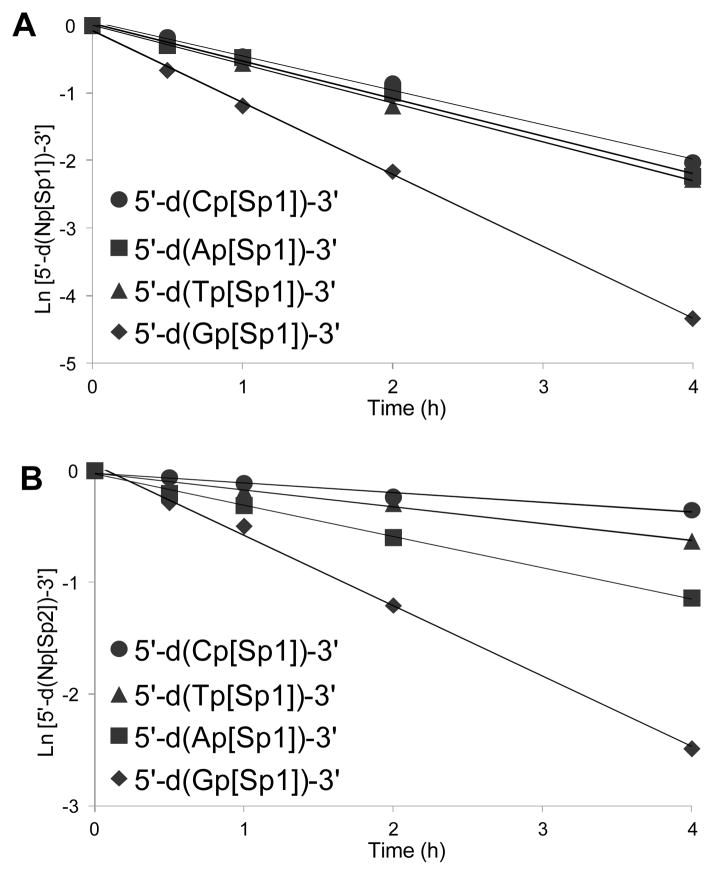
In the next set of studies, dinucleotides with a canonical nucleotide N located 5′ to the dSp lesion were hydrolyzed with nuclease P1 (Reaction I) and SAP. Again, all possible contexts were studied. The progress of these reactions was monitored by analysis of the peak areas for the starting dinucleotide and released canonical nucleoside on a C18 RP-HPLC column, and dSp nucleoside release was monitored on a Hypercarb column (see ESI,† Fig. S3). Fig. 2A and Fig. 2B provide graphs for ln[5′-d(Np[Sp])-3′] vs. time that were analyzed with Eq. 1 to provide the first-order rate constants shown in Table 2.
Fig. 2.
First-order reaction plots for digestion of 5′-d(Np[Sp])-3′-dinucleotides with nuclease P1. The reactions were conducted with 1 nmole dinucleotide, 1 unit nuclease P1 at 37 °C, pH 5.3 (NaOAc) doped with 1 mM Zn(OAc)2. (A) Plots for dSp1-containing dinucleotides. (B) Plots for dSp2-containing dinucleotides. Each value has an error of ~8% of the value.
Table 2.
First-order rate constants for nuclease P1 cleavage of the dSp-containing dinucleotides.
| Rate (h−1) | Rate dSp1 | ||
|---|---|---|---|
|
| |||
| Dinucleotide Sequence | dSp1 | dSp2 | Rate dSp2 |
|
| |||
| 5′-d(Gp[Sp])-3′ | 1.07 ± 0.06 | 0.62 ± 0.01 | 1.7 |
| 5′-d(Ap[Sp])-3′ | 0.56 ± 0.01 | 0.28 ± 0.03 | 2.0 |
| 5′-d(Tp[Sp])-3′ | 0.58 ± 0.01 | 0.15 ± 0.01 | 3.9 |
| 5′-d(Cp[Sp])-3′ | 0.51 ± 0.03 | 0.09 ± 0.01 | 5.7 |
| Eq. 1 |
As was found with SPD, the rate of digestion by nuclease P1 was affected by the 5′-flanking nucleotide. The specificity toward reaction can be summarized as decreasing in the order dG>dA~dT~dC for dSp1- and dG>dA>dT>dC for dSp2-containing dinucleotides (Table 2). In comparison to the SPD data, the diastereoselectivity of the reaction was also apparent with nuclease P1 for which digestion of dinucleotides with the dSp1 diastereomer was faster than that of dSp2. The difference is approximately two-fold for the 5′-d(Gp[Sp1])-3′ and 5′-d(Ap[Sp1])-3′ isomers compared to their dSp2-containing counterparts, four-fold for the 5′-d(Tp[Sp1])-3′ isomer compared to the dSp2 isomer, and nearly six-fold for the 5′-d(Cp[Sp1])-3′ isomer compared to the dSp2 isomer (Table 2).
Enzyme combination studies
In a final set of studies, nuclease combinations were tested to establish conditions that lead to the complete hydrolysis of the phosphodiester bond 5′ to either diastereomer of a dSp lesion with any 5′-flanking nucleotide. The 5′-d(Cp[Sp])-3′ substrate was the hardest to digest (Figs. 1, 2 and Table 2), and accordingly, all enzyme combinations tested were conducted for 18 h each, based on the reaction rate for this challenging dinucleotide. Reaction progress was monitored by following product formation via C18 RP-HPLC. Reactions conducted with SPD and SVPD (plus SAP) were not capable of completely digesting the 5′-d(Cp[Sp])-3′ phosphodiester bond, even with long reaction times (24 h). The best enzyme combination was found to be SVDP treatment followed by nuclease P1 digestion for 18 h each in the presence of SAP to provide complete disappearance of the reactant and near quantitative yield of free dSp nucleoside for both diastereomers of Sp (see ESI,† Fig. S4).
ECD spectroscopy for the dSp diastereomers
The digestion of dSp-containing ODNs were shown to be diastereoselective with both SPD and nuclease P1; however, the absolute stereochemical assignments for the dSp diastereomers have been debated in the literature via ECD spectroscopy and NMR experiments.47–49 In an attempt to couple our observed stereochemical preference for digestion of the dSp isomers with their stereochemical assignments reported in the literature we conducted a set of ECD studies on the diastereomers of the dinucleotide 5′-d(Gp[Sp])-3′ (Table 3) and the free dSp nucleoside (see ESI,† Fig. S5). First, it should be pointed out that the elution order for the dSp diastereomers is reversed between an anion-exchange HPLC column and the Hypercarb HPLC column, which we have reconfirmed in these studies (see ESI,† Fig. S6).47, 49 Here, to be consistent with our previous reports, the dSp numbering is based on the elution order of ODNs from an anion-exchange HPLC column. All sequence contexts and ODN lengths studied to date yield the same order of elution as confirmed by digestion and reinjection of the nucleosides. Our ECD data is compared to the computed literature values for the R and S diastereomers of the Sp free bases in Table 3.47, 49 From these data, the dinucleotide bearing the dSp1 diastereomer has specific ECD rotations that are consistent with R-Sp free base, and the dSp2 bearing dinucleotide has specific ECD rotations consistent with the S-Sp free base, assuming that the computed ECD spectra correctly predict the absolute configuration. Furthermore, we have confirmed that in our hands the ECD specific rotations for the free nucleosides that were prepared from the Hypercarb column are the same as those reported in the literature.49
Table 3.
Comparison of the ECD-specific rotations for the diastereomers of the dinucleotide 5′-d(Gp[Sp])-3′ and those reported in the literature.49
| ECD-specific Rotation | ||
|---|---|---|
|
| ||
| Isomer | 240 nm | 260 nm |
|
| ||
| 5′-d(Gp[Sp1])-3′a | Negative | Positive |
| 5′-d(Gp[Sp2])-3′a | Positive | Negative |
| dSp1a,b | Negative | Positive |
| dSp2a,b | Positive | Negative |
| R-Spc | Negative | Positive |
| S-Spc | Positive | Negative |
Numbering is based on the elution order from an anion-exchange HPLC column (DNAPac PA100), and this column was used to purify the samples.
These nucleosides were obtained by Hypercarb HPLC purification in which dSp2 elutes first.
These free-base experimental values were supported by TDDFT calculations.49
Discussion
In the first set of studies, the diastereomer mixture of dSp-containing 12-mers (Table 1) that have all four possible nucleotides 5′ to the dSp were digested with SVPD and SAP. In these reactions the dSp lesion was always released as a dinucleotide, 5′-d(Np[Sp])-3′, independent of sequence context or dSp stereochemistry. SVPD cannot hydrolyze the phosphodiester bond on the 5′ side of the dSp lesion, but is capable of skipping over the lesion to continue hydrolyzing the remaining part of the ODN substrate due to its single-strand nonspecific endonuclease activity.76 These results are consistent with previous digestion studies with either abasic site- or dTg-containing ODNs with SVPD in which the digestion products were always a dinucleotide with the lesion in the 3′ position.56, 62, 76 However, digestion of the two diastereomers of 5′-d(Ap[Th])-3′ as substrates for SVPD led to complete hydrolysis of the phosphodiester but with no stereochemical preference.62 Collectively these observations are interpreted to suggest that many factors such as loss of base aromaticity, glycosidic bond torsion angle and the sterics of the base play roles in determining if SVPD can digest through lesions.62, 77 Therefore, it is not surprising that the sterically bulky and hydrophilic dSp lesions were poor substrates relative to the normal nucleotides. Lastly, there was no stereochemical preference for digestion of the dSp-containing ODNs by SVPD, which is consistent with the lack of stereochemical preference for digestion of the 5R and 5S diastereomers of dTh-containing dinucleotides.62
In the second set of studies, the dinucleotides prepared from SVPD digests (5′-d(Np[Sp])-3′) were allowed to be cleaved with the 5′ →′ exonuclease SPD and dephosphorylated with SAP to yield dN and dSp nucleosides (Fig. 1). SPD showed substrate activity that was dependent on the 5′ flanking nucleotide as well as the stereochemistry of dSp. Cleavage dependence was observed in the order 5′-d(Gp[Sp])-3′ > 5′-d(Ap[Sp])-3′ > 5′-d(Tp[Sp])-3′ > 5′-d(Cp[Sp])-3′ (Fig. 1), which is consistent with a previous report that showed SPD prefers substrates with a 5′ purine over a 5′ pyrimidine.57 The data also show that dSp1-containing dinucleotides are always better substrates than dSp2 for all four dinucleotide contexts (Fig. 1). In the most extreme case the phosphodiester bond in the 5′-d(Gp[Sp1])-3′ was hydrolyzed three-fold faster than the 5′-(Gp[Sp2])-3′ isomer (Fig. 1). The effect of stereochemistry has also been observed with the 5′-d([Tg]pA)-3′ dinucleotide bearing the 5R,6S isomer that was hydrolyzed considerably slower than the 5S,6R isomer.59, 62 These observations show that the chiral context of the SPD active site does interact with diastereomeric substrates differently, as one might expect. Processing of the two diastereomers of dSp has been previously evaluated for hNEIL1 glycosylase activity, and in these studies the dSp1 isomer was reacted upon more readily, as was observed with SPD.43, 78 However, deeper arguments to understand why the dSp1 isomer is a better substrate cannot be made because the SPD crystal structure has not been determined. This enzymatic preference for one lesion diastereomer over the other has also been studied for the 5′,8-cyclo-2′-deoxypurines with pol η and the nucleotide excision repair pathway, providing another set of examples of how stereochemistry effects enzymatic reactions.79, 80
Previous kinetic studies concerning the activity of SPD with the positional substrates 5′-d([Th]pA)-3′ vs 5′-d(Ap[Th])-3′ or 5′-d([Tg]pA)-3′ vs 5′-d(Ap[Tg])-3′ showed two effects: (1) SPD binds to both bases flanking the phosphodiester to be hydrolyzed, and (2) reactivity for the lesion-containing dinucleotide is faster when the lesion is on the 5′ side.54, 62 In our studies, the propeller-shaped dSp lesion was always placed on the 3′ side, which dramatically slowed the activity of SPD relative to literature reports for native DNA substrates.56 Moreover, the better dinucleotide substrates contained a 5′-purine (Fig. 1), as expected,57, 58 which can be interpreted to say that the larger purines form better interactions with either the active site of the SPD or with the 3′-flanking dSp lesion, or both interactions are more favorable. However, without a detailed crystal structure additional insights into the molecular interactions cannot be established. Furthermore, at long reaction times (24 h) SPD can cleave substrates that have a purine nucleoside 5′ to the dSp lesion, but not those with a pyrimidine nucleoside 5′ to the lesion. Additionally, reaction efficiency was dependent on dSp stereochemistry with a 5′ purine, but not with a 5′ pyrimidine (Fig. 1).
Quantitative analysis of nuclease P1 hydrolysis of dinucleotides containing dSp at the 3′ location reveals both base specificity and diastereoselectivity. Nuclease P1 reactivity with the dSp1-bearing dinucleotides decreased according to the identity of the 5′-nucleotide in the order dG>dA~dT~dC (Table 2), while the dSp2-bearing dinucleotides decreased in the order dG>dA>dT>dC (Table 2). Additionally, the diastereoselective preference for the dSp1 diastereomer showed between two- to six-fold higher reactivity relative to the dSp2 isomer (Table 2). Because the structure of nuclease P1 has been determined by x-ray crystallography, the distorted interactions that the dSp diastereomers have with the binding pocket can be considered.81 Nuclease P1 functions as a homodimer in which one monomer binds on the 5′ side and the other on the 3′ side of the phosphodiester cleavage site. The 5′ base was found to be sandwiched between two tyrosine residues while the 3′ base π-stacks with only one phenylalanine residue. Therefore, having the nonplanar dSp lesion on the 3′ side is more easily accommodated by nuclease P1 due to the single phenylalanine interaction. Lastly, we note that nuclease P1 was capable of hydrolyzing all dinucleotide combinations at long reaction times (18 h). Consistent with this observation is the complete nuclease P1 digestion of an ODN with the d2-Ih lesion that is similar to dSp in the fact that it is sterically demanding and very hydrophilic.68 Additionally, and consistent with the current work, the diastereomers of 5′,8-cyclo-2′-deoxypurines are digested differentially by nuclease P1, and oligomers having a pyrimidine on the 5′ side of the lesion were also the slowest to digest.82–84 These observations explain why nuclease P1 should be present during any nuclease digestion method that is capable of digesting the two dSp diastereomers to completion from ODNs and DNA.30, 31, 71 We exploited this reactivity in our own studies to determine product distributions from G-quadruplex oxidations29 and Cu(II)-H2O2 mediated oxidations.64
In the present digestion studies of dSp-containing ODNs it was found that SVPD (phosphodiesterase I) and SPD (phosphodiesterase II) are both highly challenged by the dinucleotide 5′-d(Np[Sp])-3′ (Fig. 1), while nuclease P1 was capable of completing this reaction given sufficiently long reaction times (Fig. 2). Both phosphodiesterases SVPD and SPD hydrolyze the phosphodiester through a double-displacement reaction mechanism via an active site threonine residue,85, 86 while nuclease P1 hydrolyzes the phosphodiester through a Zn(II)-mediated water activation mechanism.81, 87 The spirocyclic nature of dSp causes this lesion to be very sterically demanding that is anticipated to cause a high amount of distortion in the enzyme active sites; therefore, initiation of phosphodiester bond hydrolysis by threonine is strongly slowed by SPD, and completely inhibited by SVPD. Apparently the sterically-demanding dSp substrate is less challenging for the nuclease P1 enzyme via the Zn(II)-water activation mechanism, and thus it can hydrolyze all dSp-containing dinucleotides.
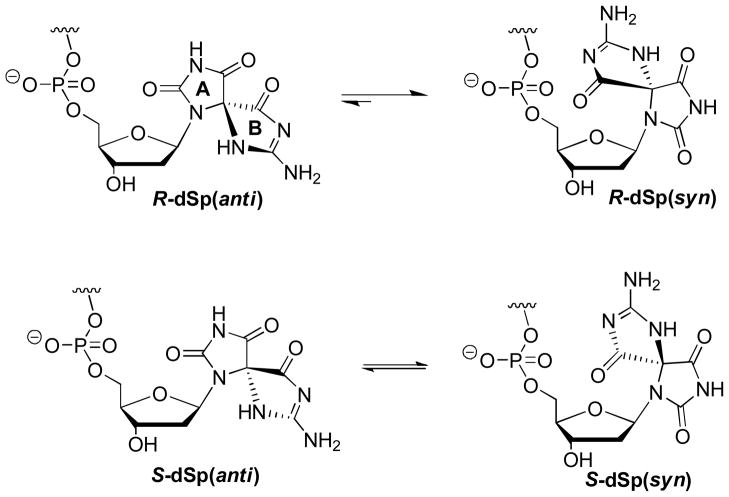
Another interesting nuance found in these studies is the degree of diastereoselectivity in hydrolyzing dinucleotides containing the two diastereomers of dSp placed on the 3′ side of the phosphodiester bond to be cleaved. Both SPD and nuclease P1 show reactivity that was greater with the dSp1 diastereomer over the dSp2 diastereomer (Figs. 1 and 2), and it seems unusual that two enzymes with unrelated active sites operating by different mechanisms would have the same diasteromeric preference. One possible explanation for these observations is that dSp1 exists in a conformation that is more amendable to phosphodiester cleavage. The glycosidic bonds for these two diastereomers can adopt either the syn or anti conformations in which the syn conformer for both diastereomers places the B-ring of dSp over the sugar and directed toward the phosphodiester reaction center (Fig. 3). As a result, the syn conformer would then inhibit the reaction and be slower for the nucleases to react upon. DFT and NOESY-NMR studies have found that the R and S diastereomers of dSp favor the syn and anti conformations differently.48 In these studies, the energetic difference between the two conformations for the S diastereomer is negligible at 0.063 kcal mol−1, while the R diastereomer favors the syn conformation by 3.37 kcal mol−1.48 Therefore, the R diastereomer is anticipated to exist in the syn conformation more often, and inhibit phosphodiester bond hydrolysis to a greater extent than the S diastereomer. Because the dSp2 (anion-exchange HPLC) diastereomer is the slowest to hydrolyze based on the present nuclease studies, it could likely be the R diastereomer of dSp. Once we take into account the HPLC column-dependent elution order, these data support the NMR-based stereochemical assignments for dSp;48 however, they run counter to the ECD spectroscopy-based stereochemical assignments for dSp.47, 49 Furthermore, our experimental ECD data for the dinucleotides and nucleosides (Table 3) are in agreement with those in the literature, which rules out any sample mishandling as an answer to this controversy.47, 49 Until an X-ray crystal structure for one of the dSp diastereomers becomes available, the absolute stereochemical assignments for dSp1 and dSp2 will remain in question.
Fig 3.
Glycosidic bond conformations for the dSp diastereomers. The relative equilibria were previously established by DFT and NOESY-NMR experiments.48
Conclusions
The studies described here add nucleases to the series of enzymes that operate differently with the two diastereomers of dSp. In previous studies, we found that dSp1 in a template ODN had a three-fold lower Km value than the same sequence containing dSp2 during the Klenow (exo−)catalyzed insertion of dATP opposite the lesion.88 DNA duplexes containing dSp1 were excised roughly two-fold faster than dSp2 in the same sequence context by the action of hNEIL1,43 and the selectivity for dSp1 is even more pronounced in non-canonical duplexes.78 This enzyme’s diastereoselectivity has been examined computationally by Broyde and workers to identify the S diastereomer to have more favorable interactions with hNEIL1;89 however, based on their data dSp2 in an ODN should be the S diastereomer of dSp.46, 49 The current studies found that dinucleotides bearing the dSp1 diastereomer were more efficiently hydrolyzed by SVPD and nuclease P1 than those with the dSp2 diastereomer. Because the R configuration of dSp favors the syn conformation,48 placing the B-ring of dSp near the phosphodiester to be hydrolyzed, it is appealing to hypothesize that dSp2 is the R diastereomer, slowing down nuclease activity due to its preference for the syn orientation. Furthermore, in vivo mutagenesis studies have shown significantly different profiles for dG → dT vs. dG → dC mutations for dSp1 and dSp2, although both diastereomers give similar levels of lesion bypass and overall extremely high levels of mutagenesis.33, 34
The practical implication of our study is that it further elucidates the requirements for the complete digestion of DNA that contains dSp lesions. This is best accomplished with nuclease P1 in combination with SVPD, and our findings are consistent with studies reported by the Tannenbaum laboratory.51 On the other hand, using SPD and SVPD to completely digest ODNs is possible with a purine nucleotide 5′ to the dSp, but not with a pyrimidine at the 5′ site next to the lesion. Among the four sequences, 5′-d(Gp[Sp])-3′ is the easiest to digest and 5′-d(Cp[Sp])-3′ is the most resistant to digestion by either SPD or nuclease P1. This may be an important factor when analyzing 5′-d(CpG)-3′ sequences that play important roles in regulatory sequences of the genome. If any of these three enzymes is used in isolation, dSp will be recovered primarily in the form of dinucleotides, 5′-d(Np[Sp])-3′ from SVPD and 5′-d([Sp]pN)-3′ from nuclease P1, and dSp will completely block the progress of SPD because it lacks endonuclease activity. These observations also interject a cautionary note to studies aimed at detecting the levels of dSp lesions from tissues exposed to oxidative stress. Because of the inefficiency of digestion and the differing rates of hydrolysis of dSp1 and dSp2 diastereomers, care must be taken in performing experiments and interpreting data from digestion studies.
Supplementary Material
Acknowledgments
This work was supported by the National Cancer Institute (CA090689).
Abbreviations
- d2-Ih
2-iminohydantoin 2′-deoxyribonucleoside
- 5-HO-dOG
5-hydroxy-8-oxo-7,8-dihydro-2′-deoxyguanosine
- dOG
8-oxo-7,8-dihydro-2′-deoxyguanosine
- dGh
5-guanidinohydantoin 2′-deoxyribonucleoside
- dSp
spiroiminodihydantoin 2′-deoxyribonucleoside
- dTg
cis 5,6-dihydroxy-5,6-dihydrothymidine (thymidine glycol)
- dTh
5,6-dihydrothymidine
- ESI-MS
electrospray ionization mass spectrometry
- HPLC
high-performance liquid chromatography
- ROS
reactive oxygen species
- SAP
shrimp alkaline phosphatase
- SPD
bovine spleen phosphodiesterase (Phosphodiesterase II)
- SVPD
snake venom phosphodiesterase (Phosphodiesterase I)
Footnotes
Electronic supplementary information available: HPLC and MS data for the 5′-d(Cp[Sp])-3′ diastereomers, ECD spectra for the 5′-d(Gp[Sp1])-3′ and 5′-d(Gp[Sp2])-3′ nucleotides, and HPLC traces that followed the nuclease-dependent reaction courses.
This manuscript is dedicated to Bernard Meunier in honor of his retirement, his recent election as vice-president of the French Academy of Sciences, and his spectacular career in biomimetic oxidation chemistry that played a major early role in catalyzing research efforts in our laboratory.
Based in part on the Ph.D. dissertation of Dr. Xin Chen, Department of Chemistry, University of Utah, 2008.
References
- 1.Beckman KB, Ames BN. Physiol Rev. 1998;78:547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- 2.Gajewski E, Rao G, Nackerdien Z, Dizdaroglu M. Biochemistry. 1990;29:7876–7882. doi: 10.1021/bi00486a014. [DOI] [PubMed] [Google Scholar]
- 3.Malins DC, Haimanot R. Cancer Res. 1991;51:5430–5432. [PubMed] [Google Scholar]
- 4.Yanagawa H, Ogawa Y, Ueno M. J Biol Chem. 1992;267:13320–13326. [PubMed] [Google Scholar]
- 5.Steenken S, Jovanovic SV. J Am Chem Soc. 1997;119:617–618. [Google Scholar]
- 6.Steenken S, Jovanovic SV, Bietti M, Bernhard K. J Am Chem Soc. 2000;122:2373–2374. [Google Scholar]
- 7.Burrows CJ, Muller JG. Chem Rev. 1998;98:1109–1152. doi: 10.1021/cr960421s. [DOI] [PubMed] [Google Scholar]
- 8.Collins AR, Cadet J, Moller L, Poulsen HE, Vina J. Arch Biochem Biophys. 2004;423:57–65. doi: 10.1016/j.abb.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 9.Helbock HJ, Beckman KB, Ames BN. Methods Enzymol. 1999;300:156–157. doi: 10.1016/s0076-6879(99)00123-8. [DOI] [PubMed] [Google Scholar]
- 10.Cadet J, Douki T, Ravanat JL. Acc Chem Res. 2008;41:1075–1083. doi: 10.1021/ar700245e. [DOI] [PubMed] [Google Scholar]
- 11.McAuley-Hecht KE, Leonard GA, Gibson NJ, Thomson JB, Watson WP, Hunter WN, Brown T. Biochemistry. 1994;33:10266–10270. doi: 10.1021/bi00200a006. [DOI] [PubMed] [Google Scholar]
- 12.Wood ML, Esteve A, Morningstar ML, Kuziemko GM, Essigmann JM. Nucleic Acids Res. 1992;20:6023–6032. doi: 10.1093/nar/20.22.6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lowe LG, Guengerich FP. Biochemistry. 1996;35:9840–9849. doi: 10.1021/bi960485x. [DOI] [PubMed] [Google Scholar]
- 14.Delaney JC, Essigmann JM. Chem Res Toxicol. 2007;21:232–252. doi: 10.1021/tx700292a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo W, Muller JG, Burrows CJ. Org Lett. 2001;3:2801–2804. doi: 10.1021/ol0161763. [DOI] [PubMed] [Google Scholar]
- 16.Luo W, Muller JG, Rachlin EM, Burrows CJ. Org Lett. 2000;2:613–616. doi: 10.1021/ol9913643. [DOI] [PubMed] [Google Scholar]
- 17.Luo W, Muller JG, Rachlin EM, Burrows CJ. Chem Res Toxicol. 2001;14:927–938. doi: 10.1021/tx010072j. [DOI] [PubMed] [Google Scholar]
- 18.Ye Y, Muller JG, Luo W, Mayne CL, Shallop AJ, Jones RA, Burrows CJ. J Am Chem Soc. 2003;125:13926–13927. doi: 10.1021/ja0378660. [DOI] [PubMed] [Google Scholar]
- 19.Niles JC, Wishnok JS, Tannenbaum SR. Org Lett. 2001;3:763–766. [PubMed] [Google Scholar]
- 20.Niles JC, Wishnok JS, Tannenbaum SR. Chem Res Toxicol. 2004;17:1510–1519. doi: 10.1021/tx0400048. [DOI] [PubMed] [Google Scholar]
- 21.Joffe A, Geacintov NE, Shafirovich V. Chem Res Toxicol. 2003;16:1528–1538. doi: 10.1021/tx034142t. [DOI] [PubMed] [Google Scholar]
- 22.Misiaszek R, Crean C, Geacintov N, Shafirovich V. J Am Chem Soc. 2005;127:2191–2200. doi: 10.1021/ja044390r. [DOI] [PubMed] [Google Scholar]
- 23.Slade PG, Priestley ND, Sugden KD. Org Lett. 2007;9:4411–4414. doi: 10.1021/ol701667t. [DOI] [PubMed] [Google Scholar]
- 24.Kupan A, Saulière A, Broussy S, Seguy C, Pratviel G, Meunier B. ChemBioChem. 2006;7:125–133. doi: 10.1002/cbic.200500284. [DOI] [PubMed] [Google Scholar]
- 25.Fleming AM, Muller JG, Dlouhy AC, Burrows CJ. J Am Chem Soc. 2012;134:15091–15102. doi: 10.1021/ja306077b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niles JC, Wishnok JS, Tannenbaum SR. Chem Res Toxicol. 2004;17:1510–1519. doi: 10.1021/tx0400048. [DOI] [PubMed] [Google Scholar]
- 27.Gremaud JN, Martin BD, Sugden KD. Chem Res Toxicol. 2010;23:379–385. doi: 10.1021/tx900362r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muller JG, Duarte V, Hickerson RP, Burrows CJ. Nucleic Acids Res. 1998;26:2247–2249. doi: 10.1093/nar/26.9.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fleming AM, Burrows CJ. Chem Res Toxicol. 2013;26:593–607. doi: 10.1021/tx400028y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hailer MK, Slade PG, Martin BD, Sugden KD. Chem Res Toxicol. 2005;18:1378–1383. doi: 10.1021/tx0501379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mangerich A, Knutson CG, Parry NM, Muthupalani S, Ye W, Prestwich E, Cui L, McFaline JL, Mobley M, Ge Z, Taghizadeh K, Wishnok JS, Wogan GN, Fox JG, Tannenbaum SR, Dedon PC. Proc Natl Acad Sci USA. 2012;109:E1820–E1829. doi: 10.1073/pnas.1207829109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kornyushyna O, Burrows CJ. Biochemistry. 2003;42:13008–13018. doi: 10.1021/bi0350755. [DOI] [PubMed] [Google Scholar]
- 33.Delaney S, Neeley WL, Delaney JC, Essigmann JM. Biochemistry. 2007;46:1448–1455. doi: 10.1021/bi061174h. [DOI] [PubMed] [Google Scholar]
- 34.Henderson PT, Delaney JC, Muller JG, Neeley WL, Tannenbaum SR, Burrows CJ, Essigmann JM. Biochemistry. 2003;42:9257–9262. doi: 10.1021/bi0347252. [DOI] [PubMed] [Google Scholar]
- 35.David SS, Williams SD. Chem Rev. 1998;98:1221–1262. doi: 10.1021/cr980321h. [DOI] [PubMed] [Google Scholar]
- 36.Leipold MD, Muller JG, Burrows CJ, David SS. Biochemistry. 2000;39:14984–14992. doi: 10.1021/bi0017982. [DOI] [PubMed] [Google Scholar]
- 37.Krishnamurthy N, Muller JG, Burrows CJ, David SS. Biochemistry. 2007;46:9355–9365. doi: 10.1021/bi602459v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hazra TK, Muller JG, Manuel RC, Burrows CJ, Lloyd RS, Mitra S. Nucleic Acids Res. 2001;29:1967–1974. doi: 10.1093/nar/29.9.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bandaru V, Zhao X, Newton MR, Burrows CJ, Wallace SS. DNA Repair. 2007;6:1629. doi: 10.1016/j.dnarep.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kathe SD, Barrantes-Reynolds R, Jarugal P, Newton MR, Burrows CJ, Bandaru V, Dizdaroglu M, Bond JP, Wallace SS. DNA Repair. 2009;8:643–653. doi: 10.1016/j.dnarep.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leipold MD, Workman H, Muller JG, Burrows CJ, David SS. Biochemistry. 2003;42:11373–11381. doi: 10.1021/bi034951b. [DOI] [PubMed] [Google Scholar]
- 42.Liu M, Imamura K, Averill AM, Wallace SS, Doublie S. Structure. 2013;21:247–256. doi: 10.1016/j.str.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krishnamurthy N, Zhao X, Burrows CJ, David SS. Biochemistry. 2008;47:7137–7146. doi: 10.1021/bi800160s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sejersted Y, Hildrestrand GA, Kunke D, Rolseth V, Krokeide SZ, Neurauter CG, Suganthan R, Atneosen-Asegg M, Fleming AM, Saugstad OD, Burrows CJ, Luna L, Bjoras M. Proc Natl Acad Sci USA. 2011;108:18802–18807. doi: 10.1073/pnas.1106880108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jia L, Shafirovich V, Shapiro R, Geacintov NE, Broyde S. Biochemistry. 2005;44:13342–13353. doi: 10.1021/bi050790v. [DOI] [PubMed] [Google Scholar]
- 46.Khutsishvili I, Zhang N, Marky LA, Crean C, Patel DJ, Geacintov NE, Shafirovich V. Biochemistry. 2013;52:1354–1363. doi: 10.1021/bi301566v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Durandin A, Jia L, Crean C, Kolbanovskiy A, Ding S, Shafirovich V, Broyde S, Geacintov NE. Chem Res Toxicol. 2006;19:908–913. doi: 10.1021/tx060078e. [DOI] [PubMed] [Google Scholar]
- 48.Karwowski B, Dupeyrat F, Bardet M, Ravanat JL, Krajewski P, Cadet J. Chem Res Toxicol. 2006;19:1357–1365. doi: 10.1021/tx060088f. [DOI] [PubMed] [Google Scholar]
- 49.Ding S, Jia L, Durandin A, Crean C, Kolbanovskiy A, Shafirovich V, Broyde S, Geacintov NE. Chem Res Toxicol. 2009;22:1189–1193. doi: 10.1021/tx900107q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Y. Chem Res Toxicol. 2002;15:671–676. doi: 10.1021/tx0155855. [DOI] [PubMed] [Google Scholar]
- 51.Yu H, Venkatarangan L, Wishnok JS, Tannenbaum SR. Chem Res Toxicol. 2005;18:1849–1857. doi: 10.1021/tx050146h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Razzell WE, Khorana HG. J Biol Chem. 1959;234:2114–2117. [PubMed] [Google Scholar]
- 53.Razzell WE, Khorana HG. J Biol Chem. 1961;236:1144–1149. [PubMed] [Google Scholar]
- 54.Liuzzi M, Weinfeld M, Paterson MC. J Biol Chem. 1989;264:6355–6363. [PubMed] [Google Scholar]
- 55.Gu F, Stillwell WG, Wishnok JS, Shallop AJ, Jones RA, Tannenbaum SR. Biochemistry. 2002;41:7508–7518. doi: 10.1021/bi020148q. [DOI] [PubMed] [Google Scholar]
- 56.Weinfeld M, Liuzzi M, Paterson MC. Nucleic Acids Res. 1989;17:3735–3745. doi: 10.1093/nar/17.10.3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bentzley CM, Johnston MV, Larsen BS. Anal Biochem. 1998;258:31–37. doi: 10.1006/abio.1998.2569. [DOI] [PubMed] [Google Scholar]
- 58.Niewiarowski WVB. Eur J Biochem. 1985;153:145–153. doi: 10.1111/j.1432-1033.1985.tb09280.x. [DOI] [PubMed] [Google Scholar]
- 59.Maccubbin AME, Paul CR, Budzinski EE, Przybyszewski J, Box HC. Radiat Res. 1991;126:21–26. [PubMed] [Google Scholar]
- 60.Mao BL, Amin BS, Cosman M, Geacintov NE. Biochemistry. 1993;32:11785–11793. doi: 10.1021/bi00095a006. [DOI] [PubMed] [Google Scholar]
- 61.Box HCB, EE, Evans MS, French JB, Maccubin AE. Biochim Biophys Acta. 1993;1161:291–294. doi: 10.1016/0167-4838(93)90227-i. [DOI] [PubMed] [Google Scholar]
- 62.Weinfeld M, Soderlind KJM, Buchko GW. Nucleic Acids Res. 1993;21:621–626. doi: 10.1093/nar/21.3.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vialas C, Claparols C, Pratviel G, Meunier B. J Am Chem Soc. 2000;122:2157–2167. [Google Scholar]
- 64.Fleming AM, Muller JG, Ji I, Burrows CJ. Org Biomol Chem. 2011;9:3338–3348. doi: 10.1039/c1ob05112a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ghude P, Schallenberger MA, Fleming AM, Muller JG, Burrows CJ. Inorg Chim Acta. 2011;369:240–246. doi: 10.1016/j.ica.2010.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ye W, Sangaiah R, Degen DE, Gold A, Jayaraj K, Koshlap KM, Boysen G, Williams J, Tomer KB, Mocanu V, Dicheva N, Parker CE, Schaaper RM, Ball LM. J Am Chem Soc. 2009;131:6114–6123. doi: 10.1021/ja8090752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rokhlenko Y, Geacintov NE, Shafirovich V. J Am Chem Soc. 2012;134:4955–4962. doi: 10.1021/ja212186w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tomaszewska A, Mourgues S, Guga P, Nawrot B, Pratviel G. Chem Res Toxicol. 2012;25:2505–2512. doi: 10.1021/tx300319y. [DOI] [PubMed] [Google Scholar]
- 69.Buchko GW, Cadet J, Berger M, Ravanat JL. Nucleic Acids Res. 1992;20:4847–4851. doi: 10.1093/nar/20.18.4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Slade PG, Hailer MK, Martin BD, Sugden KD. Chem Res Toxicol. 2005;18:1140–1149. doi: 10.1021/tx050033y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.DeFedericis HP, HB, Rajecki MJ, Budzinski EE, Iijima H, Dawidzik JB, MSG, Evans KF, Box HC. Radiat Res. 2006;165:445–451. doi: 10.1667/rr3533.1. [DOI] [PubMed] [Google Scholar]
- 72.Yu H, Venkatarangan L, Wishnok JS, Tannenbaum SR. Chem Res Toxicol. 2005;18:1849–1857. doi: 10.1021/tx050146h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pang B, Zhou X, Yu H, Dong M, Taghizadeh K, Wishnok JS, Tannenbaum SR, Dedon PC. Carcinogenesis. 2007;28:1807–1813. doi: 10.1093/carcin/bgm037. [DOI] [PubMed] [Google Scholar]
- 74.Ye Y, Muller JG, Burrows CJ. J Org Chem. 2006;71:2181–2184. doi: 10.1021/jo052484t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Taghizadeh K, McFaline JL, Pang B, Sullivan M, Dong M, Plummer E, Dedon PC. Nat Protoc. 2008;3:1287–1298. doi: 10.1038/nprot.2008.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bowman KJ, Pla RL, Guichard Y, Farmer PB, Jones GDD. Nucleic Acids Res. 2001;29:e101–106. doi: 10.1093/nar/29.20.e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ogilvie KK, Hruska FH. Biochim Biophys Acta. 1976;68:375–378. doi: 10.1016/0006-291x(76)91155-4. [DOI] [PubMed] [Google Scholar]
- 78.Zhao X, Krishnamurthy N, Burrows CJ, David SS. Biochemistry. 2010;49:1658–1666. doi: 10.1021/bi901852q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kuraoka I, Robins P, Masutani C, Hanaoka F, Gasparutto D, Cadet J, Wood RD, Lindahl T. J Biol Chem. 2001;276:49283–49288. doi: 10.1074/jbc.M107779200. [DOI] [PubMed] [Google Scholar]
- 80.Kuraoka I, Bender C, Romieu A, Cadet J, Wood RD, Lindahl T. Proc Natl Acad Sci USA. 2000;97:3832–3837. doi: 10.1073/pnas.070471597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Romier CD, Lahm R, Dahl AO, Suck D. Proteins. 1998;32:414–424. [PubMed] [Google Scholar]
- 82.Belmadoui N, Boussicault F, Guerra M, Ravanat JL, Chatgilialoglu C, Cadet J. Org Biomol Chem. 2010;8:3211–3219. doi: 10.1039/c004531d. [DOI] [PubMed] [Google Scholar]
- 83.Romieu A, Gasparutto D, Cadet J. Chem Res Toxicol. 1999;12:412–421. doi: 10.1021/tx9802668. [DOI] [PubMed] [Google Scholar]
- 84.Jaruga P, Theruvathu J, Dizdaroglu M, Brooks PJ. Nucleic Acids Res. 2004;32:e87. doi: 10.1093/nar/gnh087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Culp JS, Blytt HJ, Hermodson M, Butler LG. J Biol Chem. 1985;260:8320–8324. [PubMed] [Google Scholar]
- 86.Cummins JH, Potter BVL. Eur J Biochem. 1987;162:123–128. doi: 10.1111/j.1432-1033.1987.tb10551.x. [DOI] [PubMed] [Google Scholar]
- 87.Volbeda A, Lahm A, Sakiyama F, Suck D. EMBO J. 1991;10:1607–1618. doi: 10.1002/j.1460-2075.1991.tb07683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kornyushyna O, Berges AM, Muller JG, Burrows CJ. Biochemistry. 2002;41:15304–15314. doi: 10.1021/bi0264925. [DOI] [PubMed] [Google Scholar]
- 89.Jia L, Shafirovich V, Geacintov NE, Broyde S. Biochemistry. 2007;46:5305–5314. doi: 10.1021/bi062269m. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.