Abstract
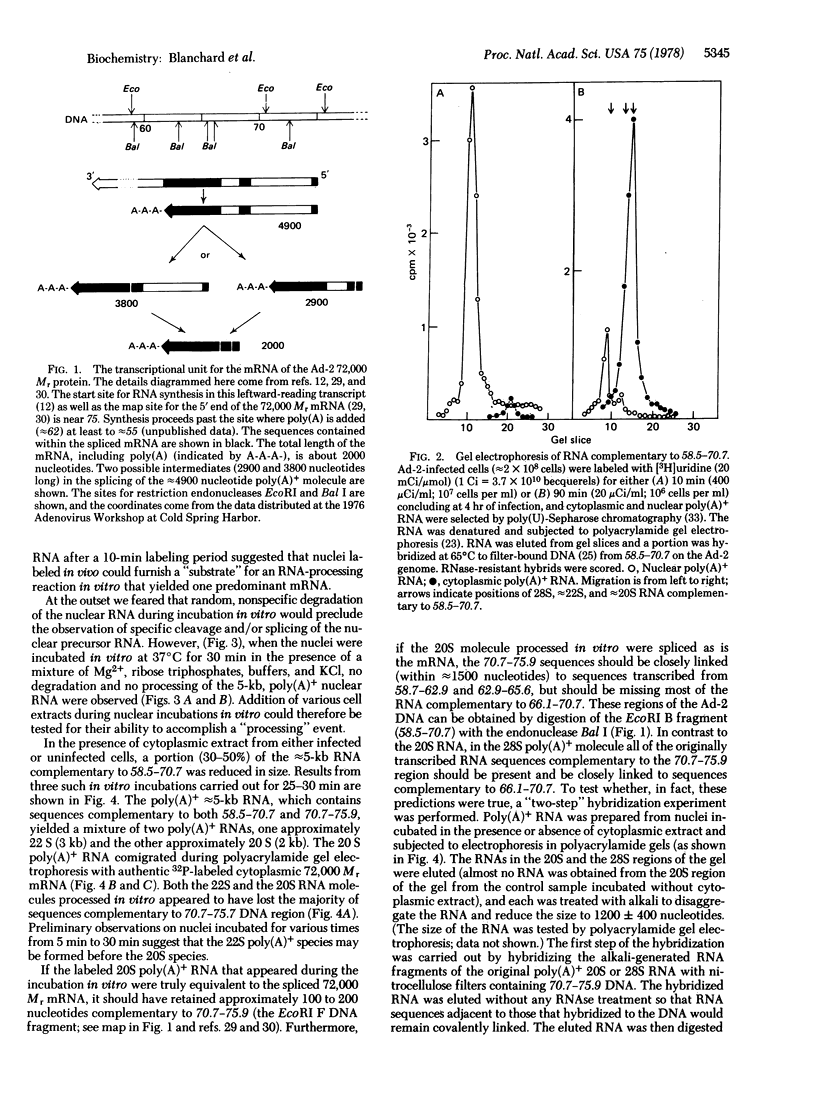
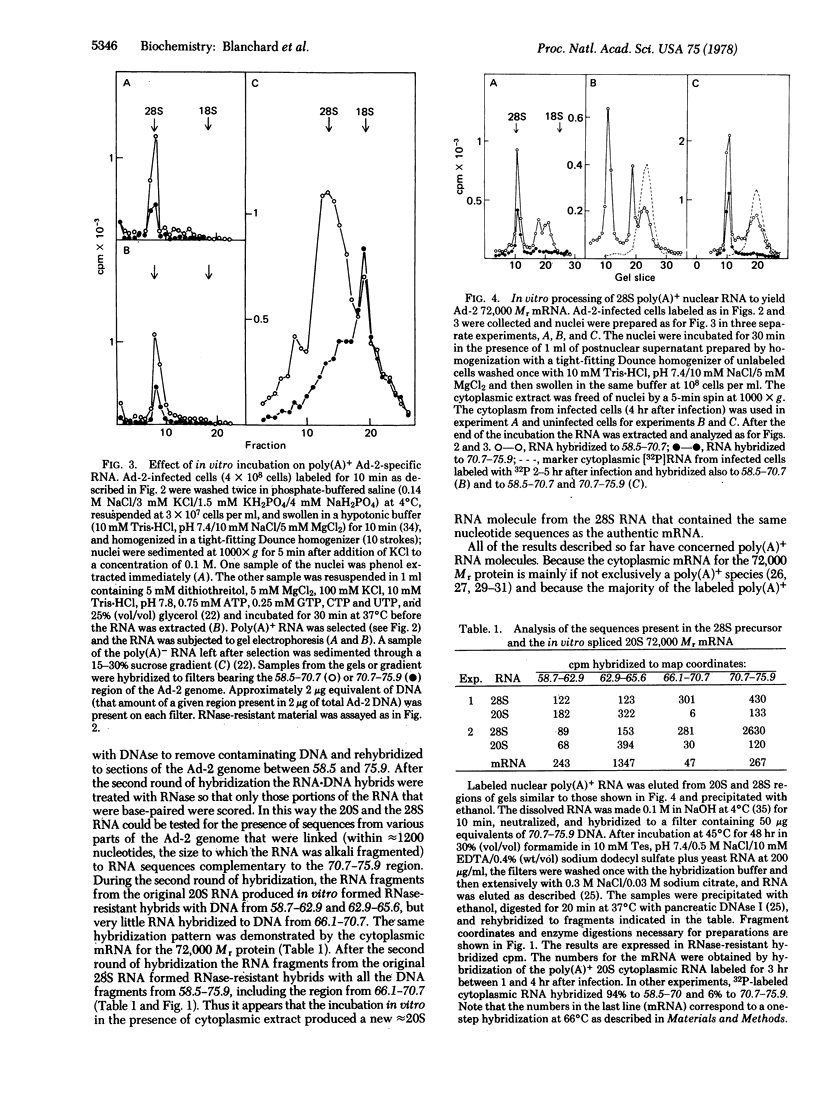
"Splicing" of the precursor to an adenovirus mRNA was accomplished in isolated cell-free extracts. Nuclei were prepared from hypotonically swollen cells that had been labeled with [3H]uridine for 10 min prior to nuclear isolation. Addition of a "cytoplasmic" fraction was required for the splicing to occur. The nuclear precursor, a poly(A)-terminated RNA molecule approximately 5 kilobases long, contained sequences complementary to the 58.5--75.9 region of the adenovirus 2 genome, including those sequences spliced out of the mature mRNA molecule. The in vitro spliced product was a poly(A)-terminated RNA molecule identical in size to the cytoplasmic 72,000 Mr protein mRNA (2 kilobases long) in which the sequences encoded in the 70.7--75.9 region of the viral genome were spliced to those encoded at 58.7--65.6, with the sequences encoded at 66.1--70.7 deleted.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aloni Y., Dhar R., Laub O., Horowitz M., Khoury G. Novel mechanism for RNA maturation: the leader sequences of simian virus 40 mRNA are not transcribed adjacent to the coding sequences. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3686–3690. doi: 10.1073/pnas.74.9.3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson J., Coutinho A., Lernhardt W., Melchers F. Clonal growth and maturation to immunoglobulin secretion in vitro of every growth-inducible B lymphocyte. Cell. 1977 Jan;10(1):27–34. doi: 10.1016/0092-8674(77)90136-2. [DOI] [PubMed] [Google Scholar]
- Bachenheimer S., Darnell J. E. Adenovirus-2 mRNA is transcribed as part of a high-molecular-weight precursor RNA. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4445–4449. doi: 10.1073/pnas.72.11.4445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastos R. N., Aviv H. Globin RNA precursor molecules: biosynthesis and process in erythroid cells. Cell. 1977 Jul;11(3):641–650. doi: 10.1016/0092-8674(77)90081-2. [DOI] [PubMed] [Google Scholar]
- Berget S. M., Moore C., Sharp P. A. Spliced segments at the 5' terminus of adenovirus 2 late mRNA. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3171–3175. doi: 10.1073/pnas.74.8.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Spliced early mRNAs of simian virus 40. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1274–1278. doi: 10.1073/pnas.75.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Structure of the adenovirus 2 early mRNAs. Cell. 1978 Jul;14(3):695–711. doi: 10.1016/0092-8674(78)90252-0. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Ultraviolet mapping of the adenovirus 2 early promoters. Cell. 1977 Sep;12(1):45–55. doi: 10.1016/0092-8674(77)90184-2. [DOI] [PubMed] [Google Scholar]
- Breathnach R., Mandel J. L., Chambon P. Ovalbumin gene is split in chicken DNA. Nature. 1977 Nov 24;270(5635):314–319. doi: 10.1038/270314a0. [DOI] [PubMed] [Google Scholar]
- Chow L. T., Gelinas R. E., Broker T. R., Roberts R. J. An amazing sequence arrangement at the 5' ends of adenovirus 2 messenger RNA. Cell. 1977 Sep;12(1):1–8. doi: 10.1016/0092-8674(77)90180-5. [DOI] [PubMed] [Google Scholar]
- Chow L. T., Roberts J. M., Lewis J. B., Broker T. R. A map of cytoplasmic RNA transcripts from lytic adenovirus type 2, determined by electron microscopy of RNA:DNA hybrids. Cell. 1977 Aug;11(4):819–836. doi: 10.1016/0092-8674(77)90294-x. [DOI] [PubMed] [Google Scholar]
- Cooke H. J., McKay R. D. Evolution of a human Y chromosome-specific repeated sequence. Cell. 1978 Mar;13(3):453–460. doi: 10.1016/0092-8674(78)90319-7. [DOI] [PubMed] [Google Scholar]
- Craig E. A., Raskas H. J. Nuclear transcripts larger than the cytoplasmic mRNAs are specified by segments of the adenovirus genome coding for early functions. Cell. 1976 Jun;8(2):205–213. doi: 10.1016/0092-8674(76)90004-0. [DOI] [PubMed] [Google Scholar]
- Curtis P. J., Mantei N., van den Berg J., Weissmann C. Presence of a putative 15S precursor to beta-globin mRNA but not to alpha-globin mRNA in Friend cells. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3184–3188. doi: 10.1073/pnas.74.8.3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliceiri G. L., Gurney T., Jr Subcellular location of precursors to small nuclear RNA species C and D and of newly synthesized 5 S RNA in HeLa cells. Biochem Biophys Res Commun. 1978 Apr 14;81(3):915–919. doi: 10.1016/0006-291x(78)91438-9. [DOI] [PubMed] [Google Scholar]
- Evans R. M., Fraser N., Ziff E., Weber J., Wilson M., Darnell J. E. The initiation sites for RNA transcription in Ad2 DNA. Cell. 1977 Nov;12(3):733–739. doi: 10.1016/0092-8674(77)90273-2. [DOI] [PubMed] [Google Scholar]
- Flint J. The topography and transcription of the adenovirus genome. Cell. 1977 Feb;10(2):153–166. doi: 10.1016/0092-8674(77)90211-2. [DOI] [PubMed] [Google Scholar]
- Foster D. N., Gurney T., Jr Nuclear location of mammalian DNA polymerase activities. J Biol Chem. 1976 Dec 25;251(24):7893–7898. [PubMed] [Google Scholar]
- Gilmore-Hebert M., Wall R. Immunoglobulin light chain mRNA is processed from large nuclear RNA. Proc Natl Acad Sci U S A. 1978 Jan;75(1):342–345. doi: 10.1073/pnas.75.1.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg S., Weber J., Darnell J. E., Jr The definition of a large viral transcription unit late in Ad2 infection of HeLa cells: mapping by effects of ultraviolet irradiation. Cell. 1977 Apr;10(4):617–621. doi: 10.1016/0092-8674(77)90094-0. [DOI] [PubMed] [Google Scholar]
- Goodman H. M., Olson M. V., Hall B. D. Nucleotide sequence of a mutant eukaryotic gene: the yeast tyrosine-inserting ochre suppressor SUP4-o. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5453–5457. doi: 10.1073/pnas.74.12.5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu M. T., Ford J. Sequence arrangement of the 5' ends of simian virus 40 16S and 19S mRNAs. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4982–4985. doi: 10.1073/pnas.74.11.4982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffreys A. J., Flavell R. A. The rabbit beta-globin gene contains a large large insert in the coding sequence. Cell. 1977 Dec;12(4):1097–1108. doi: 10.1016/0092-8674(77)90172-6. [DOI] [PubMed] [Google Scholar]
- Jelinek W., Molloy G., Fernandez-Munoz R., Salditt M., Darnell J. E. Secondary structure in heterogeneous nuclear RNA: involvement of regions from repeated DNA sites. J Mol Biol. 1974 Jan 25;82(3):361–370. doi: 10.1016/0022-2836(74)90597-x. [DOI] [PubMed] [Google Scholar]
- Kitchingman G. R., Lai S. P., Westphal H. Loop structures in hybrids of early RNA and the separated strands of adenovirus DNA. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4392–4395. doi: 10.1073/pnas.74.10.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klessig D. F. Two adenovirus mRNAs have a common 5' terminal leader sequence encoded at least 10 kb upstream from their main coding regions. Cell. 1977 Sep;12(1):9–21. doi: 10.1016/0092-8674(77)90181-7. [DOI] [PubMed] [Google Scholar]
- Knapp G., Beckmann J. S., Johnson P. F., Fuhrman S. A., Abelson J. Transcription and processing of intervening sequences in yeast tRNA genes. Cell. 1978 Jun;14(2):221–236. doi: 10.1016/0092-8674(78)90109-5. [DOI] [PubMed] [Google Scholar]
- Lewis J. B., Atkins J. F., Baum P. R., Solem R., Gesteland R. F., Anderson C. W. Location and identification of the genes for adenovirus type 2 early polypeptides. Cell. 1976 Jan;7(1):141–151. doi: 10.1016/0092-8674(76)90264-6. [DOI] [PubMed] [Google Scholar]
- Lönn U. Exclusive nuclear location of precursor 4 S RNA in vivo. J Mol Biol. 1977 Jun 5;112(4):661–666. doi: 10.1016/s0022-2836(77)80172-1. [DOI] [PubMed] [Google Scholar]
- Nevins J. R., Darnell J. E. Groups of adenovirus type 2 mRNA's derived from a large primary transcript: probable nuclear origin and possible common 3' ends. J Virol. 1978 Mar;25(3):811–823. doi: 10.1128/jvi.25.3.811-823.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penman S., Scherrer K., Becker Y., Darnell J. E. POLYRIBOSOMES IN NORMAL AND POLIOVIRUS-INFECTED HELA CELLS AND THEIR RELATIONSHIP TO MESSENGER-RNA. Proc Natl Acad Sci U S A. 1963 May;49(5):654–662. doi: 10.1073/pnas.49.5.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross J. A precursor of globin messenger RNA. J Mol Biol. 1976 Sep 15;106(2):403–420. doi: 10.1016/0022-2836(76)90093-0. [DOI] [PubMed] [Google Scholar]
- Tilghman S. M., Tiemeier D. C., Seidman J. G., Peterlin B. M., Sullivan M., Maizel J. V., Leder P. Intervening sequence of DNA identified in the structural portion of a mouse beta-globin gene. Proc Natl Acad Sci U S A. 1978 Feb;75(2):725–729. doi: 10.1073/pnas.75.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonegawa S., Maxam A. M., Tizard R., Bernard O., Gilbert W. Sequence of a mouse germ-line gene for a variable region of an immunoglobulin light chain. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1485–1489. doi: 10.1073/pnas.75.3.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela P., Venegas A., Weinberg F., Bishop R., Rutter W. J. Structure of yeast phenylalanine-tRNA genes: an intervening DNA segment within the region coding for the tRNA. Proc Natl Acad Sci U S A. 1978 Jan;75(1):190–194. doi: 10.1073/pnas.75.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall R., Darnell J. E. Presence of cell and virus specific sequences in the same molecules of nuclear RNA from virus transformed cells. Nat New Biol. 1971 Jul 21;232(29):73–76. doi: 10.1038/newbio232073a0. [DOI] [PubMed] [Google Scholar]


