Abstract
Hyperthyroidism causes increased energy intake and expenditure, although anorexia and higher weight loss have been reported in elderly individuals with hyperthyroidism. To determine the effect of age on energy homeostasis in response to experimental hyperthyroidism, we administered 200 μg tri-iodothyronine (T3) in 7- and 27-mo-old rats for 14 d. T3 increased energy expenditure (EE) in both the young and the old rats, although the old rats lost more weight (147 g) than the young rats (58 g) because of the discordant effect of T3 on food intake, with a 40% increase in the young rats, but a 40% decrease in the old ones. The increased food intake in the young rats corresponded with a T3-mediated increase in the appetite-regulating proteins agouti-related peptide, neuropeptide Y, and uncoupling protein 2 in the hypothalamus, but no increase occurred in the old rats. Evidence of mitochondrial biogenesis in response to T3 was similar in the soleus muscle and heart of the young and old animals, but less consistent in old plantaris muscle and liver. Despite the comparable increase in EE, T3's effect on mitochondrial function was modulated by age in a tissue-specific manner. We conclude that older rats lack compensatory mechanisms to increase caloric intake in response to a T3-induced increase in EE, demonstrating a detrimental effect of age on energy homeostasis.—Walrand, S., Short, K. R., Heemstra, L. A., Novak, C. M., Levine, J. A., Coenen-Schimke, J. M., Nair, K. S. Altered regulation of energy homeostasis in older rats in response to thyroid hormone administration.
Keywords: aging, food intake, spontaneous physical activity, mitochondria
Thyroid hormones play a pivotal role in the regulation of energy homeostasis, including thermogenesis and basal metabolic rate. Administration of tri-iodothyronine (T3), the most active form of thyroid hormone, to young animals increases metabolic rate, body temperature, and heart rate. Hyperthyroidism also increases spontaneous activity and nonexercise activity thermogenesis (1). Despite a reduction in adipose tissue and skeletal muscle mass, an increase in food intake has been reported in young rats after administration of T3 (2, 3). T3 may stimulate feeding through specific action on the hypothalamus (4). The increase in appetite may help compensate for the negative energy balance that typically accompanies hyperthyroidism. It remains to be determined whether age has any effect on T3's effect on energy metabolism and food intake.
Changes in circulating thyroid hormones may take place during aging. Hyperthyroidism can occur at any age, but in older people there may be fewer hyperadrenergic signs and an increased incidence of weight loss (5). Whether aging modifies the effect of hyperthyroidism on the regulation of appetite, energy balance, and tissue metabolism is unresolved. Addressing this question could help explain the clinically observed excess weight loss that occurs in the elderly with hyperthyroidism.
Mitochondria play a central role in energy metabolism and are an important target of thyroid hormones (2). Extensive mitochondrial changes occur in response to either excess endogenous or exogenous thyroid hormone (6). Tissues isolated from hyperthyroid rats display increased oxygen consumption, substrate oxidation, and activities of oxidative enzymes (6). When young, healthy male rats were made hyperthyroid by administration of T3 for 14 d (2), we observed an increased capacity for oxidative fuel metabolism, as shown by changes in mitochondrial enzymes and the ATP production rate in red slow-twitch skeletal muscle, heart, and liver. The expression of uncoupling protein 2 (UCP2) and UCP3 was also enhanced at the transcript and protein levels in skeletal muscle of hyperthyroid animals (2). Thus, thyroid hormone stimulates energy consumption by driving both coupled oxidative phosphorylation, leading to ATP production, and uncoupled respiration, leading to heat loss and energy wasting (6). In addition, the rates of skeletal muscle contraction and relaxation are stimulated by thyroid hormone, as are the energy consumption and heat production associated with activity. Quantitative and qualitative changes in substrate metabolism accommodate the increase in ATP turnover. Because of the total mass of skeletal muscle, these changes affect whole-body physiology. In the hypothalamic arcuate nucleus of the brain, thyroid hormone promotes UCP2-dependent mitochondrial uncoupling, leading to increased food intake (7), which may help offset the rapid weight loss that would otherwise occur.
We used an experimental rodent model to address the hypothesis that aging alters the effect of experimental hyperthyroidism on energy expenditure (EE), physical activity, and food intake, and that these differences could be explained in part by differential responses in hypothalamic proteins controlling appetite and tissue mitochondrial functions affecting the potential for energy utilization. Exogenous T3 was delivered by subcutaneous diffusion pump in both young and old rats, and the outcomes were compared to age-matched control rats receiving only the saline vehicle. Because old rats treated with T3 consistently ate less food than old controls ate, an additional group of old rats were pair fed (PF) with the T3 group, to determine the effects attributable to T3 treatment beyond those associated with reduced energy intake.
MATERIALS AND METHODS
Animals
Young (7 mo) and old (27 mo) male Fischer 344 (F344)/Brown Norway (BN) F1 hybrid rats (F344 X BN) were obtained from colonies maintained by Harlan Labs (Indianapolis, IN, USA) on behalf of the U.S. National Institute on Aging (NIA). F344/BN rats were used because they have a lower prevalence and a delayed onset of tumors and kidney failure in old age than do other strains (8). They also display loss of muscle mass and strength, increased abdominal fat, and increased insulin resistance, which make them good models for human aging (9). A detailed necropsy was performed on each animal; those with tumors or other pathologic changes were excluded from the analyses. Experiments were conducted after approval by the Institutional Animal Care and Use Committee of the Mayo Clinic.
Experimental protocol
Animals were delivered to our facility 7–10 d before the study, to allow acclimatization. The animals were housed in individual cages within the same controlled environment (12:12 h light-dark cycle, 20–22°C, 50–60% relative humidity). The rats were fed ad libitum or PF with the NIH31 chow diet [protein 18.5% and fat 4.5%, 73% carbohydrate (CHO)] as recommended by NIA. Food was delivered in ceramic bowls designed not to tip over. The animals and food bowls were weighed on alternate days throughout the study. Water was provided ad libitum.
Young and old animals were randomly assigned to receive either T3 or the vehicle as a placebo (n=20/group). We used T3, not thyroxine or T4 (i.e., the common treatment for hypothyroidism) because the latter acts more like a prohormone and needs to be deiodinated into T3 by thyroxine 5′-deiodinase to be fully active. It has been reported (10) that thyroxine 5′-deiodinase activity declines with aging. For these reasons and because our main objective was to study the adaptation to hyperthyroidism during aging without confounding elements, we used the T3 hormone.
Since T3 treatment may induce anorexia in old rats, a PF old group was included. Animals in the PF old rat group were treated with the vehicle only and received the same absolute amount of food as eaten by the old T3-treated animals. Because the PF rats tended to be larger at the beginning of the study and because thyroid hormone causes weight loss due to both anorexia and increased EE, lower food consumption was observed in the PF rats than in their age-matched T3-treated counterparts when expressed per gram of body weight.
T3 was administrated for 14 d via a subcutaneous osmotic pump (Alzet; Alza Sci Products, Palo Alto, CA, USA) surgically implanted above the shoulders, slightly posterior to the scapulae. The pump delivered a supraphysiological dose of 200 μg/d of T3. The dose used in the present study is the same as that used in our previous studies in young rats (1, 2, 11). This dose up-regulated major metabolic (mitochondrial) pathways at the tissue level and increased whole-body EE and food intake. These are well-recognized clinical sequelae observed during hyperthyroidism in humans. Vehicle-only (saline) pumps were implanted in control and PF animals for 14 d. The pumps were implanted without a catheter attachment, so the contents were delivered into the local subcutaneous space. Absorption of the compound (T3) by local capillaries resulted in systemic administration (2, 11). After surgery, the animals were kept on a warming bed for a few hours. Food intake was monitored daily after the surgical implantation of the pump. The animals had a slight reduction in food intake for 1–2 d and then resumed normal eating and physical activity when infused with saline.
On d 14, 24-h measurement of EE was performed on 10 rats from each group with a customized, single-chamber, indirect calorimeter (diameter, 30 cm; height, 20 cm; volume, 15 L) containing the food and water bowls. The calorimeter was housed in a sound- and light-proof, purpose-built room with filtered air. The light-dark cycle, temperature, and humidity mimicked the conditions the animals were housed in throughout the experiment. Spontaneous physical activity was simultaneously determined with the EE measurements by using a customized, high-precision array of 45 infrared activity beams crossing the cage. This system permits simultaneous detection of activity on 3 axes: forward and backward, side to side, and up and down.
After the EE measurements, the 10 rats/group were anesthetized by intraperitoneal injection of Na-pentobarbital (Nembutal; Lundbeck, Copenhagen, Denmark). Skeletal muscles (soleus, plantaris), liver, heart, and epididymal and perirenal adipose tissues were then quickly removed, and the hypothalamus was dissected from the brain. Samples of hind limb muscles, liver, and heart were kept on ice in saline-soaked gauze, so that mitochondrial ATP production could be studied in fresh tissue. The remaining portion of each tissue was immediately frozen in liquid nitrogen and used for other analyses. Blood samples were obtained via cardiac puncture.
The remaining 10 rats/group were euthanized by CO2 asphyxiation. After rapidly rapid shaving to remove body hair, each rat was entirely and immediately frozen in liquid nitrogen and placed in a double-sealed bag. They were sent on dry ice to the New York Obesity Research Center (St. Luke's-Roosevelt Hospital, New York, NY, USA; operated by Drs. Carol N. Boozer and Joseph R Vasselli) for the determination of body composition by chemical analysis. This determination was useful to determine the T3 effect on fat and fat-free mass, to allow for data normalization among groups.
Blood analysis
T3 was measured by a competitive chemiluminescence immunoassay with the ACS-180 automated immunoassay system (Bayer Diagnostics, Tarrytown, NY, USA).
DNA and RNA analyses
DNA and RNA analyses were performed as described previously (12). Briefly, DNA extraction was performed on frozen tissues with a DNA mini kit (Qiagen Inc., Valencia, CA, USA). A real-time quantitative PCR system (ABI Prism 7700; PE Biosystems, Foster City, CA, USA) was used to measure mtDNA abundance, using the mtDNA-encoded NADH dehydrogenase 1 (ND1) gene (13). The abundance of the target gene was normalized to the signal for nucleus-encoded 28S ribosomal RNA, which was coamplified in the same reaction well.
The abundance of selected mRNAs in muscle was measured with a real-time quantitative PCR system. RNA was extracted by the Trizol method (Life Technologies, Gaithersburg, MD, USA), treated with DNase (Life Technologies), and reverse-transcribed with TaqMan reverse transcription reagents (PE Biosystems). Transcripts measured included the mitochondrial components cytochrome c oxidase subunit 3 (COX3), COX4, ND4, UCP2, and UCP3, a nuclear transcription factor involved in regulation of muscle oxidative genes [peroxisome proliferator-activated receptor coactivator 1α (PGC-1α)], and the slow- and fast-twitch isoforms of the contractile protein myosin heavy chain (MHCI, MHCIIa, and MHCIIx). Samples were run in triplicate with coamplification of the target gene and 28S rRNA (as a housekeeping gene) and quantified by normalizing the target signal for the 28S rRNA signal.
Mitochondrial ATP production rate and citrate synthase (CS) activity
Mitochondria were isolated from fresh samples of skeletal muscle (soleus and plantaris), liver, and heart by using the separation procedures and buffer solutions described by Wibom et al. (14). The mitochondrial ATP production rate (MAPR) was determined by a bioluminescence technique (2). Fresh mitochondrial suspensions diluted in ATP-monitoring reagent (AMR, formula SL; BioThema AB, Dalarö, Sweden) were added to cuvettes containing AMR, substrate, and ADP. Substrates added (in a millimolar final concentration) were 10 glutamate +1 malate or 20 succinate + 0.1 rotenone, with additional blank tubes used for measuring the background. For each animal, all ATP production reactions for the 4 tissue preparations were analyzed in duplicate simultaneously. ATP production was monitored at 25°C with an automated routine in a 1251 luminometer (BioOrbit Oy, Turku, Finland). The time needed to prepare the mitochondrial suspensions (∼90 min) and perform the ATP production assay (35 min) was standard for all animals. The variation between duplicate measurements of ATP production from a given preparation was consistently <6%. Data were expressed as micromoles of ATP produced per minute per gram of tissue. CS activity in the mitochondrial preparation and a separate piece of frozen tissue was measured (2).
Mitochondrial uncoupling proteins
UCP2 and UCP3 expression in the mitochondria preparations from the soleus and plantaris muscles was measured by Western blot analysis (2, 11). Protein concentration was measured (DC Protein Assay; Bio-Rad Laboratories, Hercules, CA, USA) before samples were mixed with 4× SDS sample loading buffer (Invitrogen, Carlsbad, CA, USA), so that equal amounts of proteins were separated on NuPage Bis-Tris gels by electrophoresis (Invitrogen) and β-actin was used as a protein loading control for Western blot analysis. Proteins were transferred with an XCell II blot module (Invitrogen) to PVDF membranes. After the membranes were blocked in 5% nonfat milk, they were incubated overnight at 4°C with primary antibodies directed against UCP2 and UCP3 (Cell Signaling, Danvers, MA, USA). The blots were then incubated with horseradish peroxidase–conjugated secondary antibodies and the ECL-Plus detection system (GE Healthcare, Piscataway, NJ, USA). Images were captured on Biomax XAR film (Kodak Scientific, New Haven, CT, USA) and analyzed with Kodak Molecular Imaging software. Results were expressed as the ratio of the value for each rat to the combined values of all samples.
Hypothalamic orexigenic endpoints
To probe the potential hypothalamic origins of the blunted appetite response to T3, we dissected the hypothalamus from the rat brains. Using similar procedures Western blots and real-time quantitative PCR (with GAPDH as the reference control) were used to measure hypothalamic expression of the peptides agouti-related peptide (AgRP) and neuropeptide Y (NPY), as well as UCP2, a potential mediator of the anorexic effects of T3 (7). Because of the low Western blot detection rate of AgRP, low values (>2 se below the mean) and an outlier (>3 sd above the mean) were excluded from this analysis; for PCR, outlier values were defined as >2 sd above the mean. β-Actin was used as the protein loading control for Western blot analysis.
Statistical analysis
The statistical program Statview 4.02 (Abacus Concepts, Berkley, CA, USA) was used for all statistical analyses. Data are presented as means ± sem. Analysis of variance (ANOVA) for repeated measures was used to determine treatment effects on body mass and food intake. Two-way ANOVA and Fisher's post hoc test were used to detect interaction between T3 and age. Linear regression analysis was used to assess associations among selected outcomes. After 1 outlier was removed (>5 sem below the mean for 1 young control rat), analysis of covariance (ANCOVA) was used to compare EE between groups, with T3 and age as the independent variables and fat-free mass as the covariate. P < 0.05 was considered to be statistically significant.
RESULTS
Whole-body and hypothalamus responses
Serum T3 significantly increased in both young and old T3-treated animals, compared with vehicle-treated controls and old PF rats (Table 1). After the 14-d treatment with T3, body mass decreased by 13% in the young rats, despite a 40% increase in their food intake (Table 1). The decline in body weight was almost twice as high in the old T3 group (−24%,) as in the young T3 group. In contrast to the young rats, food intake was reduced in the old T3 rats by ∼40% (Table 1).
Table 1.
Body weight and composition, food intake, tissue weight, T3 level, energy expenditure, and physical activity in young and old rats
| Parameter | Young rats |
Old rats |
|||
|---|---|---|---|---|---|
| Control | T3 | Control | T3 | PF | |
| Body weight (g) | |||||
| Before T3 treatment | 441 ± 10 | 441 ± 9 | 601 ± 8 | 603 ± 11 | 635 ± 7 |
| After T3 treatment | 431 ± 9 | 383 ± 8* | 556 ± 7* | 457 ± 11*,# | 542 ± 6 |
| Food intake | |||||
| Before T3 treatment (g·d−1) | 19.2 ± 0.4 | 19.9 ± 0.5 | 16.7 ± 0.7 | 16.8 ± 0.9 | 15.8 ± 1.0 |
| After T3 treatment (g·d−1) | 18.1 ± 0.9 | 27.5 ± 1.5*,# | 16.6 ± 0.9 | 10.4 ± 3.5*,#,$@ | 10.4 ± 0.0 |
| After T3 treatment (mg·d−1·g BM−1) | 44.6 ± 0.3 | 52.1 ± 0.3# | 29.4 ± 0.2@ | 36.8 ± 0.6#,$@ | 24.2 ± 0.5 |
| Body composition after T3 treatment (g) | |||||
| Protein | 85.0 ± 6.7 | 76.3 ± 4.4 | 97.6 ± 5.9 | 83.1 ± 3.8# | 91.0 ± 2.0 |
| Fat | 42.7 ± 1.5 | 20.6 ± 1.2# | 133.0 ± 7.9$ | 69.3 ± 5.9#,@ | 124.8 ± 9.3 |
| Water | 282.3 ± 21.7 | 259.5 ± 7.6 | 312.2 ± 10.9 | 263.1 ± 17.1 | 291.7 ± 6.4 |
| Tissue weight after T3 treatment | |||||
| Soleus (mg) | 184.5 ± 4.9 | 145.1 ± 5.2# | 172.4 ± 4.8 | 143.4 ± 5.4# | 174.5 ± 4.7 |
| Plantaris (mg) | 440.1 ± 12.5 | 371.8 ± 12.4 | 396.6 ± 11.4$ | 327.3 ± 13.3 | 398.7 ± 9.7 |
| Liver (g) | 12.1 ± 0.4 | 12.5 ± 0.2# | 15.2 ± 0.3$ | 15.1 ± 0.8#,$@ | 12.6 ± 0.3 |
| Heart (g) | 1.0 ± 0.1 | 1.3 ± 0.1# | 1.4 ± 0.1$ | 1.8 ± 0.1#,$@ | 1.3 ± 0.1 |
| Epididymal AT (g) | 3.7 ± 0.3 | 2.3 ± 0.2# | 5.1 ± 0.2$ | 3.6 ± 0.1#,$@ | 4.7 ± 0.3 |
| Perirenal AT (g) | 3.2 ± 0.3 | 1.5 ± 0.2# | 6.3 ± 0.2$ | 3.1 ± 0.2#,$@ | 5.9 ± 0.2 |
| T3 level after T3 treatment (ng/dl) | 58.9 ± 2.4 | 505.2 ± 52.8# | 57.1 ± 2.5 | 468.4 ± 35.2#,@ | 69.0 ± 2.1 |
| Energy expenditure after T3 treatment (kcal·d−1·g FFM−1) | 0.76 ± 0.02 | 1.15 ± 0.03# | 0.82 ± 0.01 | 1.08 ± 0.04#,@ | 0.64 ± 0.01# |
| Physical activity after T3 treatment (AU·min−1) | 30.5 ± 2.2 | 30.7 ± 1.5 | 21.2 ± 0.8$ | 23.0 ± 1.4$ | 25.5 ± 2.3 |
Values are shown as means ± sem; n = 8–10 animals/group. BM, body mass; AT, adipose tissue; FFM, fat free mass; AU, arbitrary unit. Control rats received T3 vehicle as a placebo for 14 d; T3 rats received T3 for 14 d; PF rats received T3 vehicle as a placebo and the same amount of food as the T3 old group for 14 d. ANOVA with post hoc analysis.
P < 0.05 vs. before T3 treatment
P < 0.05 vs. control group (same age)
P < 0.05 vs. young (same treatment)
P < 0.05 vs. PF (same age).
Total daily EE expressed relative to fat-free body mass did not differ between the normal young and the old rats and was increased by T3 (P<0.001) in both the young (+51%) and old (+32%) animals compared with that in their age-matched controls (Table 1). In comparison, the old PF group demonstrated a 20% decline in EE compared to the old controls (P<0.05). Because the old T3-treated and PF groups were matched for energy intake, the differential changes in EE in the old rats appear to explain why T3 treatment caused greater weight loss (∼25%) than dietary energy restriction only caused in the PF group (15%, P<0.05). In a pooled analysis of all the animals, serum T3 concentration correlated positively with EE (r=0.80, P<0.0001). ANCOVA revealed significant effects of both body weight and T3 on EE (P<0.05 for the model), but no significant effect of age (P=0.157). As expected, the older rats had lower spontaneous physical activity than did their younger counterparts (∼30%, P<0.001 young vs. old rats in both control and T3-treated groups; Table 1). However, T3 treatment did not result in a change in physical activity in either young or old rats, suggesting that the increased EE was most likely attributable to tissue thermogenesis.
Whole-body fat reduction, assessed by chemical analyses, accounted for about half of the decline in body mass in the T3-treated young and old rats (Table 1). These observations were confirmed by the reduced weight of representative adipose pads of both age groups; epididymal fat mass was ∼30% less and perirenal fat mass was ∼51% less than in the controls (P<0.001). Total body protein content also tended to be lower in the T3-treated young and older groups, but the loss of lean mass was more clearly evident in hindlimb skeletal muscles. The soleus was 17–21% smaller and the plantaris 15–17% smaller in the young and old T3-treated rats vs. the controls (P<0.05). In contrast, heart mass, which was already increased in the old vs. the young animals, was enlarged in response to T3 treatment in both the young and old groups (Table 1). Liver mass was also higher in the old rats but T3 and PF treatments did not affect liver size. Pair feeding in the old rats did not result in significant changes in body composition or tissue mass compared with that of the old controls.
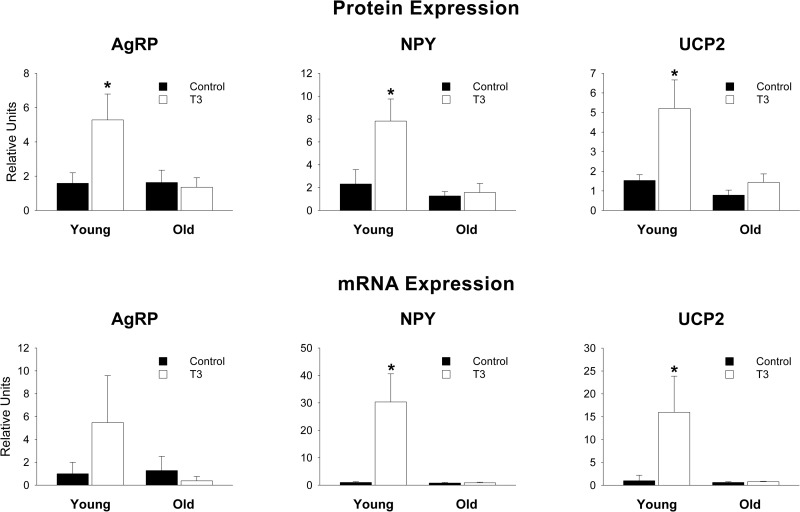
The food intake results corresponded with Western blot analyses that revealed that T3 increased hypothalamic expression of the orexigenic peptides AgRP, NPY, and UCP2 in the young rats (P<0.05 for all) but not in the old ones (P>0.045, Fig. 1). Likewise, the response pattern of hypothalamic mRNA abundance of the same genes was nearly the same as the protein, as both NPY and UCP2 expression were significantly increased by T3 in the young, but not in the old rats, with AgRP showing a similar nonsignificant trend (Fig. 1).
Figure 1.
Hypothalamic protein and mRNA levels of AgRP, NPY, and UCP2. Solid bars, control; open bars, T3-treated. T3-treated young rats had greater levels of NPY and UCP2 than all other groups. No differences were found in old T3-treated vs. old control rats. For all, P > 0.1. *P < 0.05.
Abundance of mitochondrial DNA and selected enzyme transcripts
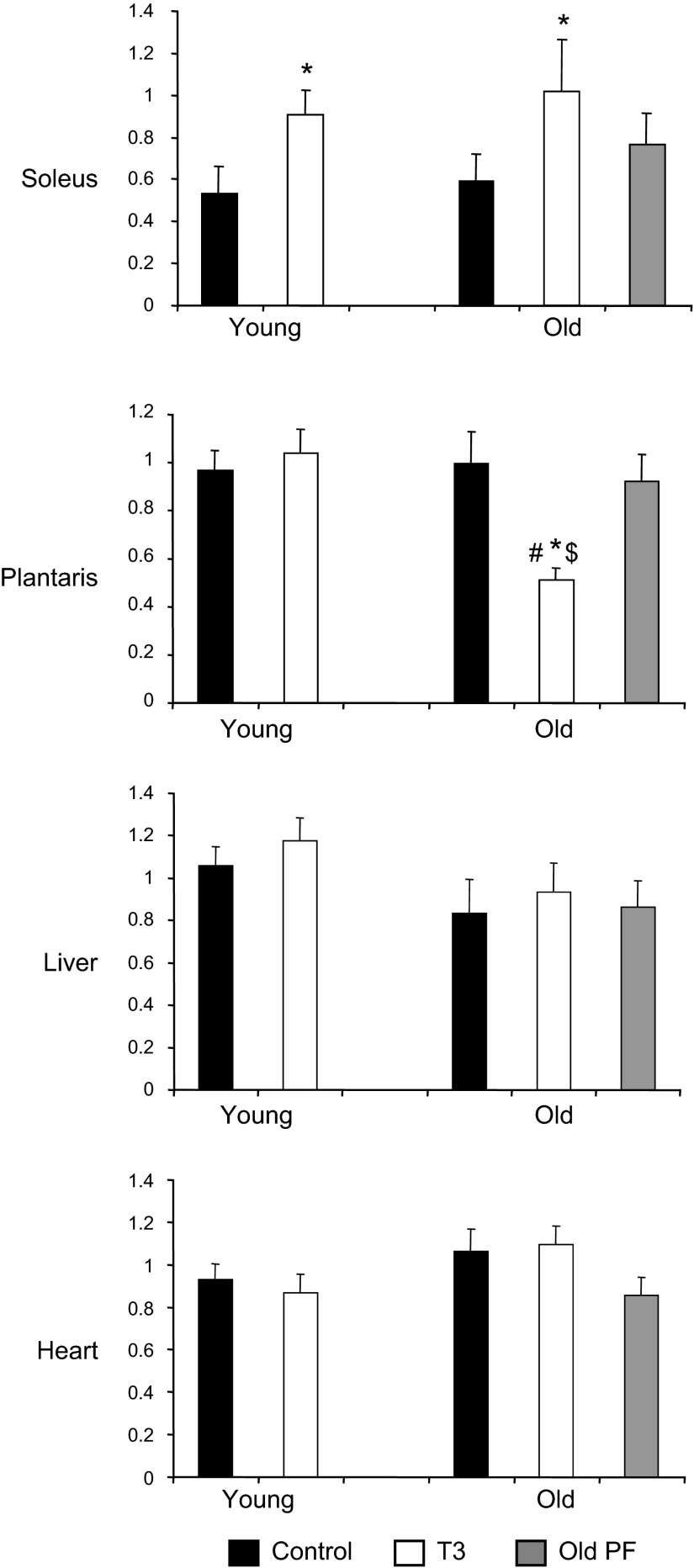
mtDNA (Fig. 2) was similarly increased by T3 in soleus muscle in both the young and old rats (+70 and +73%, respectively; P<0.01 vs. controls). In the plantaris, however, mtDNA content was unaffected by T3 in the young rats and declined in the T3-treated old ones (P<0.01). mtDNA did not differ significantly in liver and heart with age, T3 treatment, or PF.
Figure 2.
Effect of T3 on mtDNA copy number in skeletal muscle, liver, and heart tissues. Solid bars, control; open bars, T3-treated; shaded bars, PF. *P < 0.05 vs. control (same age); $P < 0.05 vs. young (same treatment); #P < 0.05 vs. PF.
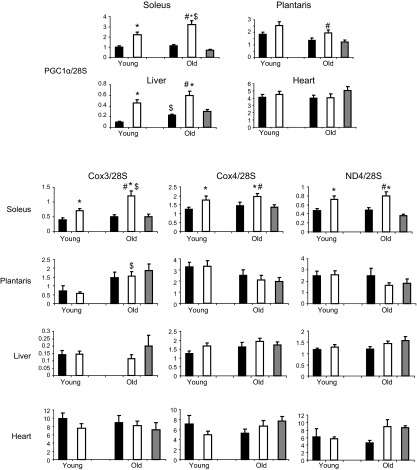
In accordance with mtDNA, transcript levels of multiple mitochondrial-regulating proteins in the soleus were increased by T3 in the young and old rats (Fig. 3), including PGC-1α (+126 and +185% in the young and old T3 rats, respectively; P<0.001 vs. controls), COX3 (+77 and +143% in the young and old T3 rats, respectively; P<0.01 vs. controls), COX4 (+41% and +34% in the young and old T3 rats, respectively; P=0.06 vs. controls), and ND4 (+53 and +65% in the young and old T3 rats, respectively; P<0.01 vs. controls). There were no other differences among groups for these same transcripts that were attributable to T3 treatment or age. Despite a marked increase in PGC-1α expression in liver tissue after T3 treatment in both age groups, the other mitochondrial gene transcripts measured were not altered by T3.
Figure 3.
Effect of T3 on PGC-1α and mitochondrial transcript levels in skeletal muscle, liver, and heart tissues of young and old rats. Solid bars, control; open bars, T3-treated; shaded bars, PF. *P < 0.05 vs. control (same age); $P < 0.05 vs. young (same treatment); #P < 0.05 vs. PF.
Neither the mtDNA abundance nor gene transcript levels in the PF group differed from those in old control rats, indicating that changes in old T3 rats were due to T3 administration and not to reduced food intake.
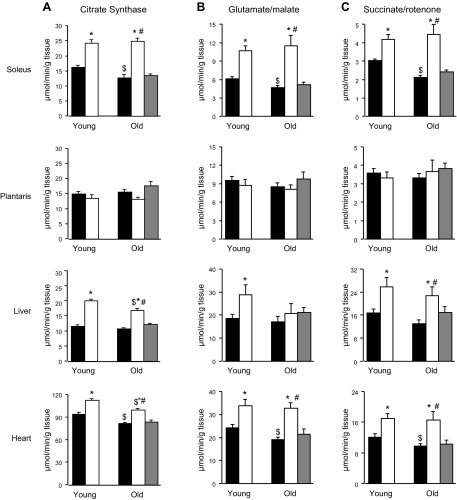
CS enzyme activity
An age-related decrease in CS activity in soleus and heart, but not in liver or plantaris, was observed in the control animals (P<0.05; Fig. 4A). T3 treatment resulted in increased CS activity in tissues of the soleus (+52 and+85% in young and old T3 rats, respectively; P<0.01 vs. controls), liver (+78 and +56% in young and old T3 rats, respectively; P<0.01 vs. controls), and heart (+19 and +22% in young and old T3 rats, respectively; P<0.05 vs. controls), but not in plantaris muscle. The reduced food intake in old PF rats did not affect CS activity.
Figure 4.
Effect of T3 on mitochondrial ATP production and CS activity in skeletal muscle, liver, and heart tissues of young and old rats. A) CS activity. B) MAPR with glutamate/malate as substrates. C) MAPR with succinate/rotenone as substrates. Solid bars, control; open bars, T3-treated; shaded bars, PF. *P < 0.05 vs. control (same age); $P < 0.05 vs. young (same treatment); #P < 0.05 vs. PF.
MAPR
The ATP production capacity varied among the tissues examined and responded differently to T3 treatment (Fig. 4B, C). MAPR was lower in soleus and heart (P<0.01 for both substrates used) in the old vs. the young control animals, but was not affected by age in plantaris muscle or liver. When succinate+rotenone, substrates that supply electrons to complex II of the respiratory chain (Fig. 4C), were used, T3-treated rats had greater MAPR in soleus muscle (+38 and +111% in the young and the old T3 rats, respectively; P<0.01 vs. controls), liver (+52 and +72% in the young and the old T3 rats, respectively; P<0.01 vs. controls), and heart (+41 and +66% in the young and the old T3 rats, respectively; P<0.01 vs. controls). Similar results were observed with glutamate+malate, which generate electrons for complex I (Fig. 4B), except that MAPR was not affected by T3 in liver of the old rats (soleus: +72 and +142% in the young and Told T3 rats, respectively; P<0.01 vs. controls; liver: +54 in the young T3 rats, P<0.05 vs. controls; and heart: +38 and +71% in the young and the old T3 rats, respectively; P<0.01 vs. controls). Like the other mitochondrial measurements, MAPR was not altered in the old PF group, showing that MAPR changes in the aged T3-treated rats were not associated with the decreased food intake.
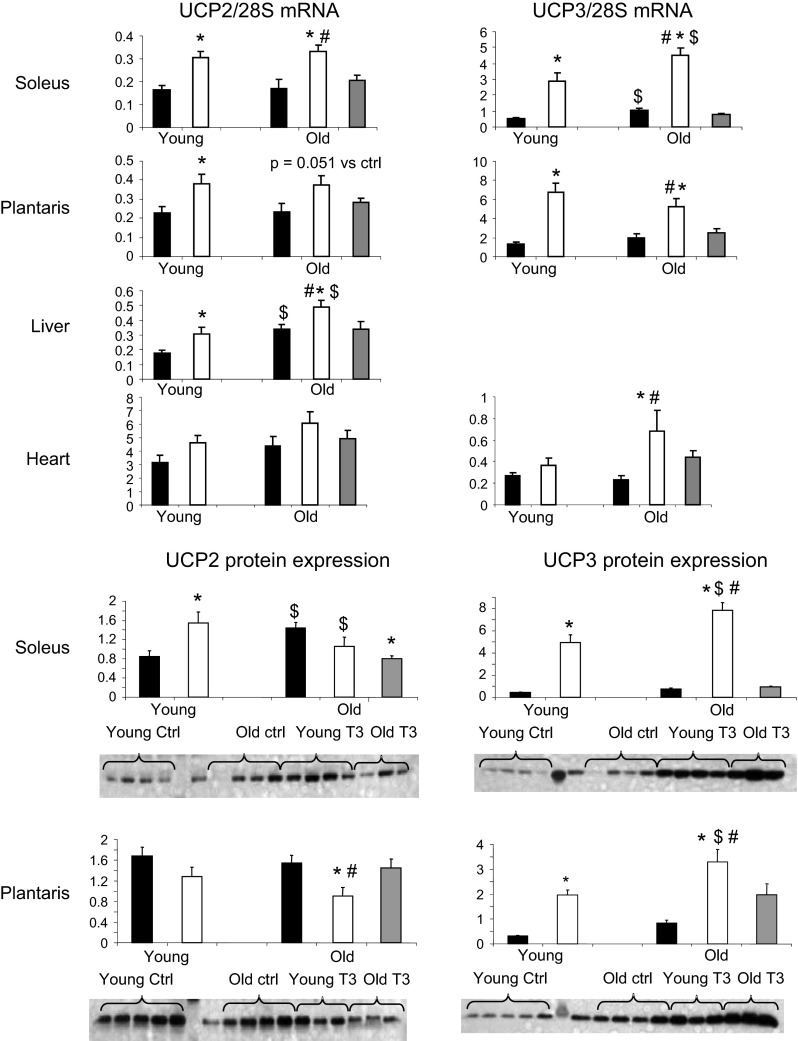
UCP transcript and protein levels
UCP2 mRNA expression was greater in T3 animals of both ages in soleus (+85 to 95%; P<0.01 vs. controls; Fig. 5), plantaris (+57 to 68%; P≤0.05 vs. controls), and liver (+45 to 72%; P≤0.05 vs. controls) tissues. The UCP2 protein level in soleus was 81% greater (P<0.001) in T3-treated young animals than in the controls (Fig. 5). UCP2 protein expression in soleus was already increased in the old vs. the young rats and did not increase further in response to T3. In the plantaris, T3 treatment had no effect on UCP2 protein content in the young rats but resulted in a 70% lower expression in the old rats (P<0.01 vs. controls).
Figure 5.
Effect of T3 on UCP2 and UPC3 mRNA level and protein expression in skeletal muscle, liver, and heart tissues of young and old rats. Solid bars, control; open bars, T3-treated; shaded bars, PF. *P < 0.05 vs. control (same age); $P < 0.05 vs. young (same treatment); #P < 0.05 vs. PF.
UCP3 transcript levels were 2.5- to 4.0-fold higher in T3 soleus and plantaris in both young and old rats (P<0.01 vs. corresponding controls) and in the heart of the old group only (+200%; P<0.001 vs. control old rats; Fig. 5). UCP3 protein response in muscle followed a pattern similar to that of mRNA, with a 3- to 6-fold increase in soleus and plantaris of both the young and old animals (P<0.001 vs. control groups). UCP3 mRNA and protein expression levels in soleus correlated positively with whole-body EE (r=0.72 for UCP3 mRNA; r=0.77 for UCP3 protein; P<0.0001 for both). Plantaris UCP mRNA (r=0.60, P=0.0007) and protein content (r=0.36, P=0.04) also correlated positively with EE. There were no differences in the PF group for either UCP2 or UCP3 expression in any of the tissues measured.
MHC transcripts
mRNA levels of MHC isoforms were measured in soleus, plantaris, and heart tissues to assess the effect of age and T3 on contractile phenotype. In control animals, the abundance of MHCI transcripts did not vary with age in any tissue, but MHCIIa transcripts were lower in soleus and plantaris of the old vs. the young rats (P<0.001; Fig. 6). In both the young and the old rats, MHCI mRNA content was reduced by T3 treatment (P<0.001 vs. controls) in plantaris and heart and in old soleus, with a trend toward reduction in young soleus. This response was attributable to T3, as MHCI was unaltered in the PF group. T3 treatment increased MHCIIa and -IIx transcripts (P<0.001) in the soleus of the young and the old rats. The PF group did not differ from the control group for these tissues.
Figure 6.
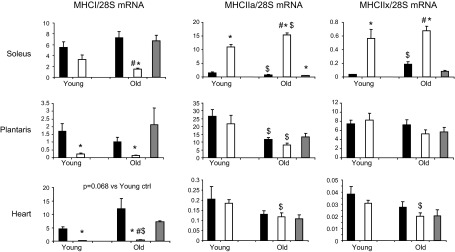
Effects of T3 on MHC mRNA in skeletal muscle and heart tissues. Solid bars, control; open bars, T3-treated; shaded bars, PF. *P < 0.05 vs. control (same age); $P < 0.05 vs. young (same treatment); #P < 0.05 vs. PF.
DISCUSSION
In the current study, both young and old rats responded similarly to a T3-induced hyperthyroid state, with increased total-body EE, muscle mitochondrial biogenesis, and oxidative capacity. In contrast, T3 promoted a rapid increase in food intake in the young rats, whereas a similar magnitude of decrease in food intake occurred in the old rats. In the older rats, unlike the young, the ability to maintain energy homeostasis by increasing the hypothalamic orexigenic peptides NPY and AgRP changed with age. Despite the substantial increase in food intake, the young animals experienced weight loss; the magnitude of weight loss was nearly 3-fold higher in the old rats. Most of the weight loss was adipose tissue in both the young and the old rats, although some lean mass catabolism was evident in the old rats, as suggested by their greater decline in whole-body protein content (14.5 g), compared to that in the old PF group (6.6 g) and the young T3 rats (8.7 g).
The present study demonstrates a clear difference between young and old rats in their ability to maintain energy homeostasis in response to an intervention that has a profound effect on EE and mitochondrial function. In the older rats, the T3-induced increase in EE was relatively less than in the young rats (32 vs. 51%, respectively), but the opposite directional changes in food intake in the young and the old animals explains the greater loss of body weight in the older group. Thus, the ability to respond to the catabolic effect of T3 with an appropriate increase in food intake was diminished in the old rats. It is unlikely that this differential feeding response to T3 was due to the relatively larger size and adiposity of the old rats. Prior studies reported that T3 does not decrease food intake in young obese rats (5, 15) and that T3 effectively increases food intake in both lean and obese young rats (15). Unlike young obese rats, old rats have a compromised ability to regain weight after food withdrawal, and central orexigenic neuropeptides are less effective in inducing appetite (16). The results from the current study demonstrated that T3 increased the hypothalamic orexigenic peptides NPY and AgRP in the young but not in the old rats. The same pattern was found for hypothalamic UCP2, a protein hypothesized to promote mitochondrial proliferation in arcuate AgRP/NPY cells, ultimately altering the excitability of these cells (7). UCP2-mediated hypothalamic mitochondrial uncoupling has been shown to increase in response to T3 (7). Notably, systemic (17, 18) or intracerebroventricular (19) leptin administration to old F344 X BN rats, the same strain as used in our study, failed both to suppress food intake and hypothalamic NPY mRNA expression, whereas it increased EE and UCP1 expression, as in the young rats. Together, the results support the hypothesis that the decreased ability of T3 to increase appetite in the old rats was due to compromised hypothalamic regulation of energy homeostasis. This corresponds to a similar clinical observation in older humans with apathetic thyrotoxicosis (20), a condition characterized by accelerated weight loss and anorexia when compared to younger people with hyperthyroidism (21).
The current study also showed that reduction in body weight in both age groups after T3 challenge was largely due to reduction in total body fat. It is well known that thyroid hormone promotes lipolysis (22, 23), but the present study shows that this T3-mediated effect is maintained with old age. The increased fat oxidation promoted by excess T3 is useful for sustaining the increased energy demand after T3 administration. In older rats, reduced energy intake in the T3-treated group was also likely to contribute to a reduction in fat depots. T3 administration also resulted in catabolism of both the soleus and the plantaris muscles (i.e., predominately type I and type II fiber muscles, respectively) in young and old animals, as has been reported (24). This corroborates results in a series of studies that demonstrated that these hindlimb muscles are highly responsive to the thyroid status in relation to protein metabolism (25). In agreement, in the present study, the loss of whole-body protein content was greater in old T3 rats compared to that in old PF rats, demonstrating the catabolic effect of T3.
We measured whether physical activity was increased by T3, since it could contribute to the elevated total EE, as we have reported (1). However, T3-treated animals in the present study were not more physically active than the euthyroid controls, although the old control rats were less active than the young control rats. In our previous study (1), total EE in young hyperthyroid animals was 5-fold higher than in the controls, whereas, in the present study, the T3-induced increase in EE was of a much lower magnitude (+31–52%). In our prior study, we used the same dose of T3 (200 μg/d) but the animal strain in the previous study was different (Sprague-Dawley), the rats were smaller (325–350 g), and the serum T3 concentration was higher (755 ng/ml) than in the present study. Thus, the lack of a measurable T3 effect on physical activity in the present study may reflect interstrain differences or variation in the dose–effect relationship. There may also be a change in physical activity detected by the use of an exercise wheel. Of note, despite a significant increase in EE, spontaneous physical activity was not significantly altered in the young or old rats after T3 administration. These data suggest that T3 treatment increased EE by stimulating basal metabolic rate. Previous studies reported a significant increase in resting metabolic rate after T3 treatment without a change in exercise efficiency (26–28).
In the current study, T3 induced significant increases in mtDNA copy number, PGC-1α and electron transport chain (ETC) gene expression, CS activity, and ATP synthesis in the soleus in both the young and the old animals. PGC-1α is a transcriptional coactivator and a master regulator of mitochondria biogenesis in many tissues (29). Therefore, increased PGC-1α gene expression appears to be a T3 target that mediates the changes in mitochondrial activity. An unexpected result in the current study was that, unlike our previous observation (12), mtDNA copy number was not reduced in the old control vs. the young control animals. As noted, we used different rat strains in the current and previous studies and, to the best of our knowledge, the present study is the first to report values for mitochondrial DNA content in skeletal muscles of young and old F344 X BN rats. In addition, there may be differential effects of aging on mitochondrial antioxidant defense systems between rat strains. For example, the age-associated change in the activity of mitochondrial antioxidant enzymes is much greater in Wistar rats than that observed in F344 rats, especially late in life (23 mo old; ref. 30). Finally, the previous studies in which mtDNA copy number declined with age showed that this change has no major effect on mitochondrial encoded transcript levels and enzyme activities in various tissues (12).
Mitochondria play a central role in the energy-transduction pathway and are a major target of thyroid hormone action (31). There was a differential response between young and old rats and among tissues in thyroid hormone response. As previously shown (2, 32), the more oxidative soleus muscle and heart were more responsive to T3 than was the less oxidative plantaris. The current study and prior reports (33, 34) show that administration of thyroid hormone to euthyroid animals results in a coordinated increase in mitochondrial gene expression, mitochondrial biogenesis, and ATP production capacity in the type I MHC, slow-twitch, red skeletal muscle fibers that predominate in muscles like the soleus.
Thyroid hormone responsive elements (TREs) have been identified on several genes, including the MHC isoforms (35, 36). As clearly shown in the present work and in previous studies (37, 38), the expression of type I MHC in the heart and skeletal muscle is regulated by T3 at the pretranslational level. Specifically, type I MHC expression is down-regulated by T3 in young and old rats in type I and II skeletal muscles and heart. This repression has been linked to a putative negative TRE located in proximity to the TATA box of the gene (39). The current study in addition has demonstrated that T3 administration had a muscle-specific effect on MHCIIa and -IIx gene transcripts, which were significantly increased in the soleus muscle only. Hence, T3 caused a type I-to-type IIa and IIx shift in the red soleus muscle, whereas a decrease in type I MHC expression was the only effect detected in the plantaris or heart. In the same way, the magnitude and specific isoform shifts resulting from hypothyroidism vary with muscle type and age (40).
In the present investigation, the plantaris, a predominantly type II, fast-twitch skeletal muscle, exhibited no detectable mitochondrial response to excess thyroid hormone in the young rats, as we previously showed (2). The differential response to T3 between the soleus and plantaris may be regulated at the transcript level, in light of the results for PGC-1α and other mitochondrial mRNA levels in these 2 muscles. Despite the age-related switch in muscle fiber type (i.e., type II to type I), the mitochondrial responses to T3 in the soleus and plantaris muscles were similar in the young and old rats, except for the mtDNA copy number in the plantaris muscle, which was reduced by T3 in the old rats. Because the mtDNA copy number was lower in the plantaris of T3-treated old rats than in their young counterparts but mitochondrial mRNA transcript levels were maintained in this tissue, there appears to be either higher transcriptional activity or enhanced mRNA stability in old plantaris after T3 treatment. The current study demonstrated that T3 increased mitochondrial ATP production in soleus similarly in the young and the old rats, as a consequence of elevated mitochondrial gene expression. An unexpected finding was that, although T3 increased mitochondrial ATP production in heart similarly in the young and the old rats, the abundance of mitochondrial DNA abundance and mitochondrial mRNA transcripts did not change in this tissue. This finding may be the result of nongenomic and nonnuclear receptor responses, since it has been reported that T3 can regulate specific cellular processes in heart tissue through direct binding to membranes or enzymes (41–44). In those studies, there was direct T3-mediated reduction of ADP concentration in the heart, mediated at least in part by the adenine nucleotide translocator (ANT), which facilitates the ADP/ATP exchange across the mitochondrial membrane.
We found that T3 induced discordant effects on ATP production measured with different substrates in liver of the old rats, with no change in ATP production when glutamate/malate was used, but an increased rate of ATP production with succinate/rotenone. This finding suggests that T3 may have distinct effects on each complex of the ETC in older animals, since glutamate/malate supplies electrons primarily to complex I, whereas succinate/rotenone supplies electrons to complex II. Although T3 stimulated mitochondrial biogenesis and ATP production in liver, the expression of selected ETC genes was not changed regardless of age. This may be attributable to T3's having a selective regulatory effect on the content or on the function (i.e., allosteric enhancement; refs. 45, 46) of proteins of the oxidative pathway according to the tissue. In contrast to UCP2, which is ubiquitously expressed (47), UCP3 is expressed primarily in striated muscle, such as skeletal muscle and heart. According to previous studies (48), there are at least 3 independent molecular actions reported for these 2 UCPs: 1) establishing a proton leak through the inner mitochondrial membrane, 2) enabling free fatty acids from the matrix to pass into the intermembrane space, and 3) contributing to Ca2+ transport across the mitochondrial membrane. Age-related increases in UCP2 and UCP3 expression in soleus muscle and liver were detected in the present study in the old rats relative to expression in the young animals. There is growing evidence that, by increasing proton leak, UCP2 and UCP3 serve to attenuate mitochondrial production of reactive oxygen species (ROS). Ablation of UCP2 and UCP3 genes in animals results in increased ROS generation in tissues such as liver (49) and skeletal muscle (50). Increased presence of UCPs in these tissues could therefore be a mechanism that attenuates ROS generation with advancing age. UCP3, because of its putative uncoupling property by proton translocation, may play a key role in the thyroid hormone–mediated increase in EE, by reducing metabolic efficiency (i.e., the uncoupling of oxidative phosphorylation). Our data suggest that advancing age modulates the ability of T3 to activate UCP3 expression in muscle cells and, therefore, the potential increase in EE by this mechanism. The age-related decline in mitochondrial function and the associated increase in ROS production in response to T3 may explain this observation.
In the current findings, hyperthyroidism affected energy metabolism in a tissue-specific manner, which may be related to both the mitochondrial content and activity of each tissue and the tissue-specific sensitivity to T3. Reduced mitochondrial adaptation to hyperthyroidism is a major age-related alteration in skeletal muscle and liver, whereas the action of T3 in heart seems to be maintained in aged rats. An important finding of the current study is the discordant effect of T3 on food intake in the young and the old animals that explains greater weight loss in the hyperthyroid state in the older rats. The results support the hypothesis that hypothalamic appetite regulation by T3 is altered with age and thus the homeostatic mechanism to maintain energy balance is altered in old age.
Acknowledgments
The authors thank Dawn Morse, Jane Kahl, and Kate Klaus for technical support with animal care and sample analyses.
Funding was provided by U.S. National Institutes of Health/National Institute on Aging grant R01-AG09531, the Mayo Foundation, the Dole-Murdock Professorship (K.S.N.), and the American Federation for Aging Research (K.R.S.).
Footnotes
- AgRP
- agouti-related peptide
- AMR
- ATP-monitoring reagent
- ANOVA
- analysis of variance
- ANCOVA
- analysis of covariance
- BN
- Brown Norway
- COX
- cytochrome c oxidase
- CS
- citrate synthase
- EE
- energy expenditure
- ETC
- electron transport chain
- F344
- Fisher 344
- MAPR
- mitochondrial ATP production rate
- MHC
- myosin heavy chain
- ND
- NADH dehydrogenase
- NIA
- National Institute on Aging
- NPY
- neuropeptide Y
- PF
- pair-fed
- PGC-1α
- peroxisome proliferator-activated receptor coactivator 1α
- ROS
- reactive oxygen species
- T3
- tri-iodothyronine
- TRE
- thyroid hormone responsive elements
- UCP
- uncoupling protein
REFERENCES
- 1. Levine J. A., Nygren J., Short K. R., Nair K. S. (2003) Effect of hyperthyroidism on spontaneous physical activity and energy expenditure in rats. J. Appl. Physiol. 94, 165–170 [DOI] [PubMed] [Google Scholar]
- 2. Short K. R., Nygren J., Barazzoni R., Levine J., Nair K. S. (2001) T3 increases mitochondrial ATP production in oxidative muscle despite increased expression of UCP-2 and -3. Am. J. Physiol. Endocrinol. Metab. 280, E761–E769 [DOI] [PubMed] [Google Scholar]
- 3. Weitzel J. M., Iwen K. A., Seitz H. J. (2003) Regulation of mitochondrial biogenesis by thyroid hormone. Exp. Physiol. 88, 121–128 [DOI] [PubMed] [Google Scholar]
- 4. Dhillo W. S. (2007) Appetite regulation: an overview. Thyroid 17, 433–445 [DOI] [PubMed] [Google Scholar]
- 5. Massoudi M., Evans E., Miller D. S. (1983) Thermogenic drugs for the treatment of obesity: screening using obese rats and mice. Ann. Nutr. Metab. 27, 26–37 [DOI] [PubMed] [Google Scholar]
- 6. Harper M. E., Seifert E. L. (2008) Thyroid hormone effects on mitochondrial energetics. Thyroid 18, 145–156 [DOI] [PubMed] [Google Scholar]
- 7. Coppola A., Liu Z. W., Andrews Z. B., Paradis E., Roy M. C., Friedman J. M., Ricquier D., Richard D., Horvath T. L., Gao X. B., Diano S. (2007) A central thermogenic-like mechanism in feeding regulation: an interplay between arcuate nucleus T3 and UCP2. Cell Metab. 5, 21–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lipman R. D., Chrisp C. E., Hazzard D. G., Bronson R. T. (1996) Pathologic characterization of Brown Norway, Brown Norway x Fischer 344, and Fischer 344 x Brown Norway rats with relation to age. J. Gerontol. Biol. Sci. 51, B54–B59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Turturro A., Witt W. W., Lewis S., Hass B. S., Lipman R. D., Hart R. W. (1999) Growth curves and survival characteristics of the animals used in the Biomarkers of Aging Program. J. Gerontol. Series 54, B492–B501 [DOI] [PubMed] [Google Scholar]
- 10. da Costa V. M., Moreira D. G., D., R. (2001) Thyroid function and aging: gender-related differences. J. Endocrinol. 171, 193–198 [DOI] [PubMed] [Google Scholar]
- 11. Short K. R., Nygren J., Nair K. S. (2007) Effect of T3-induced hyperthyroidism on mitochondrial and cytoplasmic protein synthesis rates in oxidative and glycolytic tissues in rats. Am. J. Physiol. Endocrinol. Metab. 292, E642–E647 [DOI] [PubMed] [Google Scholar]
- 12. Barazzoni R., Short K. R., Nair K. S. (2000) Effects of aging on mitochondrial DNA copy number and cytochrome c oxidase gene expression in rat skeletal muscle, liver, and heart. J. Biol. Chem. 275, 3343–3347 [DOI] [PubMed] [Google Scholar]
- 13. Short K. R., Bigelow M. L., Kahl J., Singh R., Coenen-Schimke J., Raghavakaimal S., Nair K. S. (2005) Decline in skeletal muscle mitochondrial function with aging in humans. Proc. Natl. Acad. Sci. U. S. A. 102, 5618–5623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wibom R., Lundin A., Hultman E. (1990) A sensitive method for measuring ATP-formation in rat muscle mitochondria. Scand. J. Clin. Lab. Invest. 50, 143–152 [DOI] [PubMed] [Google Scholar]
- 15. Loireau A., Dumas P., Autissier N., Michel R. (1987) Influence of thyroid status on body weight gain, food intake and serum lipid levels in genetically obese Zucker rats. J. Nutr. 117, 159–163 [DOI] [PubMed] [Google Scholar]
- 16. Wolden-Hanson T. (2006) Mechanisms of the anorexia of aging in the Brown Norway rat. Physiol. Behav. 88, 267–276 [DOI] [PubMed] [Google Scholar]
- 17. Scarpace P. J., Matheny M., Moore R. L., Tümer N. (2000) Impaired leptin responsiveness in aged rats. Diabetes 49, 431–435 [DOI] [PubMed] [Google Scholar]
- 18. Scarpace P. J., Matheny M., Shek W. (2000) Impaired leptin signal transduction with age-related obesity. Neuropharmacology 39, 1872–1879 [DOI] [PubMed] [Google Scholar]
- 19. Shek E. W., Scarpace P. J. (2000) Resistance to the anorexic and thermogenic effects of centrally administered leptin in obese aged rats. Regul. Pept. 92, 65–71 [DOI] [PubMed] [Google Scholar]
- 20. Thomas F. B., Mazzaferri E. L., Skillman T. G. (1970) Apathetic thyrotoxicosis: a distinctive clinical and laboratory entity. Ann. Intern. Med. 72, 679–685 [DOI] [PubMed] [Google Scholar]
- 21. Mooradian A. D. (2008) Asymptomatic hyperthyroidism in older adults: is it a distinct clinical and laboratory entity? Drugs Aging 25, 371–380 [DOI] [PubMed] [Google Scholar]
- 22. Villicev C. M., Freitas F. R., Aoki M. S., Taffarel C., Scanlan T. S., Moriscot A. S., Ribeiro M. O., Bianco A. C., Gouveia C. H. (2007) Thyroid hormone receptor beta-specific against GC-1 increases energy expenditure and prevents fat-mass accumulation in rats. J. Endocrinol. 193, 21–29 [DOI] [PubMed] [Google Scholar]
- 23. Wahrenberg H., Wennlund A., Arner P. (1994) Adrenergic regulation of lipolysis in fat cells from hyperthyroid and hypothyroid patients. J. Clin. Endocrinol. Metab. 78, 898–903 [DOI] [PubMed] [Google Scholar]
- 24. Hulbert A. J. (2000) Thyroid hormones and their effects: a new perspective. Biol. Rev. Camb. Philos. Soc. 75, 519–631 [DOI] [PubMed] [Google Scholar]
- 25. Angeras U., Hasselgren P. O. (1987) Protein degradation in skeletal muscle during experimental hyperthyroidism in rats and the effect of beta-blocking agents. Endocrinology 120, 1417–1421 [DOI] [PubMed] [Google Scholar]
- 26. Acheson K., Jéquier E., Burger A., Danforth E. (1984) Thyroid hormones and thermogenesis: the metabolic cost of food and exercise. Metabolism 33, 262–265 [DOI] [PubMed] [Google Scholar]
- 27. Johannsen D. L., Galgani J. E., Johannsen N. M., Zhang Z., Covington J. D., Ravussin E. (2012) Effect of short-term thyroxine administration on energy metabolism and mitochondrial efficiency in humans. PLoS ONE 7, e40837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim B. (2008) Thyroid hormone as a determinant of energy expenditure and the basal metabolic rate. Thyroid 18, 141–144 [DOI] [PubMed] [Google Scholar]
- 29. Puigserver P. (2005) Tissue-specific regulation of metabolic pathways through the transcriptional coactivator PGC1-alpha. Int. J. Obes. 29, S5–S9 [DOI] [PubMed] [Google Scholar]
- 30. Jang I., Chae K., Cho J. (2001) Effects of age and strain on small intestinal and hepatic antioxidant defense enzymes in Wistar and Fisher 344 rats. Mech. Ageing Dev. 122, 561–570 [DOI] [PubMed] [Google Scholar]
- 31. Ribeiro M. O. (2008) Effects of thyroid hormone analogs on lipid metabolism and thermogenesis. Thyroid 18, 197–203 [DOI] [PubMed] [Google Scholar]
- 32. Winder W. W., Holloszy J. O. (1977) Response of mitochondria of different types of skeletal muscle to thyrotoxicosis. Am. J. Physiol. Cell Physiol. 232, C180–C184 [DOI] [PubMed] [Google Scholar]
- 33. Bahi L., Garnier A., Fortin D., Serrurier B., Veksler V., Bigard A. X., Ventura-Clapier R. (2005) Differential effects of thyroid hormones on energy metabolism of rat slow- and fast-twitch muscles. J. Cell. Physiol. 203, 589–598 [DOI] [PubMed] [Google Scholar]
- 34. Irrcher I., Adhihetty P. J., Sheehan T. E., Joseph A. M., Hood D. A. (2003) PPARgamma coactivator-1alpha expression during thyroid hormone and contractile activity induced mitochondrial adaptations. Am. J. Physiol. Cell Physiol. 284, C1669–C1677 [DOI] [PubMed] [Google Scholar]
- 35. Long X., Boluyt M. O., O'Neill L., Zheng J. S., Wu G., Nitta Y. K., Crow M. T., Lakatta E. G. (1999) Myocardial retinoid X receptor, thyroid hormone receptor, and myosin heavy chain gene expression in the rat during adult aging. J. Gerontol. A Biol. Sci. Med. Sci. 54, B23–B27 [DOI] [PubMed] [Google Scholar]
- 36. Subramanian A., Gulick J., Neumann J., Knotts S., Robbins J. (1993) Transgenic analysis of the thyroid-responsive elements in the alpha-cardiac myosin heavy chain gene promoter. J. Biol. Chem. 268, 4331–4336 [PubMed] [Google Scholar]
- 37. Haddad F., Qin A. X., Bodell P. W., Jiang W., Giger J. M., Baldwin K. M. (2008) Intergenic transcription and developmental regulation of cardiac myosin heavy chain genes. Am. J. Physiol. Heart Circ. Physiol. 294, H29–H40 [DOI] [PubMed] [Google Scholar]
- 38. Haddad F., Qin A. X., Zeng M., McCue S. A., Baldwin K. M. (1998) Interaction of hyperthyroidism and hindlimb suspension on skeletal myosin heavy chain expression. J. Appl. Physiol. 85, 2227–2236 [DOI] [PubMed] [Google Scholar]
- 39. Edwards J. G., Bahl J. J., Flink I. L., Cheng S. Y., Morkin E. (1994) Thyroid hormone influences beta myosin heavy chain (beta MHC) expression. Biochem. Biophys. Res. Commun. 199, 1482–1488 [DOI] [PubMed] [Google Scholar]
- 40. Caiozzo V. J., Haddad F. (1996) Thyroid hormone: modulation of muscle structure, function, and adaptive responses to mechanical loading. Exerc. Sport Sci. Rev. 24, 321–361 [PubMed] [Google Scholar]
- 41. Axelband F., Dias J., Ferrão F. M., Einicker-Lamas M. (2011) Nongenomic signaling pathways triggered by thyroid hormones and their metabolite 3-iodothyronamine on the cardiovascular system. J. Cell Physiol. 226, 21–28 [DOI] [PubMed] [Google Scholar]
- 42. Portman M. A., Qian K., Krueger J., Ning X. H. (2005) Direct action of T3 on phosphorylation potential in the sheep heart in vivo. Am. J. Physiol. Heart Circ. Physiol. 288, H2484–H2490 [DOI] [PubMed] [Google Scholar]
- 43. Krueger J. J., Ning X. H., Argo B. M., Hyyti O., Portman M. A. (2001) Triidothyronine and epinephrine rapidly modify myocardial substrate selection: a (13)C isotopomer analysis. Am. J. Physiol. Endocrinol. Metab. 281, E983–E990 [DOI] [PubMed] [Google Scholar]
- 44. Sun Z. Q., Ojamaa K., Coetzee W. A., Artman M., Klein I. (2000) Effects of thyroid hormone on action potential and repolarizing currents in rat ventricular myocytes. Am. J. Physiol. Endocrinol. Metab. 278, E302–E307 [DOI] [PubMed] [Google Scholar]
- 45. Brand M. D., Steverding D., Kadenbach B., Stevenson P. M., Hafner R. P. (1992) The mechanism of the increase in mitochondrial proton permeability induced by thyroid hormones. Eur. J. Biochem. 206, 775–781 [DOI] [PubMed] [Google Scholar]
- 46. Paradies G., Petrosillo G., Ruggiero F. M. (1997) Cardiolipin-dependent decrease of cytochrome c oxidase activity in heart mitochondria from hypothyroid rats. Biochim. Biophys. Acta 1319, 5–8 [DOI] [PubMed] [Google Scholar]
- 47. Fleury C., Neverova M., Collins S., Raimbault S., Champigny O., Levi-Meyrueis C., Bouillaud F., Seldin M. F., Surwit R. S., Ricquier D., Warden C. H. (1997) Uncoupling protein-2: a novel gene linked to obesity and hyperinsulinemia. Nat. Genet. 15, 269–272 [DOI] [PubMed] [Google Scholar]
- 48. Graier W. F., Trenker M., Mallis R. (2008) Mitochondrial Ca2+, the secret behind the function of uncoupling proteins 2 and 3. Cell Calcium 44, 36–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Horimoto M., Fulop P., Derdak Z., Wands J. R., Baffy G. (2004) Uncoupling protein-2 deficiency promotes oxidant stress and delays liver regenerations in mice. Hepatology 39, 386–392 [DOI] [PubMed] [Google Scholar]
- 50. Vidal-Puig A. J., Grujic D., Zhang C. Y., Hagen T., Boss O., Ido Y., Szczepanik A., Wade J., Mootha V., Cortright R. N., Muoio D. M., Lowell B. B. (2000) Energy metabolism in uncoupling protein 3 gene knockout mice. J. Biol. Chem. 275, 16258–16266 [DOI] [PubMed] [Google Scholar]