Abstract
The single-chain triplebody HLA-ds16-hu19 consists of three single-chain Fv (scFv) antibody fragments connected in a single polypeptide chain. This protein with dual-targeting capacity mediated preferential lysis of antigen double-positive (dp) over single-positive (sp) leukemic cells by recruitment of natural killer (NK) cells as effectors. The two distal scFv modules were specific for the histocompatibility protein HLA-DR and the lymphoid antigen CD19, the central one for the Fc gamma receptor CD16. In antibody-dependent cellular cytotoxicity (ADCC) experiments with a mixture of leukemic target cells comprising both HLA-DR sp HuT-78 or Kasumi-1 cells and (HLA-DR plus CD19) dp SEM cells, the triplebody mediated preferential lysis of the dp cells even when the sp cells were present in ≤20-fold numerical excess. The triplebody promoted equal lysis of SEM cells at 2.5-fold and 19.5-fold lower concentrations than the parental antibodies specific for HLA-DR and CD19, respectively. Finally, the triplebody also eliminated primary leukemic cells at lower concentrations than an equimolar mixture of bispecific single-chain Fv fragments (bsscFvs) separately addressing each target antigen (hu19-ds16 and HLA-ds16). The increased selectivity of targeting and the preferential lysis of dp over sp cells achieved by dual-targeting open attractive new perspectives for the use of dual-targeting agents in cancer therapy.
Keywords: triplebodies, expanded natural killer cells, dual-targeting, selective cytotoxicity, preferential lysis
Introduction
Antibodies and antibody-derived agents have become useful tools for the treatment of cancer.1-8 Further improvement is needed to develop successful agents also for those types of cancer that do not respond to available products, and to create agents with enhanced selectivity for cancer cells and reduced reactivity for healthy cells to minimize systemic damage. Most of the approved antibody-based agents currently used in oncology are based on bivalent, monospecific IgGs. One idea to improve selectivity is to target a cancer cell via two different antigens present on its surface in greater combined density than on corresponding healthy cells by using “dual-targeting” agents. The underlying hypothesis is that dual-targeting agents should preferentially eliminate the antigen double-positive (dp) cancer cells over healthy normal cells present in the same environment and thus achieve both goals: increased efficacy and reduced systemic toxicity.
Numerous different formats of dual-targeting antibodies and antibody-derived agents have been developed over the past 25 years. First milestones were the creation of hybrid-hybridomas or quadromas and of chemically-coupled Fab-fragments.9-13 The production of recombinant heterodimeric immunoglobulins through the expression of two different H- and L-chains in the same cell was initially hampered by the “chain association problem.” In this case only 12.5% of the resulting total antibody proteins are the desired heterodimers, the rest are unwelcome chain combinations.14 A first breakthrough to overcome this hurdle was the “knobs-in-holes” (kih) strategy, which increased the yield of the desired heterodimers to over 95%.15-17 Another milestone was the design of the so-called “Morrison-format” (IgG-scFvs).18 Here, an scFv of one specificity is fused C-terminally to the H-chain of an antibody of another specificity, thus generating a new tetravalent molecule with two regular antigen combining sites of the first specificity plus two scFv-binding sites of the second. The Dual-Variable Domain Igs (DVD-Igs), which carry two different antigen combining sites in tandem on each half-antibody, represent a major new development.19-21 DVD-Igs are tetravalent structures with two copies each of the two different binding sites, and they carry a homodimeric Fc-domain. Another new format is the Strand Exchange Engineered Domain (SEED) antibody22,23 in which structurally-related subdomains of CH3 from an IgG and an IgA antibody are pieced together in alternating, reciprocal patterns. Co-expression of two such mosaic H-chains leads to an efficient enforced formation of heterodimers. To enforce correct L-chain association in heterodimeric antibodies, the kih-technology was refined by the use of a common light-chain compatible with both different H-chains.15 The two-in-one antibodies, heterodimeric duo-bodies generated by controlled Fab-arm exchange, and Cross-mabs are also recent major advances in protein engineering.14,24-26 These agents still carry an Fc domain and thus achieve their anti-cancer effects through the recruitment of effector cells and other effector functions (complement). Additional dual-targeting molecular formats that lack Fc-domains and carry scFv-fragments or domain-antibody (dab) fragments as targeting domains have also been developed. In total, more than 50 different bi- and multi-specific formats have been published, as discussed in several recent reviews.14,27,28
Many dual-targeting agents achieve their anti-cancer effects by recruiting effector functions through their Fc-domains or through specialized domains incorporated for the purpose into the molecular format. Two other important mechanisms for the elimination of cancer cells have also been combined with dual-address systems. One is the dual blockage of two different cell surface receptors needed to transduce growth- and proliferation-promoting signals into the cell. The other is the delivery of toxic components into the cell, mediated by dual targeting. A bispecific scFv fusion protein (bsscFv) with one binding site each for erbB2 and erbB3 illustrates the first concept.29 This fusion protein interacted more strongly with double-positive (dp) cells (erbB2 plus erbB3) than with single-positive (sp) cells carrying only one of these antigens, or with normal cells carrying both, but in reduced cell surface densities. It inhibited the proliferation of the targeted cells more strongly than mono-targeting controls carrying two identical binding sites for only one of these targets. The agent achieved its potent effects by simultaneous blockage of the interactions of both of these growth factor receptors with their respective ligands, and thereby depriving the cancer cell of two important growth-promoting signals, resulting in a synergistic anti-proliferative outcome. MM-111 (Merrimack) is the first dual-targeting agent based on dual blockage of proliferation-promoting signals to have entered clinical trials.30 This bispecific scFv-fusion protein blocks the EGFR-HER3 signaling unit. MM-141, a dual-specific antibody simultaneously targeting the growth factor receptors Erb-B3 and Igf1-R functions along similar principles.31 Simultaneous blockage of these receptors caused stronger anti-cancer effects than treatment with the corresponding single antibodies alone or in combination. Along the same lines, so-called “two-in-one” molecules that combine two different antigen specificities in a single hybrid binding site have been developed.24 An example is MEHD7945A, which blocks ligand binding to both the HER3 and EGF receptors on the same cell. This agent displayed superior anti-cancer activity in cell culture assays over a combination treatment with the corresponding mono-specific parental HER3- and EGFR-targeting antibodies, and it recently entered a Phase 2 study in patients with head-and-neck cancer.32
Finally, dual-targeting agents have also been designed to import toxic payloads into cancer cells, e.g., fusion proteins between a diphtheria toxin (DT) fragment and two tandem scFvs specific for CD19 and CD22 for import into malignant human B-lymphoid cells.33 An impressive array of similar agents has been designed and tested using bispecific scFvs against EpCAM, various growth-factor and cytokine receptors, as well as an urokinase-type plasminogen activator receptor (uPAR), to import toxic cargo into various types of human cancer cells in culture and in mice xenografted with human cancer tissues.34-38 For most of these agents, investigators have shown that the dual-targeting proteins had more potent anti-cancer activity than the corresponding mono-targeting control agents. We are not aware, however, of any studies attempting to demonstrate directly that the dual-targeting agent indeed preferentially eliminated antigen dp over sp cells when both were present in the same reaction mixture, mimicking the simultaneous presence of both types of cells in an infiltrated cancerous tissue.
Our team has developed dual-targeting agents of a distinct format, so-called single chain triplebodies. These fusion proteins consist of three different scFv modules connected in tandem by flexible linkers into a single polypeptide chain.39-43 The distal scFv binding modules can either bind two copies of the same target antigen, or one copy each of two different targets. In the latter case, the triplebody is said to be “dual-targeting.” The central scFv serves to recruit an effector cell and to trigger its functions. The prototypical dual-targeting triplebody 123-ds16-33, in which “ds” refers to a disulfide-stabilized scFv, connects CD33 and CD123 on the surface of AML cells with NK cells through CD16, the low affinity Fcγ RIII receptor.40 Another triplebody, 33-ds16-ds19, targets the antigen combination (CD19 plus CD33), uniquely present on mixed-lineage leukemia (MLL) cells.41 A third example of a dual-targeting triplebody is HLA-ds16-hu19, in which “hu” refers to a humanized scFv, targeting HLA-DR and CD19, a pair of antigens present on many leukemia and lymphoma cells.42 This agent, designed for the sole purpose of testing our hypothesis that dual-targeting permits increased selectivity of targeting, but not designed for clinical use, is further investigated in the present study.
We previously reported that dual targeting mediated by triplebody HLA-ds16-hu19 leads to preferential binding of the agent to antigen dp over antigen sp target cells present in the same reaction mixture.42 Here, we wished to investigate, whether this preferential binding also caused an increased selectivity of lysis mediated by effector cells, such as natural killer (NK) cells. This enhanced cytolytic function does not follow necessarily from the fact that the agent binds the target cells with enhanced selectivity. Conceivably, a dual-targeting triplebody could bind a particular pair of target antigens on a cell surface and produce selectivity of binding, but this binding could remain unproductive for the formation of a lytically active synapse with a cytolytic effector cell. An example of this are scFv-IgGs in the “Morrison-format.”18 After binding of these agents to the corresponding antigens, they were still capable of binding C1q through their Fc-domain, although to a reduced extent, but unlike the parental IgG, they were unable to mediate lysis of antigen-coated sheep red blood cells.18 The explanation given was that there probably was sterical interference between the C-terminally fused scFv and a lytically fully productive interaction of the antigen-coated red blood cells opsonized with the agent and the full complement system beyond C1q. The authors discussed that the 5 amino acid flexible linker, rich in glycines and serines, inserted between the C-terminus of the H-chain and the scFv in this format probably permitted some interaction of the Fc-domain with complement (and also with FcγRI), but not the unimpaired full interaction seen for the parental antibody. For our triplebody, the flexible linker between the CD16-specific central scFv and the terminal scFv was 20 amino acids in length (G4S)4, and thus 4 times longer than in the Morrison format. This choice was made to provide increased flexibility and reduced sterical hindrance. Therefore, we wished to determine, whether this improved design permitted greater accessibility of the central scFv to CD16 on the NK cell and unimpaired effector cell mediated lysis. Consequently, the aim of the present study was to investigate whether the enhanced selectivity of binding of our triplebody to antigen dp cells is followed by an enhanced selectivity of redirected lysis. It is necessary to show increased selectivity of lysis, and not only increased selectivity of binding, if we aim at creating new agents with stronger activity for cancer cells than for corresponding healthy cells, accompanied by reduced systemic toxicities compared with conventional agents, as we have stated as a goal in the beginning.
For the particular pair of target antigens and target cell lines used here, preferential lysis of the dp target cells was indeed observed, even when the sp cells were present in the reaction in large numerical excess. Future studies can build upon this precedent and investigate, whether triplebodies can be used for a broader range of antigen combinations on different types of cancer cells to achieve selectivity of cancer cell lysis accompanied by reduced systemic toxicity.
Results
Ex-vivo expansion of MNCs and NK cells for use as effectors in ADCC reactions
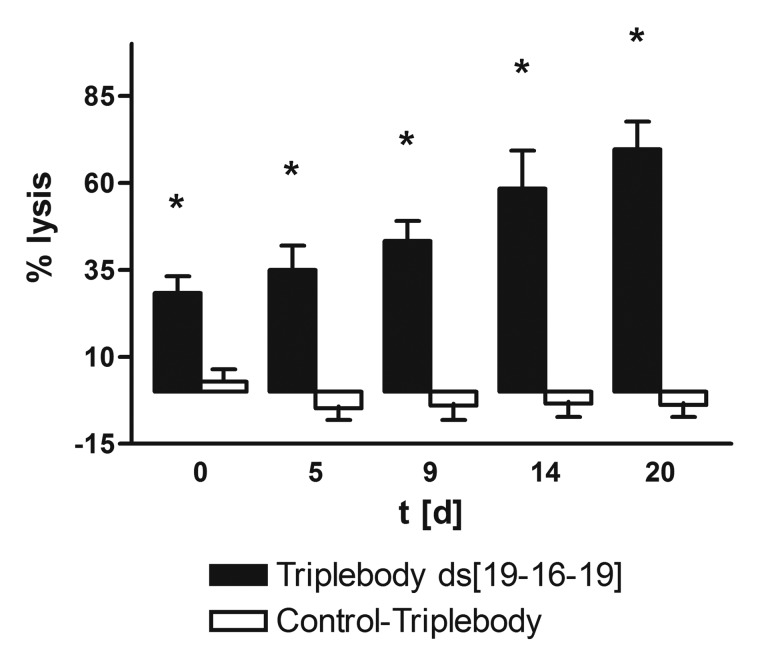
To produce sufficient numbers of effector cells for ADCC assays, MNCs were expanded ex vivo by a modified published procedure, described in the methods section and in supplement Figure 1.44 The ability of the NK cell subset of this expanded MNC population, which varied between 5 and 25% depending on the healthy donor, to mediate ADCC was assessed on days 0, 5, 9, 14, and 20. The triplebody ds19-ds16-ds19 and the control triplebody 7-ds16-7, specific for CD7, were used at a saturating concentration of 1 nM.39 Target cells were the acute B-lymphoblastic leukemia-derived cell-line SEM, positive for CD19 and negative for CD7.50 The potential of the NK cell subset for specific lysis increased with the length of the expansion period from 28 (± 5) to 70 (± 8) % on day 20 (Fig. 1) under standardized assay conditions with no specific lysis of the SEM cells mediated by the control triplebody 7-ds16-7. All experiments reported below were performed with MNCs and in one case with immuno-magnetically enriched NK cells expanded in this manner.
Figure 1. Lysis of CD19-positive leukemic SEM cells by ex vivo expanded MNCs as effectors mediated by triplebody ds[19-16-19]. The ability of the enriched and expanded MNCs to mediate ADCC was measured in a 3 h Calcein-AM release assay using a constant effector-to-target cell (E:T) ratio of 10:1, where E refers to the total MNCs, and 1 nM for each of the triplebodies. Black bar: Triplebody ds[19–16–19]; open bar: control triplebody 7-16-7. The calcein-labeled SEM target cells were CD7-negative. Data represent the mean ± SEM (Standard Error of the Mean) of 6 expansions. *Statistically significant (p ≤ 0.006) lysis.
The dual-targeting triplebody mediates stronger specific lysis than the parental antibodies
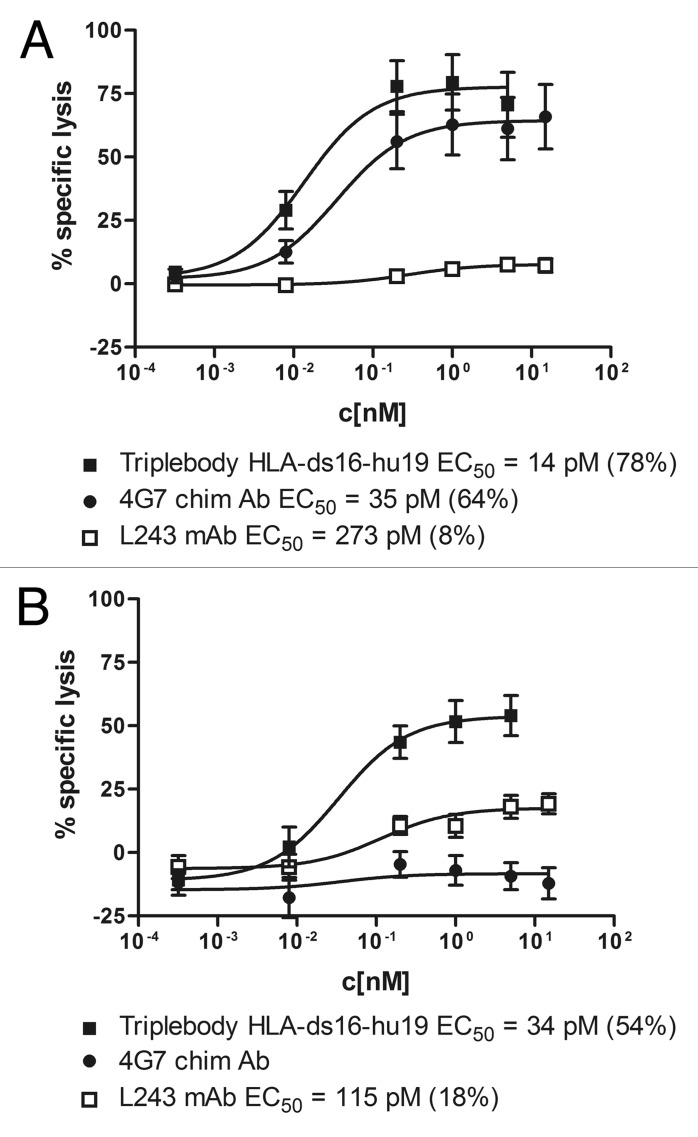
Our team previously reported that dual-targeting generated preferential binding of a triplebody to dp over sp cells.42 Here, we set up experiments to test the working hypothesis that dual-targeting triplebodies also mediate preferential lysis of dp over sp targets present in a mixed environment via NK cells as effectors. To test this contention, specific lysis of antigen-dp SEM cells and of antigen-sp HuT-78 cells was measured in ADCC assays using the dual-targeting triplebody HLA-ds16-hu19 and suitable controls in combination with ex vivo expanded MNCs as the source of effectors. The test was performed in three stages. In stage 1 (Fig. 2), the specific cytolytic activity of the triplebody for antigen-dp and sp cells was compared with the activities of the parental antibodies. Dose-response curves were recorded, and the EC50 values (concentrations for half-maximum lysis) were determined. For the experiment reported below, sp and dp cells were present in equal numbers in the ADCC assay, but only one of the two lines was labeled with Calcein AM. Reciprocal experiments were performed, with both the dp cells labeled and the sp cells unlabeled and vice versa (Fig. 2A and 2B, respectively). Lysis of the labeled cells was measured by Calcein-release. The experiment was set up in this manner to generate data more closely comparable to the preferential lysis data generated in the next two stages (Figs. 3 and 4), which were also performed with mixed populations of sp and dp cells. In the first experiment (Fig. 2A), dp SEM cells were labeled and mixed 1:1 with unlabeled sp HuT-78 cells. In the reciprocal experiment (Fig. 2B), dp cells were left unlabeled and mixed with labeled sp cells. The mixtures were then incubated with expanded MNCs plus either the triplebody or the parental chimeric CD19 antibody 4G7, or the murine HLA-DR antibody L243, in three parallel reactions, respectively.45,46 As a result, the labeled dp cells were efficiently eliminated by the triplebody and the chimeric CD19 antibody in a concentration-dependent manner, whereas the HLA-DR antibody produced only marginal lysis (Fig. 2A). EC50 values achieved with labeled SEM cells were 14, 35, and 273 pM, for the triplebody and the CD19 and HLA-DR antibodies, respectively (Fig. 2A). Therefore, the triplebody achieved an equal degree of specific lysis of dp cells at a 2.5-fold lower concentration than the chimeric CD19 antibody and at a 19.5-fold lower concentration than the HLA-DR antibody. From these data, we conclude that the combination of a weakly binding HLA-DR specific scFv module with a strongly-binding CD19-specific scFv into a triplebody generated a final agent that was twice as potent for the lysis of dp cells as the best of the parental antibodies.
Figure 2. Dose-dependent induction of ADCC of (CD19- plus HLA-DR) dp SEM cells in the presence of HLA-DR sp HuT-78 cells by the triplebody and the parental antibodies. (A) Calcein-AM labeled SEM cells, mixed with unlabeled HuT-78 cells, were used as targets to compare efficacy of the triplebody at a constant E:T ratio of 10:1. Effector cells here were ex vivo expanded MNCs. The triplebody (■), the chimeric CD19 antibody 4G7 (●), and the monoclonal HLA-DR antibody L243 (□) triggered ADCC in a dose-dependent manner. The EC50-value of the triplebody was significantly (p ≤ 0.02) lower than the corresponding values of the parental antibodies. (B) Unlabeled SEM cells, mixed with Calcein-AM labeled HuT-78 cells, were used as targets to compare efficacy of the triplebody at a constant E:T ratio of 10:1. The triplebody (□), the chimeric 4G7 (●) and the monoclonal antibody L243 (□) triggered ADCC in a dose-dependent manner. Values given in brackets are the maximum lysis of the labeled cells. Data points represent mean percentage of specific lysis ± SEM (Standard Error of the Mean), obtained with expanded MNCs from 6 different experiments.
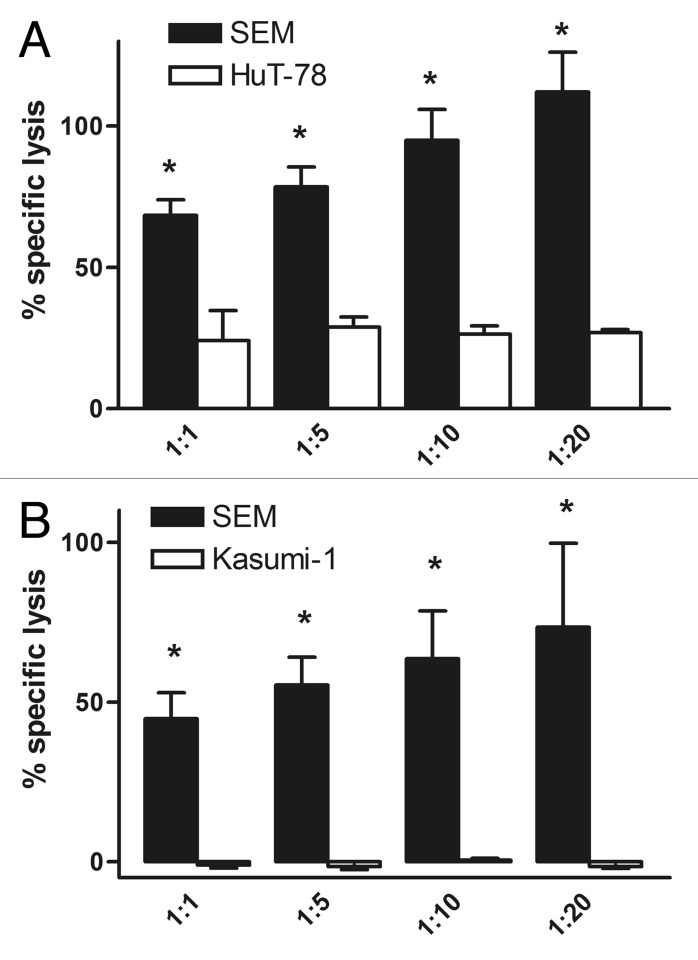
Figure 3. Preferential lysis of antigen dp cells by the dual-targeting triplebody HLA-ds16-hu19. For the first arm of the experiment, antigen dp SEM cells were labeled with Calcein-AM and mixed with unlabeled HLA-DR sp (A) HuT-78 cells, or (B) Kasumi-1 cells, respectively, at different ratios ranging from 1:1 to 1:20. For the second arm, sp HuT-78 or Kasumi-1 cells were labeled with Calcein-AM and mixed with unlabeled dp SEM cells. The triplebody HLA-ds16-hu19 was added to a final concentration of 1 nM and expanded MNCs were added to reach an E:T ratio of 10:1. The dual-targeting triplebody induced statistically significant greater lysis of the dp SEM cells (black bar) than of the sp cells (open bar). Data points represent mean percentage of specific lysis ± SEM (Standard Error of the Mean) obtained from 4 different experiments for the HuT-78 cells and 6 different assays for the Kasumi-1 cells. *Statistically significant (p ≤ 0.01) differences of lysis induced by the dual-targeting triplebody (A) and statistically significant (p ≤ 0.04) differences of lysis induced by the dual-targeting triplebody (B).
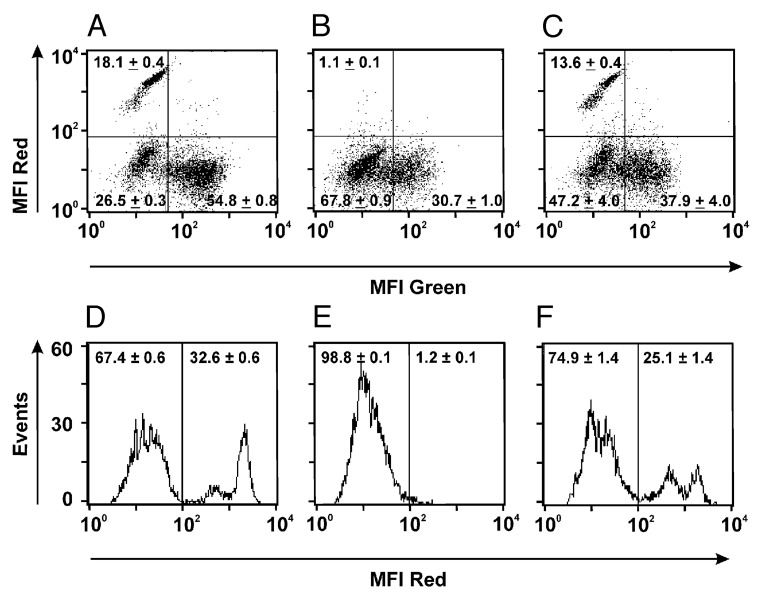
Figure 4. Preferential elimination of dp cells by the dual-targeting triplebody HLA-ds16-hu19. SEM cells (dp for CD19 and HLA-DR) were mixed with HuT-78 cells (sp for HLA-DR) in equal numbers and incubated with isolated and expanded NK cells (E:T ratio 1:1) alone (A, D), with isolated and expanded NKs plus the dual-targeting triplebody (B, E), or with isolated and expanded NKs plus the bsscFv mix consisting of equal amounts of the bsscFvs HLA-16 and 19–16 (C, F). Mixtures were incubated for 3 h at 37 °C and subsequently stained with directly fluorescent-conjugated antibodies. Living cells were detected by flow cytometry (10,000 events collected). SEM cells were detected in the upper left quadrant stained with a PE-conjugated CD19 ab. NK cells were visualized with an FITC-conjugated CD16 antibody and clustered in the lower right quadrant. The unstained HuT-78 cells clustered in the lower left quadrant (A-C). For histograms (D-F) the gated cells (gated for CD19) were used. The vertical bar in the center of each panel represents the boundary between antigen-positive and antigen-negative cells, as defined by the isotype controls, carried along in parallel. Numbers represent the mean percentage ± SEM of the cells in the respective regions obtained from 3 independent experiments. SEM stands here for “Standard Error of the Mean.”
In the reciprocal experiment, the sp HuT-78 cells were lysed well by the triplebody and weakly by the parental HLA-DR antibody L243, but not by the chimeric CD19 antibody, consistent with the absence of CD19 from these cells (Fig. 2B). In this experiment, the EC50 values for the triplebody and the HLA-DR antibody were 34 and 115 pM, respectively (Fig. 2B). Therefore, the triplebody showed 3.4-fold greater cytolytic potency than the parental HLA-DR antibody for lysis of this sp cell line, probably because its CD16-specific binding site binds more strongly to CD16 on the NK cells than the Fc-portion of the murine antibody L243 is bound by CD16 on the human NK cells. A comparison of both experiments (Fig. 2A, B) shows that the triplebody lysed the sp cells with an EC50 of 34 pM, mediated by its HLA-DR specific binding site, and the dp cells with an EC50 of 14 pM, i.e., more than twice as well, mediated by the combination of both binding sites. These data demonstrate that both binding sites in the triplebodies function, and that each can either act in isolation, as, for example, the HLA-DR binding site for lysis of the sp cell line HUT-78 in Figure 2B, or that they can act in concert, as for the dp cell line SEM in Figure 2A.
Preferential lysis of double-positive over single-positive cells is achieved by dual-targeting
In stage 2 of our test, dp SEM cells were mixed with sp HuT-78 cells (Fig. 3A) or sp Kasumi-1 cells (Fig. 3B), respectively, in differing proportions, ranging from 1:1 to 1:20. The SEM cells carried ~61 000 molecules of HLA-DR per cell and 90 000 molecules of CD19. The HuT-78 cells, a human T cell leukemia-derived cell line, and Kasumi-1 cells, an AML-derived cell line, carried ~72 000 and ~7 000 molecules of HLA-DR per cell, respectively, and lacked detectable CD19. In one arm of the experiment (Fig. 3, black bars), the dp SEM cells were labeled with Calcein-AM, and the sp cells were left unlabeled. Both lines were mixed at different ratios. In the other arm (Fig. 3, white bars), HLA-DR sp cells were labeled and mixed with unlabeled dp cells in the same numeric proportions. The mixtures were then treated with the dual-targeting triplebody HLA-ds16-hu19 in a 1 nM saturating concentration. Expanded MNCs were added and ADCC reactions were allowed to proceed. As a result, the triplebody mediated a greater degree of specific lysis of the dp cells (black bars) than of the sp cells (open bars), when both were simultaneously present in the reaction mixtures. This experiment was performed 4 times for the HuT-78 cells (Fig. 3A) and 6 times for Kasumi-1 cells (Fig. 3B). Statistical significance for the difference in lysis of the two cell populations was achieved, as indicated by the asterisks in Figure 3. Due to the fact that the HuT-78 cells carried about 10-times more HLA-DR than Kasumi-1 cells, a higher degree of specific lysis was achieved for Hut-78 cells (Fig. 3A). From these results taken together, we conclude that the dual-targeting triplebody preferentially lysed dp cells, even when the sp cells were present in the reaction mixture in an up to 20-fold numerical excess.
In stage 3 of the experiment, lysis of dp SEM cells and sp HuT-78 cells by triplebody HLA-ds16-hu19 and appropriate controls was analyzed by cytofluorimetry. To this effect, dp and sp target cells were mixed in a 1:1 ratio. Purified, expanded NK cells were added with an E:T ratio of 1:1 to the reaction mixture either in the absence of a therapeutic agent (Fig. 4A and D), or to the mixture containing the triplebody in a saturating 1 nM dose (Fig. 4B and E). The third reaction mixture contained the bsscFvs HLA-ds16 and hu19-ds16 in a 1:1 proportion and each target binding site (CD19- and HLA-DR-specific scFvs) in a 1 nM concentration (Fig. 4C and F). The lysis reaction was permitted to proceed for 3 h, and thereafter the composition of the mixture was analyzed by cytofluorimetry. The mixture was stained with a PE-conjugated CD19 antibody and a FITC-conjugated CD16 antibody. The three component populations were clearly distinguished (Fig. 4A). The incubation with triplebody and NK cells eliminated the dp cells to near completion (Fig. 4B, top left quadrant). By contrast, the samples incubated with the bsscFv mix showed only a small decrease of dp cells (Fig. 4C, top left quadrant). Gated on the CD19-positive targets, two separate subpopulations were seen, which together accounted for 32.6% of the fluorescent cells (Fig. 4D). Together, these are the dp SEM cells. The difference between the two subsets is unknown, but they probably represent subsets of SEM cells with different CD19 surface antigen densities in different stages of the cell cycle. The population of events to the left of the vertical bar (indicating the boundary between CD19pos and CD19neg cells, as defined by the isotype control) in Figure 4D (67.4%) are the sum of the HUT-78- and the NK cells that survived the reaction. In the reaction containing the triplebody, the dp cells were eliminated to near completion (only 1.2% of total surviving cells remained CD19 pos; Fig. 4E). By contrast, the reaction incubated with the 1:1 bsscFv mix showed a decrease of dp cells from 32.6 to 25.1% (Fig. 4F) due to redirected lysis mediated by the bsscFv components. We take these data to provide direct experimental support for the working hypothesis, and conclude that selective elimination of the dp target cells is a unique property, which is achieved exclusively through a molecular format connecting the two different target binding sites into one single, dual-targeting agent. This selectivity is not achieved by an equimolar combination of the separate mono-targeting agents. Thus, a dual-targeting agent achieves unique functional properties (selectivity of lysis) through its unique molecular architecture allowing the physical cross-linking to the two targets on the same cell, which is not achieved in an equivalent fashion by the combination of the corresponding mono-targeting agents.
This experiment (Fig. 4) provides non-identical but complementary information as the Calcein-release experiments (Fig. 3), because in the release experiments the numbers of dead cells were quantitated, whereas in the FACS experiment surviving cells were quantitated. Both experiments therefore measured non-identical, complementary source parameters and thus provided two independent, mutually reinforcing sets of experimental data in support of the hypothesis.
The dual-targeting triplebody HLA-ds16-hu19 eliminates primary leukemic cells
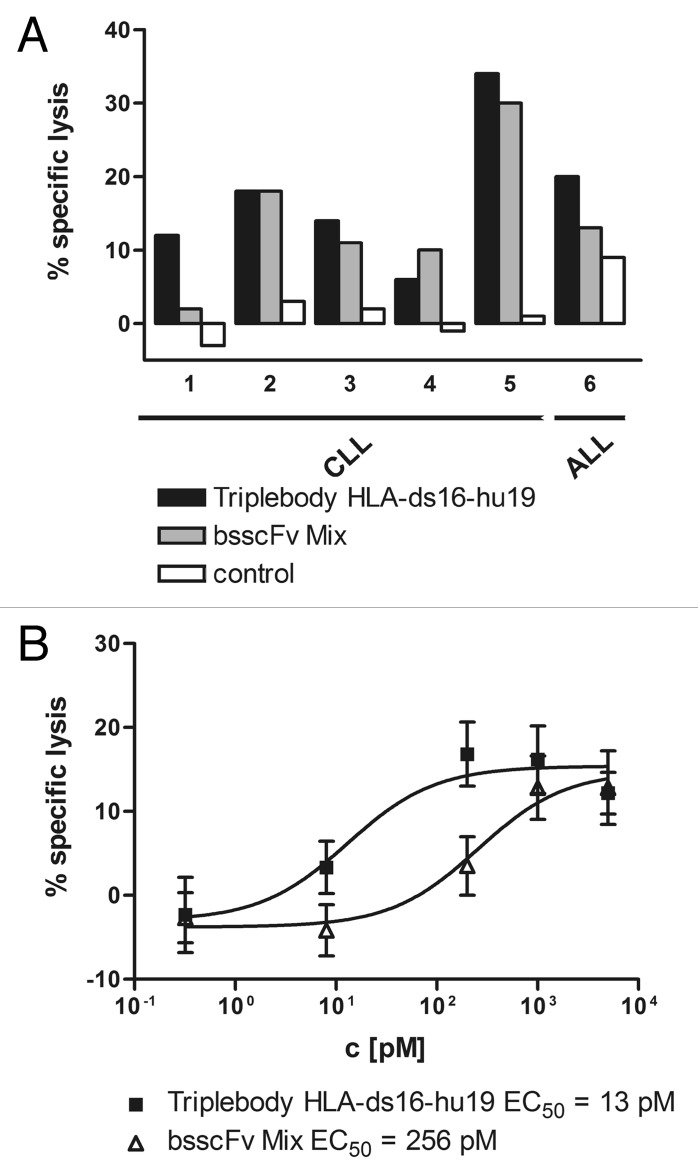
Finally, the triplebody was tested for its ability to mediate redirected lysis (ADCC) of freshly isolated cells from patients with two different leukemias, chronic B-lymphocytic leukemia (B-CLL) and acute lymphocytic leukemia (ALL). For these experiments, isolated MNCs from peripheral blood or bone marrow of 6 patients were used as targets in ADCC reactions. No deliberate effort was made to match the histocompatibility type and killer cell Ig-like receptor (KIR) patterns of tumor cells and donors of the NK cells.
For comparison, a 1:1 mixture of the bsscFvs HLA-ds16 and hu19-ds16 was carried along side-by-side. As a result, both the triplebody and the bsscFv mix showed potent specific lysis of primary leukemic cells at a saturating concentration of 1 nM of CD19- and HLA-DR-specific binding heads (Fig. 5A). For 4 of 6 samples, the triplebody (black bars) mediated stronger lysis than the bsscFv mix (gray bars), and for the remaining 2 of 6 samples the bsscFv mix mediated equally strong or more potent lysis (Fig. 5A). The difference in potency of the two molecular formats was visualized more strikingly when the data for all patient samples were condensed into a single aggregate figure showing dose-response curves for the agents (Fig. 5B). The EC50 values derived from these data were ~13 and ~256 pM for the triplebody and the bsscFv mix, respectively. From these results we conclude that the dual-targeting agent showed a tendency to mediate more potent specific lysis of primary leukemic cells from patients with B-lineage malignancies than an equimolar combination of agents capable of monospecific targeting only.
Figure 5. Potent lysis of primary leukemic cells by the triplebody and the bsscFv mix. (A) Peripheral blood (patients 1–4, 6) or bone marrow (patient 5) cells freshly drawn from B-CLL patients (1–5) and a B-ALL patient (6), were lysed by the dual-targeting triplebody (black bar) and the bsscFv mix (gray bar) at the concentration of 1 nM, using expanded MNCs at an E:T ratio of 10:1. No lysis was induced by the control triplebody 7–16–7 (open bar) specific for CD7, a leukocyte surface antigen not present on the target cells. Data points are presented as mean values from triplicate determinations. (B) Dose-dependent induction of ADCC by the dual-targeting triplebody and the bsscFv mix of primary leukemic cells from different patients; aggregate data. The triplebody (■) induced lysis with 11-fold lower EC50 values than the bsscFv mix (Δ). Data points represent mean percentage of specific lysis ± SEM averaged over the 6 patients obtained with expanded MNCs at an E:T ratio of 10:1. Specific lysis is total lysis minus spontaneous lysis mediated by the MNCs alone, in the absence of added protein agents.
Discussion
For the three dual-targeting triplebodies 123-ds16–33, 33-ds16-19 and HLA-ds16-hu19, our team previously observed simultaneous binding to one copy each of the different antigens on the same target cell. This was demonstrated by comparing the avidity of the entire triplebody for antigen dp target cells with the monovalent affinities of each target antigen binding site carried in the triplebody for antigen sp cells. In addition, the avidity of binding of the triplebody for an antigen dp cell was compared with the monovalent affinity of the same scFv binding module carried in a corresponding bispecific scFv (bsscFv). For example, for triplebody 33-ds16-ds19 the monovalent affinity (equilibrium binding constant KD) of the CD33 binding module was 24 nM measured on a CD33 sp cell, the monovalent affinity of the CD19 binding module was 35 nM measured on a CD19 sp cell, and the avidity of the entire triplebody measured on antigen dp cells (SEM) was 21 nM. Therefore, the entire triplebody bound antigen dp cells with greater binding strength (lower KD value) than each of the individual scFv binding modules. This greater overall binding strength can only be achieved if the target antigen binding modules of the triplebody simultaneously bind 2 target antigens on the same cell. The difference was small in this case because the SEM cells carried only a 10-fold lower surface antigen density of CD33 than of CD19. Similarly, for the triplebody HLA-ds16-hu19 of the present study, the overall avidity of the molecule (equilibrium binding constant KD of the entire triplebody for an antigen dp cell line) was determined. Equilibrium binding curves for HLA-ds16-hu19 were recorded by calibrated flow cytometry, and the KD value for antigen dp target cells was 37 ± 4 nM. By comparison, the monovalent affinity of the CD19-specific scFv carried in the bsscFv 19-16 was 61 ± 13 nM and the monovalent affinity of the HLA-DR-specific scFv carried in the bsscFv HLA-DR-16 was 71 ± 6 nM. Therefore, the triplebody interacted with antigen dp cells with an approximately 2-fold greater binding strength than each of its single target antigen binding sites interacted with an antigen sp-cell. This again is only possible, if both target antigen binding sites of the triplebody contribute by simultaneously binding two antigens on the same target cell.40-42
Our team had previously reported that this dual-targeting capacity of a triplebody can contribute to an enhanced selectivity of binding to antigen dp cells over antigen sp cells, when both are present in a mixed environment.42 However, the hypothesis that the dual-targeting feature should endow triplebodies with the ability to preferentially eliminate antigen dp over antigen sp cells present in the same reaction mixture and mediated by effector cells has not been tested so far. The present study was designed to test this prediction.
For the experiments reported here, effector cells of constant quality were needed. To meet this requirement, a published method for the ex vivo expansion of MNCs was adapted.44 Starting with 108 MNC cells, 750 × 108 cells were obtained on average after 3 weeks of ex vivo expansion (Fig. 1, supplement). These cell numbers and the numerical expansion factors, which were in the range of 700- to 800-fold, compared favorably with published results.44,47 Furthermore, our cells, which were expanded in the continuous presence of IL-2, had an increased specific cytolytic potential of about 2-fold, possibly due to upregulation of CD16 or other activating molecules on the surface of the NK cells, such as NKp30, NKp44 and NKp46 through the action of IL-2 and crosstalk with the CD4-positive T cells that were present during the 21 d expansion period (Fig. 1).45 These expanded NK cells have been stored frozen and can be thawed again and retain much of their cytolytic function, and even retain the capacity for further numerical expansion in culture. They therefore represent a valuable source of effectors of constant quality.
Two methods to quantitate cell death were employed in this study. One relied on Calcein-release as a measure of death, the other on staining of cell surface antigens with fluorescent antibodies followed by cytofluorimetry to measure surviving cells. Both methods measure different source parameters and each comes with their own systemic errors. Therefore, by employing both, we hoped to generate a more reliable data set because we experienced in earlier studies that different methods for the quantification of cell death can produce significantly divergent data. In the present study, both methods produced mutually reinforcing, complementary data.
As a first step toward testing the hypothesis, the ability of the triplebody HLA-ds16-hu19 to mediate lysis of dp and sp cells in redirected lysis assays was compared with the parental antibodies (Fig. 2A and B). A first result was that by combining a scFv-binding site for HLA-DR, derived from parental antibody L243 with weak lytic potential, with a binding module for CD19, a triplebody was generated with a 2.5-fold enhanced EC50 over the parental CD19 molecule. This result suggests that the triplebody can draw on the combined surface antigen densities of CD19 plus HLA-DR on the target cells, which endows it with far stronger binding. Consequently, the triplebody gained increased cytolytic potential, even if the lytic potential of the parental HLA-DR antibody was low. This surprising observation supports our contention that dual-targeting enhances binding and subsequent cytolysis mediated by effector cells, because it increases the available combined target antigen density for the agent on the cancer cell.
Next, the ability of the dual-targeting triplebody to mediate specific lysis of dp cells in the presence of an excess of sp cells was studied (Fig. 3). The dual-targeting agent mediated preferential lysis of dp SEM cells over sp HuT-78 or Kasumi-1 cells carrying only HLA-DR when both were present in the same reaction mixture, even when the sp cells were present in numerical excesses of ≤ 20-fold (Fig. 3). Thus, selective lysis can indeed be achieved by dual-targeting, confirming the working hypothesis. The fact that this effect was observed for two different types of HLA-DR sp leukemic cell lines, HUT-78 and Kasumi, further strengthens our belief that this conclusion is valid.
The final experiment providing proof for our hypothesis was a FACS experiment in which the fates of both the dp and sp target cells after redirected lysis mediated by the triplebody were visualized when both were present in the same reaction mixture. Instead of using the entire population of expanded MNCs, here the NK cells were enriched first by using immunomagnetic beads and then expanded, and an E:T ratio of 1:1 was used. As a result, the dp cells were clearly separated from the sp cells and NK cells in the FACS profile (Fig. 4A, D). The dp cells in this figure are those to the right of the vertical dividing line (Fig. 4D; 32.6%), the sp cells plus NK cells those to the left (67.4%). After incubation with the dual-targeting triplebody, nearly the entire dp population had disappeared (Fig. 4E; right half). In striking contrast, incubation with an equimolar mixture of the bsscFvs HLA-ds16 and hu19-ds16, mixed in a 1:1 proportion, produced only a small decrease of the dp cells from 32.6 to 25.1% (Fig. 4F) due to the combined cytolytic activities of the bsscFv agents. We interpret these data to provide direct evidence in favor of the hypothesis that selectivity of lysis of dp over sp cells can be uniquely achieved by new formats of agents incorporating both binding sites into one physically-linked new molecule, such as the single polypeptide chain of our triplebodies, or the common immunoglobulin scaffold of DVD-Igs, duo-mabs, Cross-mabs, or two-in-one antibodies described in the introduction.
The selectivity of lysis was not achieved by an equimolar cocktail of the corresponding mono-targeting agents (bsscFvs), even if these were present in saturating concentrations, as was the case in our experiment. We do not yet fully comprehend the molecular basis of this surprising finding, because one might have expected that at saturating concentrations of the mono-targeting bsscFvs, the NK cells may have functioned as an integrating surface and achieved the same overall lytic effect, regardless whether the target cells were opsonized with a dual-targeting agent or a sum of two mono-targeting agents. We would have expected that these differences between the triplebody and the cocktail of bsscFvs might gradually disappear as more and more saturating doses of both agents were used. The unexpected result of this experiment was that preferential lysis was still observed for the triplebody over the cocktail of the corresponding bsscFvs, even under conditions when both were provided in saturating concentrations.
Selective lysis of dp over sp cells is a fairly recent observation in the field of antibody-based agents for cancer therapy. No well-documented published precedent cases have come to our attention. In the closest related case, a dual-targeting immunotoxin, DT2219, a single-chain fusion protein consisting of a fragment of diphtheria toxin (DT) fused to tandem scFvs specific for CD22 and CD19, has been designed.33 This dual-targeting agent produced a stronger elimination of CD19- plus CD22- dp lymphoma cells than the mono-targeting control molecules DT1919 and DT2222 with two binding sites each for CD19 or CD22, respectively. However, preferential elimination of dp over sp cells, simultaneously present in the same mixture, has not been demonstrated for this agent. Another related case is the bsscFv with one binding site each for ErbB2 and ErbB3 mentioned in the introduction.29 In this case, preferential binding of dp over sp cells simultaneously present in the mixture has been reported. This agent, however, still differed from ours, because it induced cell death through blockage of two receptors for essential growth-promoting ligands, not through effector cell-mediated lysis. Most pairs of suitable target antigens on the surface of cancer cells that differ in their abundance of expression from the corresponding normal cells are not likely to be growth factor receptors, which could be blocked in a similar fashion by new therapeutic agents. Therefore, the potential clinical applications of the strategy developed by Robinson and more recently followed by others20,26,30,31 are likely to be more restricted than those of dual-targeting triplebodies, which can also target antigens that are not receptors for essential growth- and survival-promoting ligands.
Taken together, these results permit the conclusion that preferential effector cell-mediated lysis of dp over sp cells has been achieved by the dual-targeting agent in the present precedent case. In our academic setting (Fig. 3A), the dp cells were lysed roughly 3- to 4-times as well as the sp cells in the same reaction mixture. Based on the results of this study, we expect new and highly specific anticancer agents that rely on the enhanced capabilities and the unique selectivity of recognition permitted by dual-targeting to be designed in the future. Other agents that exploit dual targeting for the blockage of signaling events essential for proliferation and survival, or that induce apoptosis by direct dual signaling, following the examples outlined in the introduction, may also be developed as new anti-cancer agents.
Materials and Methods
Cell lines and hybridomas
Chinese hamster ovary (CHO) cells, stably transfected with cDNA for human Fcγ RIIIa, were from Dr J van de Winkel (Utrecht, the Netherlands). The hybridoma 4G7 CD19, mIgG1;48 was from Dr R Levy (Stanford University). Kasumi-1 cells (from the DSMZ; German Collection of Microorganisms and Cell Lines) were cultured in medium consisting of 80% Roswell Park Memorial Institute 1640 Glutamax-I medium (RPMI, Invitrogen), 20% fetal calf serum (FCS; Invitrogen), 100 U/ml penicillin (Invitrogen) and 100 µg/ml streptomycin (Invitrogen). The hybridomas L 243 (HLA-DR, mIgG2a) and 3G8 FcgRIII, CD16, mIgG1,49 and the HuT-78 cells were from the American Type Cell Culture Collection (ATCC). CHO cells, the hybridomas, the cell lines SEM50 and HuT-78 were cultured in RPMI containing 10% FCS, 100 U/ml of penicillin and 100 µg/ml of streptomycin.
Construction of recombinant fusion proteins
Escherichia coli strain XL-1 blue (Stratagene) was used as the host for the amplification of the plasmids and for cloning. For construction and eukaryotic expression, the vector pSecTag2HygroC (Invitrogen) was employed. Expression plasmids for the triplebody HLA-ds16-hu19 and the bsscFvs HLA-ds16 and hu19-ds16 were generated as previously described.42
Expression and purification of recombinant fusion-proteins
For expression of bsscFvs HLA-ds16, hu19-ds16, the triplebody HLA-ds16-hu19, and the control triplebody 7-ds16–7,39 HEK 293T cells were transiently transfected with the expression plasmids using the calcium phosphate technique including chloroquine.51 Supernatants containing the secreted proteins were collected and the recombinant proteins were enriched as previously described.42
Flow cytometry analysis
Immunofluorescence analysis was performed on a FACS-Calibur instrument using CellQuest software (Becton Dickinson) as described.52 For each sample 104 events were collected, and whole cells were analyzed using appropriate scatter gates to exclude cellular debris and aggregates. The recombinant proteins were detected using a penta-His antibody and a phycoerythrin (PE)-conjugated goat anti-mouse IgG (Dako) unless otherwise stated. To compare the different cell populations, the expanded mononuclear cells (MNCs) were analyzed by cytofluorimetry (FACS analysis) using directly coupled antibodies CD16-FITC, CD3-FITC and CD56-PE (Miltenyi Biotec).
Target cells from fresh blood and bone marrow
Citrate buffered peripheral blood or bone marrow samples, drawn from patients, were obtained after receiving informed consent, and with the approval of the Ethics Committee of the University of Munich. Leukemic cells were enriched by Lymphoflot (Biotesty) ficoll density centrifugation according to manufacturers’ instructions, and suspended in RPMI containing 10% FBS and penicillin and streptomycin at 100 U/ml and 100 µg/ml, respectively. Viability was verified by Trypan blue exclusion and exceeded 95%.
Ex-vivo expansion of mononuclear cells (MNCs) and immuno-magnetic enrichment of NK cells
To produce sufficient numbers of effector cells for ADCC assays, MNCs were expanded ex vivo by a modified published procedure.44 To obtain MNCs, citrate buffered peripheral blood samples or a leukapheresis sample were drawn from healthy volunteers after obtaining informed consent. The procedure was approved by the Ethics Committee of the University of Erlangen medical center. In one case, NK cells were enriched by immuno-magnetic beads, following manufacturer’s instructions (Miltenyi Biotec). These MNCs or the enriched NK cells were seeded at a density of 106 cells/ml in RPMI medium containing 5% human serum (Invitrogen), 0.5% penicillin and streptomycin, and 500 U/ml IL-2, and incubated at 37 °C over 5 d in the presence of the OKT3 antibody (eBioscience) at a concentration of 10 ng/ml. On day 5, the cells were sedimented (1000 rpm, 5 min) and washed with PBS twice. They were resuspended in medium and adjusted every second day to 106 cells per ml. After 21 d the cells were harvested and frozen in aliquots of 108 cells in 75% human serum and 25% freezing-medium (60% RPMI, 40% DSMO and 12% w/v glucose). After seeding 108 cells, the total cell number recovered in our expansions was (750 ± 130) × 108 on average (Fig. S1A). NK, T, and NKT cells were identified as the CD56+/CD16+, CD56-/CD3+ and CD56+/CD3+ subsets, respectively. On day 0, NK-, T-, and NKT cells accounted for 17 (± 3), 62 (± 5), and 6 (± 2) % of the total MNCs, respectively. After expansion, the frequencies of the NK and T cells remained nearly unchanged with 18 (± 3) and 66 (± 2)%, respectively, and the NKT cells showed a small increase to 26 (± 3)% on day 20 (Fig. S1B). The amplification in cell numbers relative to the input was 629 (± 195)-fold and 704 (± 218)-fold for NK and T cells, respectively (Fig. S1C). The ability of the NK cells to mediate ADCC was assessed on days 0, 5, 9, 14, and 20. The triplebody ds19-ds16-ds19 and the control triplebody 7-ds16-7, specific for CD7, were used at a saturating concentration of 1 nM.39 The potential of the NK cell subset for specific lysis increased with the length of the expansion period from 28 (± 5) to 70 (± 8)% on day 20 (Fig. 1) under standardized assay conditions. No specific lysis of the SEM cells was mediated by the control. All experiments reported below were performed with MNCs or enriched NK cells, respectively, expanded by this protocol.
Non-radioactive ADCC assay
Non-radioactive ADCC assays, using expanded MNCs as effectors at a constant effector-to-target (E:T) ratio of 10:1, were performed using a 3 h Calcein-AM (Invitrogen) release assay. Calcein-AM was dissolved in 50 µl DSMO and diluted in 3.3 ml RPMI to reach a final concentration of 15 µM. Target cells were labeled with 15 µM Calcein-AM for 30 min at 37 °C. After two washing steps with RPMI medium, cells were adjusted to 2 × 105/ml. Effector cells, triplebodies, and RPMI medium were added to round-bottom microtiter plates. Assays were started by adding the target cell suspension, to reach a final reaction volume of 200 µL. After 3 h at 37 °C assays were stopped by centrifugation 100 µl supernatant were collected, and Calcein-AM release was measured in triplicate samples in a fluorimeter/ELISA plate reader, and expressed in relative light units (RLU) at 485/535 nm. Percentage of specific cellular cytotoxicity was calculated by using the formula: Percent specific lysis = (Experimental RLU - Basal RLU) × 100/(Maximal RLU-Basal RLU).
Maximum release was determined by adding 10% Triton X-100 to the target cells and basal release was measured by omitting the effector cells. Dose-response curves were recorded using several 5- or 25-fold serial dilutions of the molecules. Background lysis induced by effector cells alone was subtracted from each data point. EC50 values were calculated by using a nonlinear regression curve fit (variable slope).
Graphical and statistical analysis
Graphical and statistical analyses were performed using Graph Pad Prism Software (Graph Pad Software Inc). Differences between groups were analyzed using unpaired or, where appropriate, paired Student t-test. P-values ≤0.05 were considered statistically significant.
Supplementary Material
Acknowledgments
We thank Dr J G van de Winkel for the CD16 transfected CHO cells, Dr M Herrmann for valuable advice on the interpretation of FACS data, and Drs M. Peterlin and T Braciak for critical comments on the manuscript.
Financial support
This research was supported by grants from the DFG (Deutsche Forschungsgemeinschaft; German Research Community, SFB 643, partial project C3), a research grant No. 2007.049.1 from the Wilhelm Sander Foundation, Neustadt, Germany, and support from the Stiftung Deutsche Krebshilfe, and the Association “Kaminkehrer helfen krebskranken Kindern” (Chimney Sweeps support children with cancer) to GHF.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental Materials
Supplemental materials may be found here: www.landesbioscience.com/journals/mabs/article/26768
Footnotes
Previously published online: www.landesbioscience.com/journals/mabs/article/26768
References
- 1.Adams GP, Weiner LM. Monoclonal antibody therapy of cancer. Nat Biotechnol. 2005;23:1147–57. doi: 10.1038/nbt1137. [DOI] [PubMed] [Google Scholar]
- 2.Adler MJ, Dimitrov DS. Therapeutic antibodies against cancer. Hematol Oncol Clin North Am. 2012;26:447–81, vii. doi: 10.1016/j.hoc.2012.02.013. [vii.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beck A, Wurch T, Bailly C, Corvaia N. Strategies and challenges for the next generation of therapeutic antibodies. Nat Rev Immunol. 2010;10:345–52. doi: 10.1038/nri2747. [DOI] [PubMed] [Google Scholar]
- 4.Reichert JM. Antibody-based therapeutics to watch in 2011. MAbs. 2011;3:76–99. doi: 10.4161/mabs.3.1.13895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reichert JM. Antibodies to watch in 2013: Mid-year update. MAbs. 2013;5:513–7. doi: 10.4161/mabs.24990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scott AM, Allison JP, Wolchok JD. Monoclonal antibodies in cancer therapy. Cancer Immun. 2012;12:14. [PMC free article] [PubMed] [Google Scholar]
- 7.Scott AM, Wolchok JD, Old LJ. Antibody therapy of cancer. Nat Rev Cancer. 2012;12:278–87. doi: 10.1038/nrc3236. [DOI] [PubMed] [Google Scholar]
- 8.Weiner LM, Surana R, Wang S. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nat Rev Immunol. 2010;10:317–27. doi: 10.1038/nri2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glennie MJ, McBride HM, Worth AT, Stevenson GT. Preparation and performance of bispecific F(ab’ gamma)2 antibody containing thioether-linked Fab’ gamma fragments. J Immunol. 1987;139:2367–75. [PubMed] [Google Scholar]
- 10.Lindhofer H, Mocikat R, Steipe B, Thierfelder S. Preferential species-restricted heavy/light chain pairing in rat/mouse quadromas. Implications for a single-step purification of bispecific antibodies. J Immunol. 1995;155:219–25. [PubMed] [Google Scholar]
- 11.Linke R, Klein A, Seimetz D. Catumaxomab: clinical development and future directions. MAbs. 2010;2:129–36. doi: 10.4161/mabs.2.2.11221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ménard S, Canevari S, Colnaghi MI. Hybrid antibodies in cancer diagnosis and therapy. Int J Biol Markers. 1989;4:131–4. doi: 10.1177/172460088900400301. [DOI] [PubMed] [Google Scholar]
- 13.Milstein C, Cuello AC. Hybrid hybridomas and their use in immunohistochemistry. Nature. 1983;305:537–40. doi: 10.1038/305537a0. [DOI] [PubMed] [Google Scholar]
- 14.Klein C, Sustmann C, Thomas M, Stubenrauch K, Croasdale R, Schanzer J, Brinkmann U, Kettenberger H, Regula JT, Schaefer W. Progress in overcoming the chain association issue in bispecific heterodimeric IgG antibodies. MAbs. 2012;4:653–63. doi: 10.4161/mabs.21379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carter P. Bispecific human IgG by design. J Immunol Methods. 2001;248:7–15. doi: 10.1016/S0022-1759(00)00339-2. [DOI] [PubMed] [Google Scholar]
- 16.Merchant AM, Zhu Z, Yuan JQ, Goddard A, Adams CW, Presta LG, Carter P. An efficient route to human bispecific IgG. Nat Biotechnol. 1998;16:677–81. doi: 10.1038/nbt0798-677. [DOI] [PubMed] [Google Scholar]
- 17.Ridgway JB, Presta LG, Carter P. ‘Knobs-into-holes’ engineering of antibody CH3 domains for heavy chain heterodimerization. Protein Eng. 1996;9:617–21. doi: 10.1093/protein/9.7.617. [DOI] [PubMed] [Google Scholar]
- 18.Coloma MJ, Morrison SL. Design and production of novel tetravalent bispecific antibodies. Nat Biotechnol. 1997;15:159–63. doi: 10.1038/nbt0297-159. [DOI] [PubMed] [Google Scholar]
- 19.Digiammarino EL, Harlan JE, Walter KA, Ladror US, Edalji RP, Hutchins CW, Lake MR, Greischar AJ, Liu J, Ghayur T, et al. Ligand association rates to the inner-variable-domain of a dual-variable-domain immunoglobulin are significantly impacted by linker design. MAbs. 2011;3:487–94. doi: 10.4161/mabs.3.5.16326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gu J, Ghayur T. Generation of dual-variable-domain immunoglobulin molecules for dual-specific targeting. Methods Enzymol. 2012;502:25–41. doi: 10.1016/B978-0-12-416039-2.00002-1. [DOI] [PubMed] [Google Scholar]
- 21.Wu C, Ying H, Grinnell C, Bryant S, Miller R, Clabbers A, Bose S, McCarthy D, Zhu RR, Santora L, et al. Simultaneous targeting of multiple disease mediators by a dual-variable-domain immunoglobulin. Nat Biotechnol. 2007;25:1290–7. doi: 10.1038/nbt1345. [DOI] [PubMed] [Google Scholar]
- 22.Davis JH, Aperlo C, Li Y, Kurosawa E, Lan Y, Lo KM, Huston JS. SEEDbodies: fusion proteins based on strand-exchange engineered domain (SEED) CH3 heterodimers in an Fc analogue platform for asymmetric binders or immunofusions and bispecific antibodies. Protein Eng Des Sel. 2010;23:195–202. doi: 10.1093/protein/gzp094. [DOI] [PubMed] [Google Scholar]
- 23.Muda M, Gross AW, Dawson JP, He C, Kurosawa E, Schweickhardt R, Dugas M, Soloviev M, Bernhardt A, Fischer D, et al. Therapeutic assessment of SEED: a new engineered antibody platform designed to generate mono- and bispecific antibodies. Protein Eng Des Sel. 2011;24:447–54. doi: 10.1093/protein/gzq123. [DOI] [PubMed] [Google Scholar]
- 24.Eigenbrot C, Fuh G. Two-in-One antibodies with dual action Fabs. Curr Opin Chem Biol. 2013;17:400–5. doi: 10.1016/j.cbpa.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 25.Labrijn AF, Meesters JI, de Goeij BE, van den Bremer ET, Neijssen J, van Kampen MD, Strumane K, Verploegen S, Kundu A, Gramer MJ, et al. Efficient generation of stable bispecific IgG1 by controlled Fab-arm exchange. Proc Natl Acad Sci U S A. 2013;110:5145–50. doi: 10.1073/pnas.1220145110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schaefer W, Regula JT, Bähner M, Schanzer J, Croasdale R, Dürr H, Gassner C, Georges G, Kettenberger H, Imhof-Jung S, et al. Immunoglobulin domain crossover as a generic approach for the production of bispecific IgG antibodies. Proc Natl Acad Sci U S A. 2011;108:11187–92. doi: 10.1073/pnas.1019002108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beck A, Carter PJ, Gerber HP, Lugovskoy AA, Wurch T, Junutula JR, et al. 8 (th) Annual European Antibody Congress 2012: November 27-28, 2012, Geneva, Switzerland. MAbs 2013; 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kontermann R. Dual targeting strategies with bispecific antibodies. MAbs. 2012;4:182. doi: 10.4161/mabs.4.2.19000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robinson MK, Hodge KM, Horak E, Sundberg AL, Russeva M, Shaller CC, von Mehren M, Shchaveleva I, Simmons HH, Marks JD, et al. Targeting ErbB2 and ErbB3 with a bispecific single-chain Fv enhances targeting selectivity and induces a therapeutic effect in vitro. Br J Cancer. 2008;99:1415–25. doi: 10.1038/sj.bjc.6604700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McDonagh CF, Huhalov A, Harms BD, Adams S, Paragas V, Oyama S, Zhang B, Luus L, Overland R, Nguyen S, et al. Antitumor activity of a novel bispecific antibody that targets the ErbB2/ErbB3 oncogenic unit and inhibits heregulin-induced activation of ErbB3. Mol Cancer Ther. 2012;11:582–93. doi: 10.1158/1535-7163.MCT-11-0820. [DOI] [PubMed] [Google Scholar]
- 31.Xu L, Kohli N, Rennard R, Jiao Y, Razlog M, Zhang K, Baum J, Johnson B, Tang J, Schoeberl B, et al. Rapid optimization and prototyping for therapeutic antibody-like molecules. MAbs. 2013;5:237–54. doi: 10.4161/mabs.23363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schaefer G, Haber L, Crocker LM, Shia S, Shao L, Dowbenko D, Totpal K, Wong A, Lee CV, Stawicki S, et al. A two-in-one antibody against HER3 and EGFR has superior inhibitory activity compared with monospecific antibodies. Cancer Cell. 2011;20:472–86. doi: 10.1016/j.ccr.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 33.Vallera DA, Todhunter DA, Kuroki DW, Shu Y, Sicheneder A, Chen H. A bispecific recombinant immunotoxin, DT2219, targeting human CD19 and CD22 receptors in a mouse xenograft model of B-cell leukemia/lymphoma. Clin Cancer Res. 2005;11:3879–88. doi: 10.1158/1078-0432.CCR-04-2290. [DOI] [PubMed] [Google Scholar]
- 34.Oh S, Stish BJ, Sachdev D, Chen H, Dudek AZ, Vallera DA. A novel reduced immunogenicity bispecific targeted toxin simultaneously recognizing human epidermal growth factor and interleukin-4 receptors in a mouse model of metastatic breast carcinoma. Clin Cancer Res. 2009;15:6137–47. doi: 10.1158/1078-0432.CCR-09-0696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stish BJ, Chen H, Shu Y, Panoskaltsis-Mortari A, Vallera DA. A bispecific recombinant cytotoxin (DTEGF13) targeting human interleukin-13 and epidermal growth factor receptors in a mouse xenograft model of prostate cancer. Clin Cancer Res. 2007;13:6486–93. doi: 10.1158/1078-0432.CCR-07-0938. [DOI] [PubMed] [Google Scholar]
- 36.Stish BJ, Chen H, Shu Y, Panoskaltsis-Mortari A, Vallera DA. Increasing anticarcinoma activity of an anti-erbB2 recombinant immunotoxin by the addition of an anti-EpCAM sFv. Clin Cancer Res. 2007;13:3058–67. doi: 10.1158/1078-0432.CCR-06-2454. [DOI] [PubMed] [Google Scholar]
- 37.Todhunter DA, Hall WA, Rustamzadeh E, Shu Y, Doumbia SO, Vallera DA. A bispecific immunotoxin (DTAT13) targeting human IL-13 receptor (IL-13R) and urokinase-type plasminogen activator receptor (uPAR) in a mouse xenograft model. Protein Eng Des Sel. 2004;17:157–64. doi: 10.1093/protein/gzh023. [DOI] [PubMed] [Google Scholar]
- 38.Tsai AK, Oh S, Chen H, Shu Y, Ohlfest JR, Vallera DA. A novel bispecific ligand-directed toxin designed to simultaneously target EGFR on human glioblastoma cells and uPAR on tumor neovasculature. J Neurooncol. 2011;103:255–66. doi: 10.1007/s11060-010-0392-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kellner C, Bruenke J, Stieglmaier J, Schwemmlein M, Schwenkert M, Singer H, Mentz K, Peipp M, Lang P, Oduncu F, et al. A novel CD19-directed recombinant bispecific antibody derivative with enhanced immune effector functions for human leukemic cells. J Immunother. 2008;31:871–84. doi: 10.1097/CJI.0b013e318186c8b4. [DOI] [PubMed] [Google Scholar]
- 40.Kügler M, Stein C, Kellner C, Mentz K, Saul D, Schwenkert M, Schubert I, Singer H, Oduncu F, Stockmeyer B, et al. A recombinant trispecific single-chain Fv derivative directed against CD123 and CD33 mediates effective elimination of acute myeloid leukaemia cells by dual targeting. Br J Haematol. 2010;150:574–86. doi: 10.1111/j.1365-2141.2010.08300.x. [DOI] [PubMed] [Google Scholar]
- 41.Schubert I, Kellner C, Stein C, Kügler M, Schwenkert M, Saul D, Mentz K, Singer H, Stockmeyer B, Hillen W, et al. A single-chain triplebody with specificity for CD19 and CD33 mediates effective lysis of mixed lineage leukemia cells by dual targeting. MAbs. 2011;3:21–30. doi: 10.4161/mabs.3.1.14057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schubert I, Kellner C, Stein C, Kügler M, Schwenkert M, Saul D, Stockmeyer B, Berens C, Oduncu FS, Mackensen A, et al. A recombinant triplebody with specificity for CD19 and HLA-DR mediates preferential binding to antigen double-positive cells by dual-targeting. MAbs. 2012;4:45–56. doi: 10.4161/mabs.4.1.18498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singer H, Kellner C, Lanig H, Aigner M, Stockmeyer B, Oduncu F, Schwemmlein M, Stein C, Mentz K, Mackensen A, et al. Effective elimination of acute myeloid leukemic cells by recombinant bispecific antibody derivatives directed against CD33 and CD16. J Immunother. 2010;33:599–608. doi: 10.1097/CJI.0b013e3181dda225. [DOI] [PubMed] [Google Scholar]
- 44.Alici E, Sutlu T, Björkstrand B, Gilljam M, Stellan B, Nahi H, Quezada HC, Gahrton G, Ljunggren HG, Dilber MS. Autologous antitumor activity by NK cells expanded from myeloma patients using GMP-compliant components. Blood. 2008;111:3155–62. doi: 10.1182/blood-2007-09-110312. [DOI] [PubMed] [Google Scholar]
- 45.Lang P, Barbin K, Feuchtinger T, Greil J, Peipp M, Zunino SJ, Pfeiffer M, Handgretinger R, Niethammer D, Fey GH. Chimeric CD19 antibody mediates cytotoxic activity against leukemic blasts with effector cells from pediatric patients who received T-cell-depleted allografts. Blood. 2004;103:3982–5. doi: 10.1182/blood-2003-05-1735. [DOI] [PubMed] [Google Scholar]
- 46.Robbins PA, Evans EL, Ding AH, Warner NL, Brodsky FM. Monoclonal antibodies that distinguish between class II antigens (HLA-DP, DQ, and DR) in 14 haplotypes. Hum Immunol. 1987;18:301–13. doi: 10.1016/0198-8859(87)90077-2. [DOI] [PubMed] [Google Scholar]
- 47.Koehl U, Esser R, Zimmermann S, Tonn T, Kotchetkov R, Bartling T, Sörensen J, Grüttner HP, Bader P, Seifried E, et al. Ex vivo expansion of highly purified NK cells for immunotherapy after haploidentical stem cell transplantation in children. Klin Padiatr. 2005;217:345–50. doi: 10.1055/s-2005-872520. [DOI] [PubMed] [Google Scholar]
- 48.Meeker TC, Miller RA, Link MP, Bindl J, Warnke R, Levy R. A unique human B lymphocyte antigen defined by a monoclonal antibody. Hybridoma. 1984;3:305–20. doi: 10.1089/hyb.1984.3.305. [DOI] [PubMed] [Google Scholar]
- 49.Fleit HB, Wright SD, Unkeless JC. Human neutrophil Fc gamma receptor distribution and structure. Proc Natl Acad Sci U S A. 1982;79:3275–9. doi: 10.1073/pnas.79.10.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Greil J, Gramatzki M, Burger R, Marschalek R, Peltner M, Trautmann U, Hansen-Hagge TE, Bartram CR, Fey GH, Stehr K, et al. The acute lymphoblastic leukaemia cell line SEM with t(4;11) chromosomal rearrangement is biphenotypic and responsive to interleukin-7. Br J Haematol. 1994;86:275–83. doi: 10.1111/j.1365-2141.1994.tb04726.x. [DOI] [PubMed] [Google Scholar]
- 51.Sambrook J, Russel DW. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY, 2001. [Google Scholar]
- 52.Bruenke J, Fischer B, Barbin K, Schreiter K, Wachter Y, Mahr K, Titgemeyer F, Niederweis M, Peipp M, Zunino SJ, et al. A recombinant bispecific single-chain Fv antibody against HLA class II and FcgammaRIII (CD16) triggers effective lysis of lymphoma cells. Br J Haematol. 2004;125:167–79. doi: 10.1111/j.1365-2141.2004.04893.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.