Abstract
The cytofluorometric analysis of dissociated tumor samples identified distinct immunophenotypes among the most common variants of pediatric brain tumor. These findings suggest that immunotherapeutic regimens against pediatric brain malignancies should be tailored to individual tumor types.
Keywords: flow cytometry, immune cell characterization, immunotherapy, pediatric brain tumor
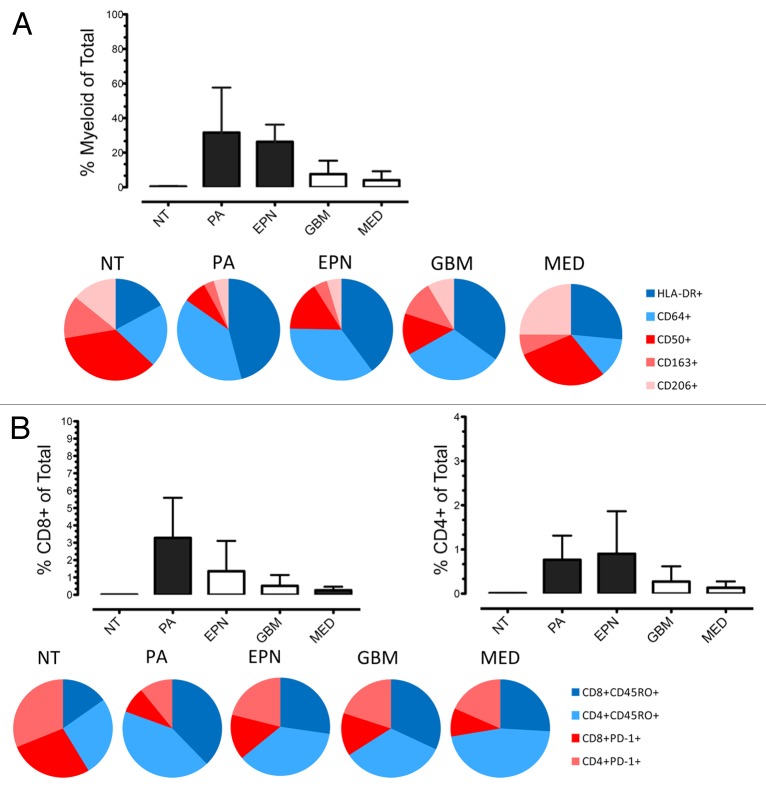
Tumor-infiltrating leukocytes (TIL) are a source of immune effector cells that may be exploited for the treatment of pediatric brain tumors. The immune contexture underlying pediatric brain tumors, however, has not been extensively investigated. The majority of research on the immune infiltrate of brain tumors has been conducted in adult glioblastoma (GBM), identifying a predominantly immunosuppressed phenotype. Whether other malignancies of the brain and/or GBMs from patients in other age groups display a similar immunophenotype has not been studied in detail. We recently addressed this paucity of knowledge by systematically characterizing the immunophenotype of common pediatric brain neoplasms, including GBM, pilocytic astrocytoma (PA), ependymoma (EPN), and medulloblastoma (MED), as well as of material from pediatric patients with non-malignant epilepsy.1 The cytofluorometric analysis of dissociated samples provided a comparative and semi-quantitative measure of the phenotype, frequency, and inferred functionality of infiltrating leukocytes. This study identified distinct immunophenotypes in different types of pediatric brain malignancy (Fig. 1). In addition, we demonstrated that the immunosuppressive phenotype that normally characterizes adult GBMs does not necessarily affect all pediatric brain tumors. Specifically, PA and EPN, which are relatively common in the pediatric population, exhibited a significantly more robust infiltration by myeloid and lymphoid cells than GBMs, MEDs and non-malignant, epileptic brains. Additionally, PAs and EPNs displayed a classically activated myeloid cell-skewed functional phenotype, as denoted by the expression of HLA-DR and CD64. In contrast, GBMs and MEDs contained reduced amounts of myeloid cells, in thus far resembling non-malignant brain tissues from epileptic patients. As compared with PAs and EPNs, GBMs and MEDs exhibited an immunosuppressive myeloid phenotype, characterized by a relatively high proportion of the M2 macrophage markers CD163 and CD206 (Fig. 1A). Although the expression of functional T-cell markers was relatively homogeneous across distinct pediatric brain neoplasms, the relative frequency of tumor-infiltrating T-cells was significantly higher in PAs and EPNs than in GBMs, MEDs and brain tissues from epileptic patients (Fig. 1B). The differential immunophenotype of pediatric brain tumors has major implications for both passive and active immunotherapy, which should be carefully considered for the development of clinical immunotherapeutic approaches against these malignancies.
Figure 1. Distinct profiles of myeloid cells and T lymphocytes are found in pediatric brain tumors. (A and B) Multicolor flow cytometry was used to measure the extent and functional phenotype of myeloid cells infiltrating the non-malignant brain (NT), obtained from epilepsy resections, as well as pilocytic astrocytomas (PAs), ependymomas (EPNs), glioblastomas (GBMs) and medulloblastomas (MEDs), obtained from the surgical resection of primary neoplasms in pediatric patients. The relative amount of tumor-infiltrating CD45+CD11b+ myeloid cells (A), CD3+CD8+ cytotoxic T cells (B) and CD3+CD4+ helper T cells (B) is illustrated. Black columns indicate values that were significantly different as compared with NT samples (P < 0.05). Pie charts represent the proportion of tumor-infiltrating myeloid cells (A) and T cells (B) that express the indicated immunophenotypic markers of activation (in blue) or immature state/immunosuppression (in red).
Passive immunotherapeutic approaches are currently dominated by anticancer antibodies. Antibody-based cancer therapies have been established over the past 15 y and are now one of the most successful and important strategies for the treatment of patients with hematological and solid malignancies. The success of antibody-based immunotherapy against pediatric cancers was recently demonstrated in a Phase III clinical trial testing the efficacy of GD2 ganglioside-targeting antibodies in neuroblastoma patients.2 The antineoplastic activity of antibodies can result from a direct action on malignant cells (for instance, through the inhibition of key receptors) or from the activation of antibody-dependent cell-mediated cytotoxicity (ADCC). ADCC is considered the dominant mechanism-of-action of multiple therapeutic antibodies and relies on the engagement of Fcγ receptors expressed by immune effector cells.3 Our work is particularly pertinent to this mechanism, identifying the abundant expression of the Fcγ receptor CD64 on the majority of myeloid cells infiltrating PAs, EPNs, and GBMs. CD64 expression levels have previously been correlated with improved disease outcome among EPN patients in a gene expression microarray study.4 The levels of CD64 expression by the immune cells that infiltrate brain neoplasms, however, had not previously been appreciated, at least in part due to technical limitations. Indeed, CD64 cannot be measured by immunohistochemistry on formalin-fixed paraffin-embedded material. The finding that PA-, EPN-, and GBM-infiltrating myeloid cells are primed for ADCC is therefore novel, suggesting that these tumors may respond favorably to antibody-based immunotherapy.
TILs represent a source of effector T cells that presumably have been selected for their ability to recognize and respond to specific tumor-associated antigens. The adoptive transfer of tumor-infiltrating autologous T cells expanded ex vivo has provided the best results in malignant melanoma patients, inducing objective response rates as high as 50% in some patient subsets.5 The relatively abundant cell infiltration of PAs and EPNs by T cells might facilitate the use of adoptive immunotherapy in patients affected by these tumor types. Although the majority of clinical trials testing adoptive T-cell transfer and anticancer vaccines in patients with brain malignancies involves adult GBM, a growing number of studies that enroll pediatric patients with tumors other than GBM is being initiated.6-8 Based on our findings, the relatively active immune contexture of PAs and EPNs would, at least theoretically, provide a relatively permissive microenvironment for such immunotherapeutic approaches. Conversely, the immunosuppressed microenvironment of MEDs may be less favorable for adoptive T-cell therapy and anticancer vaccines. Such an inference is supported by the preliminary results of a clinical trial testing dendritic cell-based vaccines in pediatric patients with brain tumors. In this context, glial malignancies responded more favorably to vaccination than MEDs and primitive neuroectodermal tumors.6 A number of strategies to reprogram the tumor immunophenotype by depleting immunosuppressive cell populations has been shown to provide clinical benefits to patients affected by a variety of tumors. Recent clinical successes in this direction include the use of antibodies that block immunosuppressive receptors such as cytotoxic T lymphocyte-associated protein 4 (CTLA4) or programmed cell death 1 (PDCD1, best known as PD-1) in melanoma patients.9,10 These approaches may be more appropriate for GBM and MED patients, as the microenvironment of these malignancies appears to be skewed toward an immunosuppressive phenotype.
In conclusion, our results confirm the importance of performing an in-depth characterization of the immunophenotype of pediatric brain tumors, and emphasize that we should not assume that all these malignancies display an immunophenotypic similarity to adult GBMs. The success of immunotherapy in pediatric neuro-oncology is likely to rely on approaches that are tailored to different tumor types. It is even possible that distinct molecular subtypes of particular tumors (4 have been identified for MED, just to cite an example) may require different immunotherapeutic approaches, a hypothesis that is currently under investigation in our laboratory.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Citation: Griesinger AM, Donson AM, Foreman NK. Immunotherapeutic implications of the immunophenotype of pediatric brain tumors. OncoImmunology 2013; 2:e27256; 10.4161/onci.27256
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/27256
References
- 1.Griesinger AM, Birks DK, Donson AM, Amani V, Hoffman LM, Waziri A, Wang M, Handler MH, Foreman NK. Characterization of distinct immunophenotypes across pediatric brain tumor types. J Immunol. 2013;191:4880–8. doi: 10.4049/jimmunol.1301966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu AL, Gilman AL, Ozkaynak MF, London WB, Kreissman SG, Chen HX, Smith M, Anderson B, Villablanca JG, Matthay KK, et al. Children’s Oncology Group Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010;363:1324–34. doi: 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med. 2000;6:443–6. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 4.Donson AM, Birks DK, Barton VN, Wei Q, Kleinschmidt-Demasters BK, Handler MH, Waziri AE, Wang M, Foreman NK. Immune gene and cell enrichment is associated with a good prognosis in ependymoma. J Immunol. 2009;183:7428–40. doi: 10.4049/jimmunol.0902811. [DOI] [PubMed] [Google Scholar]
- 5.Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, Royal RE, Kammula U, White DE, Mavroukakis SA, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–57. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ardon H, De Vleeschouwer S, Van Calenbergh F, Claes L, Kramm CM, Rutkowski S, Wolff JE, Van Gool SW. Adjuvant dendritic cell-based tumour vaccination for children with malignant brain tumours. Pediatr Blood Cancer. 2010;54:519–25. doi: 10.1002/pbc.22319. [DOI] [PubMed] [Google Scholar]
- 7.Caruso R, Wierzbicki V, Marrocco L, Salvati M. Paragangliomas of the cauda equina. Report of one case and review of the literature. J Exp Clin Cancer Res. 2006;25:269–75. [PubMed] [Google Scholar]
- 8.Peres E, Wood GW, Poulik J, Baynes R, Sood S, Abidi MH, Klein J, Bhambhani K, Dansey R, Abella E. High-dose chemotherapy and adoptive immunotherapy in the treatment of recurrent pediatric brain tumors. Neuropediatrics. 2008;39:151–6. doi: 10.1055/s-0028-1093333. [DOI] [PubMed] [Google Scholar]
- 9.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–44. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]