Abstract
The objective of this study is to investigate and assess the use of in situ deposit nanosilver (nAg2O) or nanocopper oxides (nCuO) into nonwoven polyester fabric (NWPF) as a safe and effective antibacterial filter of pollution from domestic wastewater. The bactericidal effect of both nAg2O and nCuO was examined against Gram-negative bacteria (Escherichia coli, Salmonella typhi) and Gram-positive bacteria (Enterococcus faecalis, Staphylococcus aureus) using agar diffusion disk method. In addition, the capability of nAg2O and nCuO as disinfectants for secondary treated domestic wastewater was investigated as a case study. Transmission electron microscope (TEM) confirmed the formation of nAg2O and nCuO particles with average particle sizes of 15 and 41 nm, respectively. Disk diffusion results showed that nAg2O had a higher bactericidal effect than nCuO. Moreover, the disinfection of secondary treated wastewater using 1.27 mg/cm3 of nAg2O in the nonwoven fabric was capable of hindering 99.6% and 91.7% of total and fecal coliforms within 10 minutes with a residual value of 18 and 15 MPN-index/100 mL, respectively. The residual total and fecal coliform concentrations were far less than that stated in the national and international limits for wastewater reuse in agriculture purpose.
1. Introduction
Municipal wastewater is one of the major sources of aquatic pollution, particularly in developing countries. Microbial contamination of water poses a serious threat to public health. Accordingly, wastewater should be disinfected to prevent the transmission of infectious diseases and to ensure that water is safe for human contact and the environment [1]. Although disinfection methods currently used in water and wastewater treatment can effectively control microbial pathogens, research in the past few decades has revealed a dilemma between effective disinfection and formation of harmful disinfection byproducts (DBPs). Chemical disinfectants commonly used by the water industry such as free chlorine, chloramines, and ozone can react with various constituents in natural water to form DBPs, many of which are carcinogens. In some literature, 600 DBPs have even been reported [2, 3]. The resistance of some pathogens, such as Cryptosporidium and Giardia, to conventional chemical disinfectants requires an extremely high disinfectant dosage, leading to aggravated DBP formation. Therefore, there is an urgent need to reevaluate conventional disinfection methods and to consider innovative approaches that enhance the reliability and robustness of disinfection while avoiding DBP formation [4].
Moreover, the emergence of nanoscience and nanotechnology in the last decade presents opportunities for exploring the bactericidal effect of metal nanoparticles. This has been attributed to their small size and high surface to volume ratio, which allows them to interact closely with microbial membranes, and is not merely due to the release of metal ions in solution [5]. Many of the present problems concerning water quality could be resolved or greatly ameliorated using nanosorbents, nanocatalysts, bioactive nanoparticles, nanostructured catalytic membranes, and nanoparticles enhanced filtration along with other products and processes resulting from the development of nanotechnology. Innovations in the development of novel technologies to disinfect water are among the most exciting and promising approaches.
Furthermore, nanotechnology-derived products that reduce the concentrations of toxic compounds to sub-ppb levels can be used to support the achievement of water quality standards and health advisories [6]. Metal nanoparticles with bactericidal activity can be immobilized and coated on surfaces, which may find application in various fields, that is, medical instruments and devices, water treatment, and food processing. Metal nanoparticles may also be combined with polymers to form composites for better utilization of their antimicrobial activity [7–10].
The objective of this study was to in situ deposit nanometal oxides, such as nanoparticles of silver and copper oxides, into nonwoven polyester fabric, to investigate and asses the capability of nanoparticles, deposited in an innovative fabric, as disinfectants for the removal of bacterial indicators of pollution from secondary treated domestic wastewater.
2. Experimental Methods
2.1. Materials
Nonwoven polyester fabric (NWPF) was purchased from the local market. Silver nitrate and copper chloride were purchased from Merck (Germany). The used hydrogen peroxide (H2O2) and other laboratory chemicals were of analytical grade purchased from Sigma-Aldrich Chemical Company.
2.2. Methods
2.2.1. In Situ Deposition of Nanosilver Oxide or Nanocopper Oxide into Nonwoven Polyester Fabric
The nanometal oxides deposition was performed by padding the polyester fabric samples in an aqueous solution containing 50 mmol of metal salts solution (silver nitrate or copper chloride) and 1.5 wt. % (w/v) of polyvinyl pyrrolidone (PVP) as stabilizing agent. The fabric was then squeezed to a wet pick up 100%. The fabric was padded twice in the reducing-oxidizing bath containing 4 g/L sodium hydroxide and 10 mL H2O2 (35%) at pH 9.5, then squeezed to a wet pick up of 100%. The treated fabric was dried at 50°C for 10 minutes, then thoroughly washed with water for 45 minutes at 50°C and dried for 20 minutes at 50°C.
2.2.2. Characterization of Treated Polyester Fabric
Treated polyester fabric with nanometal oxides was examined qualitatively and quantitatively using energy dispersive X-ray spectrum (SEM-EDX), coupled with a scanning electron microscope (type JXA-840—electron probe micro-analyzer—JOEL) and a transmission electron microscope (TEM) that gave images of cross-section of treated fabric samples. Total silver concentrations were determined according to Standard Methods for the Examination of Water and Wastewater [11], using an atomic absorption spectrometer, Varian SpectrAA (220), with a graphite furnace accessory and equipped with deuterium arc background corrector.
2.2.3. Water-Leaching Test
Triplicate samples of one gram of fabric were transferred to shaking bottles with 10 mL de-ionized water. The bottles were then sealed with Para film lids and secured, rotated end-over-end for 48 h at 30 rpm, and then filtered through a 0.45 μm membrane filter. Leached silver was analyzed in the filtrate [11]. For each series of metal measurements, an absorption calibration curve was constructed. It was composed of a blank and three or more standards from Merck (Germany). Accuracy and precision of the silver measurement were confirmed using external standard reference material 1643e, from National Institute Standards and Technology (NIST).
2.2.4. Bacterial Strains
Two Gram-negative bacterial strains (Escherichia coli ATCC 11229 and Salmonella typhi ATCC 13311) and two Gram-positive bacterial strains (Enterococcus faecalis ATCC 29212 and Staphylococcus aureus ATCC 6538) were used as reference strains for studying the bactericidal effect of nAg2O and nCuO. All bacterial strains were grown overnight (37°C for 24 h) in nutrient broth (Himedia, India). The nutrient agar (Himedia, India) composed of peptic digest of animal tissue (5.0 g), sodium chloride (5.0 g), beef extract (1.5 g), yeast extract (1.5 g), and agar (15.0 g/L).
2.2.5. Determination of the Efficiency of Treated Polyester Fabric Using Disk Diffusion Test
A comparison between treated NWPF with nAg2O and nCuO using a disk of 6 mm diameter of fabric was carried out using agar diffusion disk test [12]. 0.1 mL of each overnight grown bacterial suspension (104–105 CFU/mL) was spread over the surface of nutrient agar Petri dishes (Himedia, India). Disks of both nAg2O and nCuO were then placed over the surfaces of petri dishes. The dishes were inversely incubated at 37°C for 24 h. The antibacterial effect was qualified based on the formation of inhibition zone around the disks.
2.2.6. Effect of Contact Time for Bacterium-Nanoparticle Interaction
Three overnight grown bacterial suspensions of Staphylococcus aureus, Escherichia coli, and Salmonella typhi with initial counts 3.1 × 104, 4.6 × 104, and 6.4 × 103 CFU/mL, respectively, were examined. Three 26 cm2 surface area slides was immersed into three tubes containing 100 mL of examined bacterial suspensions at different contact times (15 min to 24 h). After each contact time, the count of each bacterial suspension was determined using poured plate count method according to Standard Methods for the Examination of Water and Wastewater [11].
2.2.7. Application of Polyester Fabric Loaded with Nanometal Oxides as a Disinfectant for a Secondary Treated Domestic Wastewater (Case Study)
Domestic wastewater effluent treated in a Packed bed upflow anaerobic sludge blanket (P-UASB) followed by Inclined Plate Settler (IPS) then Multistage Roughing Fine Sand Filtration (MSRFF) was used as a real source for testing the efficiency of antibacterial filter [13]. Physicochemical and bacterial indicators (total coliforms and fecal coliforms) of raw and treated wastewater were determined. Slides with a 26 cm2 surface area were immersed in 200 mL of treated wastewater (25 samples) for a contact time of 10 min. Total and fecal coliforms were examined using the MPN-method according to Standard Methods for the Examination of Water and Wastewater [11].
3. Result and Discussion
3.1. Characterization of Treated Polyester Fabrics
In situ deposition of nanometal oxides into NWPF with a thickness of 1.82 mm was used for disinfection of various microbial strains of Gram-positive and Gram-negative bacteria. Also, disinfection of a real secondary treated wastewater, as a case study, was investigated. Cross-section image of dried fabric was taken with a scanning electron microscope in high vacuum mode after coating with approximately 10 nm of gold to observe fabric asymmetry and its pore structure (Figure 1). It was found that the pore size of the fabric ranged from 5.3 to 9.9 μm with an average pore size of 7.2 μm.
Figure 1.
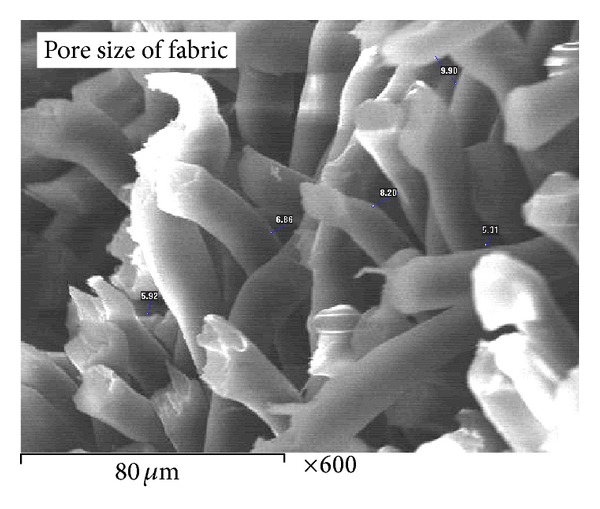
SEM of polyester fabric.
In addition, the X-ray diffraction(XRD) patterns of the NWPF before and after loading with nanometal oxides are shown in Figure 2. The recorded XRD patterns indicated no change after loading with nanometal oxides and no relevant peaks of metals were observed. This indicated that metals did not affect fabric crystallinity. However, there was a slight shift in peaks position, which revealed that metals particles were incorporated in the fabric. TEM micrographs of fabric loaded with nanometal oxides (Figures 3(a) and 3(b)) confirmed the formation of nanosilver and nanocopper particles of about 15 nm and 41 nm on average. Furthermore, the total concentration of silver and copper in the treated fabric was 4635 and 4610 mg/kg, respectively.
Figure 2.
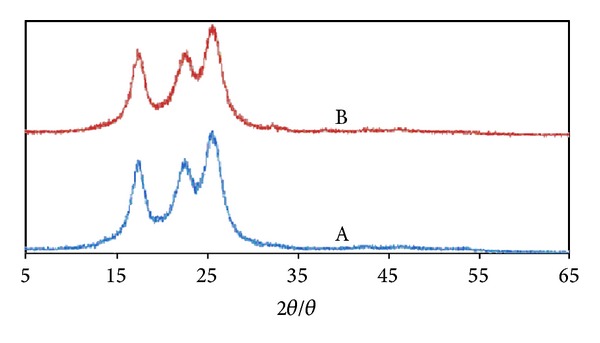
XRD patterns of polyester fabric (A) before loading and (B) after loading with nanometal oxides.
Figure 3.
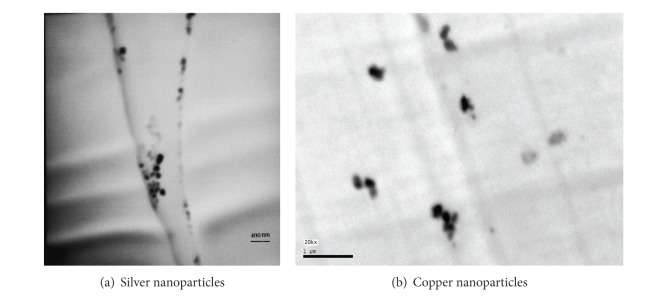
Transmission electron micrograph of cross-section fabric (a) after loading with nanosilver oxide and (b) after loading with nanocopper oxide.
3.2. Effect of the Two Types of Nanometal Oxides on the Removal of Bacteria Using Disk Diffusion Method
The two types of in situ nanometal oxides fabrics, namely, nAg2O and nCuO were examined for the removal of bacterial indicators of pollution using the disk diffusion test. Table 1 shows that nAg2O had a higher bactericidal effect than nCuO against both Gram-negative and Gram-positive examined strains. It was also clear that both of nAg2O and nCuO have higher bactericidal effect against Gram-negative strains than Gram-positive strains. This might be due to the fact that Gram-positive bacteria are more resistant than Gram-negative bacteria since they have thicker cell wall. Our results are in good agreement with other studies [14, 15].
Table 1.
Effect of nAg2O and nCuO on microbial removal using agar diffusion disk test.
| Types of polyester nanoparticles filters | Gram-negative | Gram-positive | ||
|---|---|---|---|---|
| Escherichia coli | Salmonella typhi | Staphylococcus aureus | Enterococcus faecalis | |
| nAg2O | +++ | +++ | ++ | + |
| nCuO | ++ | + | ++ | − |
+: low effect (<5 mm diameter); ++: moderate effect (5–15 mm diameter); +++: high effect (>15 mm diameter); −: no effect.
3.3. Mobility of Nano-Ag-Oxide and Its Effect on the Morphology of Polyester Fabric
It is recognized that nanoparticles may have undesirable and unforeseen effects on the environment and in the ecosystem [16, 17]. Therefore, water-leaching test was carried out in this study in order to estimate the actual mobility of nano-Ag-oxide that can be obtained by applying simple one-stage leaching test. The test involved mixing of the fabric with deionized water at a liquid to solid ratio (L : S) 10 : 1 and agitation of the mixture for 12, 24, and 48 h then filtration and determination of dissolved species in the filtrate. The results obtained showed that the concentration of silver in the leachate did not exceed 0.01 mg/L even after 48 h exposure. These results suggest that the bactericidal action requires a close contact of microorganisms with the microbial fabric filter rather than being due to the release of metal ions in solution [4, 5].
3.3.1. Morphology of Fabric
The morphology of fabric using the SEM micrographs after nano-Ag-oxide (nAg2O) loading is presented in Figure 4. The SEM image after loading with nAg2O indicated that it was well distributed inside the fabric. For the determination of silver content, energy-dispersive X-ray spectroscopy (EDX) analysis was conducted on 30 μm of fabric. The calculated silver ratio was 0.43% which was almost in agreement with that determined by atomic absorption spectrometry (0.46%). This indicated that fabricated filter contains 1.27 mg/cm3 of silver nanoparticles.
Figure 4.
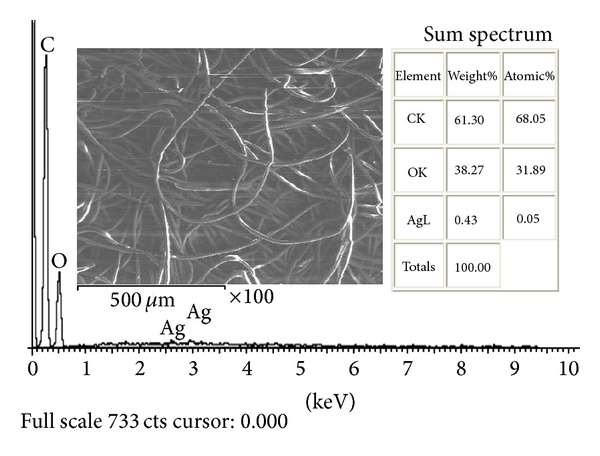
SEM-EDX analysis of fabric after nAg2O loading.
To confirm the morphology of the fabric used, it was treated with gold (Au) layer and recognized by Scanning Electron Microscopy (SEM). The energy dispersive spectrometer (EDS) spectrum for the fabric after firing was also recorded. The SEM image indicated that Ag was well distributed inside the fabric as shown in Figure 5.
Figure 5.
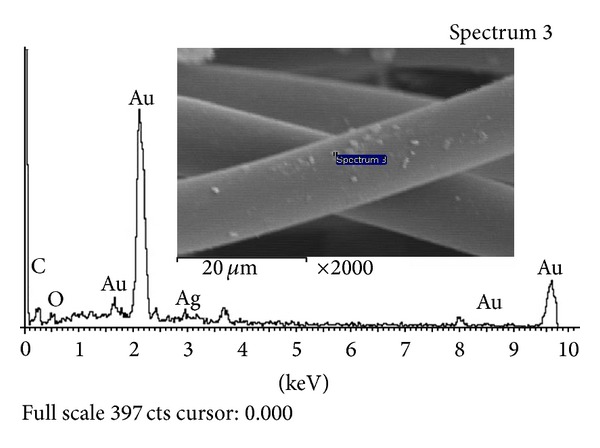
SEM-EDX micrograph with Au layer for fabric after nAg2O loading.
3.4. Effect of Contact Time for Bacterium-Nano-Ag2O Interaction
The effect of contact time of nano-Ag2O fabric and three bacterial strains (Staphylococcus aureus, Escherichia coli and Salmonella typhi) at different contact times varying from 15 min to 24 h is shown in Table 2. A control of fabric without nano-Ag2O was used. The percent reductions in bacterial density after contact time of 60 min were 64.5, 73.9, and 67.2% for Staphylococcus aureus, Escherichia coli, and Salmonella typhi, respectively. Gradual increase of the contact time improved the removal efficiency of the different bacterial strains under investigation. An almost quantitative reduction of bacterial density was achieved after 180 min. The results indicated that n-Ag2O had a strong disinfectant effect on the examined bacterial strains. These results are in agreement with other studies [18, 19].
Table 2.
Effect of contact time for bacterial reduction using nano-Ag-oxide.
| Bacterial strain (CFU/100 mL) |
Contact time | CFU/100 mL | % of removal | |
|---|---|---|---|---|
| Control of fabric without nAg2O | Fabric with nAg2O | |||
| Staphylococcus aureus 3.1 × 104 | *C 0 | 3.0 × 104 | 2.4 × 104 | 22.7 |
| 15 min | 3.3 × 104 | 2.1 × 104 | 32.3 | |
| 30 min | 3.4 × 104 | 1.6 × 104 | 48.4 | |
| 60 min | 3.7 × 104 | 1.1 × 104 | 64.5 | |
| 120 min | 4.1 × 104 | 1.1 × 103 | 96.5 | |
| 180 min | 4.7 × 104 | 8.6 × 102 | 97.2 | |
| 24 h | 5.1 × 104 | 1.2 × 102 | 99.6 | |
|
| ||||
| Escherichia coli 4.6 × 104 | C 0 | 4.6 × 104 | 3.2 × 104 | 30.4 |
| 15 min | 4.8 × 104 | 2.1 × 104 | 54.3 | |
| 30 min | 5.0 × 104 | 1.6 × 104 | 65.2 | |
| 60 min | 5.4 × 104 | 1.2 × 104 | 73.9 | |
| 120 min | 5.8 × 104 | 4.2 × 103 | 90.8 | |
| 180 min | 6.1 × 104 | 6.2 × 102 | 98.7 | |
| 24 h | 6.9 × 104 | 1.1 × 102 | 99.8 | |
|
| ||||
| Salmonella typhi 6.4 × 103 | C 0 | 6.4 × 103 | 5.2 × 103 | 18.8 |
| 15 min | 6.5 × 103 | 3.9 × 103 | 39.1 | |
| 30 min | 6.8 × 103 | 3.7 × 103 | 42.2 | |
| 60 min | 7.1 × 103 | 2.1 × 103 | 67.2 | |
| 120 min | 7.4 × 103 | 1.0 × 103 | 84.4 | |
| 180 min | 7.7 × 103 | 6.2 × 102 | 90.3 | |
| 24 h | 1.1 × 104 | 10.1 × 10 | 99.8 | |
*C 0: the concentration at zero retention time; minutes: min; hours: h.
3.5. Efficiency of Nanosilver Oxide Fabric for Disinfection of Secondary Treated Domestic Wastewater: A Case Study
Physicochemical and bacterial indicators analyses of raw and secondary treated domestic wastewater effluent are shown in Table 3. Effluent from the integrated treatment system was disinfected using the NWPF loaded with n-Ag2O.
Table 3.
Physicochemical and bacterial indicators of raw and treated wastewater*.
| Parameters | Raw wastewater | Treated effluent** |
|---|---|---|
| pH | 7.0 ± 0.14 | 7.3 ± 0.22 |
|
| ||
| Total suspended solids (TSS) mg/L | 200 ± 50.88 | 10.90 ± 4.39 |
|
| ||
| Chemical oxygen demand (COD) mgO2/L | 320 ± 52.9 | 60.30 ± 9.10 |
|
| ||
| Biological oxygen demand (BOD) mgO2/L | 190 ± 28.39 | 34.60 ± 5.23 |
|
| ||
| Total coliform (MPN-index/100 mL) | 2.8 × 107 | 1.1 × 103 |
|
| ||
| Fecal coliform (MPN-index/100 mL) | 1.5 × 107 | 2.8 × 102 |
*Average of 25 samples; **effluent from the integrated treatment system.
The effect of exposure time of nano-Ag-oxide incorporated in nonwoven polyester fabric, on the bacteria, was investigated. The results depicted in Table 4 show that the total coliform was reduced by around two orders of magnitude (from 5700 to 18 MPN-index/100 mL) after 10 min with a removal rate of 99.6%.
Table 4.
Effect of contact time for reduction of total and fecal coliforms from secondary treated effluent using nano-Ag-oxide.
| Time | Control | Nano-Ag-oxide | ||||
|---|---|---|---|---|---|---|
| Total coliforms (MPN-index/100 mL) | Fecal coliforms (MPN-index/100 mL) | Total coliforms | Fecal coliforms | |||
| Conc. (MPN-index/100 mL) |
% of removal | Conc. (MPN-index/100 mL) |
% of removal | |||
| Zero time | 4.1 × 103 | 1.8 × 102 | 4.1 × 103 | 0.0 | 1.8 × 102 | 0.0 |
| 2 min | 4.6 × 103 | 1.7 × 102 | 1.1 × 103 | 73.2 | 1.4 × 102 | 22.2 |
| 5 min | 5.1 × 103 | 2.1 × 102 | 7.5 × 102 | 81.7 | 1.1 × 102 | 38.9 |
| 10 min | 5.7 × 103 | 2.6 × 102 | 1.8 × 10 | 99.6 | 1.5 × 10 | 91.7 |
| 15 min | 6.2 × 103 | 3.1 × 102 | 1.5 × 10 | 99.6 | 1.0 × 10 | 94.4 |
| 20 min | 6.4 × 103 | 4.1 × 102 | 1.1 × 10 | 99.7 | 5.3 | 97.1 |
| 30 min | 6.8 × 103 | 5.8 × 102 | 1.0 × 10 | 99.8 | 5 | 97.2 |
Also, fecal coliforms were reduced by one order of magnitude (from 260 to 15 MPN-index/100 mL) after 10 min. These residual values are far less than the concentration of coliforms stated in the Egyptian Code of Standards (501-2005) for reuse of wastewater for agricultural purposes (1000 MPN-index/100 mL) [20]. However, an exposure time of 30 minutes achieved almost complete removal for both total coliforms and fecal coliform. From this study, it can be concluded that a very satisfactory disinfection technology for a secondary treated wastewater can be achieved using nAg2O fabric filter with a suitable contact time. The results are in agreement with the studies for the use of silver as a disinfectant of generated wastewater especially from hospitals containing infectious microorganisms [21–24].
The results were also confirmed by the image shown in Figure 1 taken by a scanning electron microscope, where the image of the fabric filter has a small pore size (7.2 μm), allowing it to act as a bacterial filter which reserves coliforms cells. Also, the small pore size of the filter allows more contact time between coliforms and nanoparticles that lead to more death of coliforms cells and consequently lead to nanoparticle incorporating in the cell membrane of microbes causing leakage of intracellular substances and eventually causing cell death [25–27]. Figure 6 shows the cross-section of treated polyester fabric taken after disinfection of coliforms where the dead cells were incorporated in the fabric and at the surface too.
Figure 6.
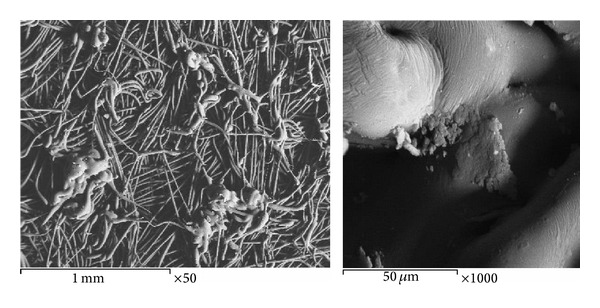
Cross-sections of treated polyester fabric taken after disinfection.
4. Conclusion
The results indicated that bactericidal effect of nano-Ag-oxide is a promising alternative technique to the traditional chemical disinfectants which generate harmful disinfection byproducts. Silver nanoparticles are stable and are not washed away by water leaching test after 48 h. In situ deposition of nanometal oxides into nonwoven polyester fabrics proved to be a very effective antibacterial filter against Escherichia coli, Salmonella typhi, Staphylococcus aureus, and Enterococcus faecalis providing enough time to penetrate the multistage cell wall. However, after only 10 minutes and by using 1.27 mg/cm3 of nano-Ag-oxide fabric, more than 90% of coliforms removal was achieved from secondary treated wastewater. The residual value did not exceed 18 and 15 MPN-index/100 mL, respectively. These residual values are far less than 1000 MPN-index/100 mL which is the concentration of coliforms stated in the Egyptian Code of Standards (501-2005) for reuse of wastewater in agricultural purposes.
Acknowledgments
The authors would like to thank the Science and Technology Development Fund and the Egyptian Academy of Scientific Research and Technology for funding this project under Grant no. 1088.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Kolar M, Urbanek K, Latal T. Antibiotic selective pressure and development of bacterial resistance. International Journal of Antimicrobial Agents. 2001;17:357–363. doi: 10.1016/s0924-8579(01)00317-x. [DOI] [PubMed] [Google Scholar]
- 2.Krasner SW, Weinberg HS, Richardson SD, et al. Occurrence of a new generation of disinfection byproducts. Environmental Science and Technology. 2006;40(23):7175–7185. doi: 10.1021/es060353j. [DOI] [PubMed] [Google Scholar]
- 3.Trussell RR. Treatment for the control of disinfectant byproducts and disinfectant residuals. In: Craun GF, editor. Safety of Water Disinfection: Balancing Chemical and Microbial Risks. Washington, DC, USA: International Life Science Institute (ILSI); 1993. [Google Scholar]
- 4.Li Q, Mahendra S, Lyon DY, et al. Antimicrobial nanomaterials for water disinfection and microbial control: potential applications and implications. Water Research. 2008;42(18):4591–4602. doi: 10.1016/j.watres.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 5.Morones JR, Elechiguerra JL, Camacho A, et al. The bactericidal effect of silver nanoparticles. Nanotechnology. 2005;16(10):2346–2353. doi: 10.1088/0957-4484/16/10/059. [DOI] [PubMed] [Google Scholar]
- 6.Savage N, Diallo MS. Nanomaterials and water purification: opportunities and challenges. Journal of Nanoparticle Research. 2005;7(4-5):331–342. [Google Scholar]
- 7.Vaseashta A, Dimova-Malinovska D. Nanostructured and nanoscale devices, sensors and detectors. Science and Technology of Advanced Materials. 2005;6(3-4):312–318. [Google Scholar]
- 8.Comini E. Metal oxide nano-crystals for gas sensing. Analytica Chimica Acta. 2006;568(1-2):28–40. doi: 10.1016/j.aca.2005.10.069. [DOI] [PubMed] [Google Scholar]
- 9.Raveh A, Zukerman I, Shneck R, Avni R, Fried I. Thermal stability of nanostructured superhard coatings: a review. Surface and Coatings Technology. 2007;201(13):6136–6142. [Google Scholar]
- 10.Gouda M, Hebeish A. Preparation and evaluation of CuO/chitosan nanocomposite for antibacterial finishing cotton fabric. Journal of Industrial Textiles. 2010;39(3):203–214. [Google Scholar]
- 11.APHA, AWWA, WEF. Standard Methods for Examination of Water and WasteWater. 22nd edition. Washington D.C: 2005. [Google Scholar]
- 12.Case CL, Johnson TR. Laboratory Experiments in Microbiology. San Francisco, Calif, USA: Benjamin Cummings; 1984. [Google Scholar]
- 13.Abou-Elela SI, Fawzy ME, Abdel-Halim W. Packed bed up-flow anaerobic sludge blanket combined with multistage sand fine roughing filtration for municipal wastewater treatment and reuse. International Journal of Sustainable Development and Planning. 2013;8:506–516. [Google Scholar]
- 14.Kumar VS, Nagaraja BM, Shashikala V, et al. Highly efficient Ag/C catalyst prepared by electro-chemical deposition method in controlling microorganisms in water. Journal of Molecular Catalysis A. 2004;223(1-2):313–319. [Google Scholar]
- 15.Li Z, Lee D, Sheng X, Cohen RE, Rubner MF. Two-level antibacterial coating with both release-killing and contact-killing capabilities. Langmuir. 2006;22(24):9820–9823. doi: 10.1021/la0622166. [DOI] [PubMed] [Google Scholar]
- 16.Long TC, Saleh N, Tilton RD, Lowry GV, Veronesi B. Titanium dioxide (P25) produces reactive oxygen species in immortalized brain microglia (BV2): implications for nanoparticle neurotoxicity. Environmental Science and Technology. 2006;40(14):4346–4352. doi: 10.1021/es060589n. [DOI] [PubMed] [Google Scholar]
- 17.Porter AE, Muller K, Skepper J, Midgley P, Welland M. Uptake of C60 by human monocyte macrophages, its localization and implications for toxicity: studied by high resolution electron microscopy and electron tomography. Acta Biomaterialia. 2006;2(4):409–419. doi: 10.1016/j.actbio.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 18.Denyer SP, Stewart GSAB. Mechanisms of action of disinfectants. International Biodeterioration and Biodegradation. 1998;41(3-4):261–268. [Google Scholar]
- 19.Russell AD. Bacterial resistance to disinfectants: present knowledge and future problems. Journal of Hospital Infection. 1999;43(1):S57–S68. doi: 10.1016/s0195-6701(99)90066-x. [DOI] [PubMed] [Google Scholar]
- 20.Egyptian Code (ECP) 501-2005. Egyptian Standards for Use of Treated Wastewater in Agriculture. 2005. [Google Scholar]
- 21.Lin Y-SE, Vidic RD, Stout JE, McCartney CA, Yu VL. Inactivation of Mycobacterium avium by copper and silver ions. Water Research. 1998;32(7):1997–2000. [Google Scholar]
- 22.Lin Y-SE, Vidic RD, Stout JE, Yu VL. Individual and combined effects of copper and silver ions on inactivation of Legionella pneumophila. Water Research. 1996;30(8):1905–1913. [Google Scholar]
- 23.Blanc DS, Carrara P, Zanetti G, Francioli P. Water disinfection with ozone, copper and silver ions, and temperature increase to control Legionella: seven years of experience in a university teaching hospital. Journal of Hospital Infection. 2005;60(1):69–72. doi: 10.1016/j.jhin.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 24.Gouda M. Nano-zirconium oxide and nano-silver oxide/cotton gauze fabrics for antimicrobial and wound healing acceleration. Journal of Industrial Textiles. 2012;41(3):222–240. [Google Scholar]
- 25.Sondi I, Salopek-Sondi B. Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. Journal of Colloid and Interface Science. 2004;275(1):177–182. doi: 10.1016/j.jcis.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 26.Cho K-H, Park J-E, Osaka T, Park S-G. The study of antimicrobial activity and preservative effects of nanosilver ingredient. Electrochimica Acta. 2005;51(5):956–960. [Google Scholar]
- 27.Yoon K-Y, Hoon Byeon J, Park J-H, Hwang J. Susceptibility constants of Escherichia coli and Bacillus subtilis to silver and copper nanoparticles. Science of the Total Environment. 2007;373(2-3):572–575. doi: 10.1016/j.scitotenv.2006.11.007. [DOI] [PubMed] [Google Scholar]


