Abstract
Tendon injuries are often associated with significant dysfunction and disability due to tendinous tissue’s very limited self-repair capacity and propensity for scar formation. Dental-derived mesenchymal stem cells (MSCs) in combination with appropriate scaffold material present an alternative therapeutic option for tendon repair/regeneration that may be advantageous compared to other current treatment modalities. The MSC delivery vehicle is the principal determinant for successful implementation of MSC-mediated regenerative therapies. In the current study, a co-delivery system based on TGF-β3-loaded RGD-coupled alginate microspheres was developed for encapsulating periodontal ligament stem cells (PDLSCs) or gingival mesenchymal stem cells (GMSCs). The capacity of encapsulated dental MSCs to differentiate into tendon tissue was investigated in vitro and in vivo. Encapsulated dental-derived MSCs were transplanted subcutaneously into immunocompromised mice. Our results revealed that after 4 weeks of differentiation in vitro, PDLSCs and GMSCs as well as the positive control human bone marrow mesenchymal stem cells (hBMMSCs) exhibited high levels of mRNA expression for gene markers related to tendon regeneration (Scx, DCn, Tnmd, and Bgy) via qPCR measurement. In a corresponding in vivo animal model, ectopic neo-tendon regeneration was observed in subcutaneous transplanted MSC-alginate constructs, as confirmed by histological and immunohistochemical staining for protein markers specific for tendons. Interestingly, in our quantitative PCR and in vivo histomorphometric analyses, PDLSCs showed significantly greater capacity for tendon regeneration than GMSCs or hBMMSCs (P<0.05). Altogether, these findings indicate that periodontal ligament and gingival tissues can be considered as suitable stem cell sources for tendon engineering. PDLSCs and GMSCs encapsulated in TGF-β3-loaded RGD-modified alginate microspheres are promising candidates for tendon regeneration.
Keywords: tissue engineering, tendon regeneration, dental mesenchymal stem cells, alginate hydrogel, RGD tripeptide, microencapsulation
1. Introduction
Tendons are specialized tissues that connect bone to muscle, transmitting the forces generated by these structures to allow for body movement [1]. Tendons are frequently injured during sports and other rigorous physical activities. These injuries are often very difficult to manage because tendons heal through fibrotic scar formation rather than a regenerative process, leading to the formation of poor-quality tissue with low mechanical strength [1–3]. As a result, tendon injuries can lead to long-term pain, discomfort, and disability for patients. This limited self-repair capacity necessitates the development of alternative therapeutic strategies to functionally repair injured tendons [3–5]. In comparison to currently available treatment modalities, research indicates that the application of mesenchymal stem cells (MSCs) may present an advantageous alternative therapeutic option for the regeneration and repair of tendon tissue [6]. MSCs are pluripotent cells that can differentiate into multiple lineages, depending on the nature of the environmental signals they receive. Specifically with regards to their capacity to form tendon, studies have shown that direct implantation of MSCs functionally improves tendon defects [6–8]. However, identifying an optimal cell source is one of the crucial factors in tendon regeneration and repair, since not all MSCs have equal capacity to form tendons. Tendon stem/progenitor cells (TSPCs), which possess both self-renewal and multilineage differentiation capacity, have been identified and extracted from human and mouse tendons [9]. Several studies have proposed the application of this type of progenitor cell for tendon regeneration [8,9]. In addition, other types of stem cells have been proposed for tendon tissue engineering, including human bone marrow mesenchymal stem cells (hBMMSCs) and skin fibroblasts [10, 11].
It is well known that MSCs are found in a wide range of post-natal tissue types, including the dental and craniofacial tissues [12–15]. Among the dental-derived MSCs, periodontal ligament stem cells (PDLSCs) and gingival mesenchymal stem cells (GMSCs) are of particular interest as they are accessible through the oral cavity and can be harvested easily; indeed, they can often be obtained as discarded biological samples in dental clinics [13–16]. Furthermore, both in vitro and in vivo studies have confirmed the multilineage differentiation capabilities of these dental-derived stem cells [13–16]. Additionally, since the periodontal ligament is composed of tendon-like collagen bundles that are capable of bearing occlusal forces, PDLSCs show unique potential as a source of MSCs for tendon regenerative treatments [17].
In order to induce the differentiation of MSCs into a specific and desired lineage/phenotype, it is necessary to deliver an appropriate and specific signal. It has been reported that stem cell-mediated tissue regeneration is partially controlled by the recipient local microenvironment, including the presence of growth factors, immune cells and cytokines [18–21]. For this reason, exogenous growth factors have been delivered along with stem cells to achieve a desired differentiation route. Here we focus on the potential of the transforming growth factor-beta (TGF-β) signaling pathway to drive differentiation into tendon. Studies have reported that the TGF-β pathway plays an important role in tendon regeneration [22,23]. In addition, it has been shown that disruption of TGF-β signaling pathway results in the loss of most tendons and ligaments. Moreover, TGFβ signaling is a potent inducer of Scleraxis (Scx), a basic helix-loop-helix (bHLH) transcription factor gene that is a unique marker for the tendon cell fate in vitro and in vivo, demonstrating a vital role for the TGF-β signaling pathway in tendon repair and regeneration [23–25]. Among the members of the TGF-β superfamily, TGF-β3 is of particular interest as it is an isoform that has been associated with promoting dermal wound and tendon healing without fibrotic scar formation [26,27]. Other studies have confirmed that differentiation of MSCs driven by TGF-β3 can promote tendon tissue formation and may provide a promising modality of treatment for tendon regeneration and repair [24–27].
In order to develop a promising microenvironment for tendon regeneration based on dental-derived MSCs, we sought to engineer a microenvironment with the physiochemical characteristics of the extracellular micro-milieu. We utilized alginate hydrogel coupled with RGD (arginine- glycine-aspartic acid tripeptide) in order to modify the niche properties and to direct the cell phenotype through differentiation [28–30]. Hydrogel biomaterials like the one we selected have been widely used for tissue engineering. Alginates are natural hetero-polysaccharides isolated from brown sea algae that possess unique properties including injectability and biodegradability [28–32]. Moreover, alginates can provide a 3D scaffold that facilitates the spatial distribution of MSCs, thus resulting in a structural organization that resembles the native in vivo microenvironment. Alginate microspheres have been used extensively for controlled delivery of growth factors (e.g., TGF-β) and have an excellent track record of safety [33, 35]. Also, it is has been reported that presence of cell-binding peptides, such as RGD, in the structure of the alginate scaffold could be advantageous because these peptides mimic the cell-matrix interaction typical of the ECM [31–33].
Recently, our research group reported that that RGD-coupled alginate hydrogel can be used to encapsulate PDLSCs and GMSCs for cartilage regeneration via in vitro and in vivo analyses [34]. However, a literature search revealed no reports assessing the application of PDLSCs or GMSCs encapsulated in RGD-coupled alginate microspheres, loaded with TGF-β3, in tendon regeneration. Therefore, in the present study, we developed a co-delivery system that provides a 3D architecture of RGD-coupled alginate hydrogel loaded with TGF-β3 ligands for the encapsulation of dental MSCs (PDLSCs and GMSCs). It was hypothesized that the tendon regeneration capacity of PDLSCs and GMSCs encapsulated in RGD coupled alginate microspheres, loaded with TGF-β3, making this a promising combination for tendon tissue engineering applications. Considering the fact that GMSCs and are easily harvested from the oral cavity and can often be obtained as discarded biological samples, these MSC sources can be considered ideal for stem cell banking purposes provided they show promise in MSC-based tissue regeneration. This approach was designed to optimize tendon regeneration for potential application in the repair of the appendicular skeleton.
2. Materials and methods
2.1. Progenitor cell isolation and culture
Human PDLSCs and GMSCs were isolated and cultured according to previously published procedures [15,16]. The gingival tissues and teeth were obtained from twenty healthy male patients (18–25 years old) undergoing third molar extractions with IRB approval from the University of Southern California. Only subjects without any history of periodontal disease were included in this study.
2.2. Biomaterial fabrication and cell encapsulation
In this study, RGD-coupled alginate (NovaMatrix FMC Biopolymer, Norway) was used as the scaffold material. The alginate was purified and partially oxidized (2%) to increase its degradability according to published methods in the literature [28–30]. Subsequently, the alginate was concentrated, freeze-dried under reduced pressure, and mixed with TGF-β3 (Invitrogen, Carlsbad, CA) (50 μg/mL).
PDLSCs, GMSCs, and hBMMSCs (as a positive control) were encapsulated separately in alginate loaded with TGF-β3 ligand. Cells were encapsulated at a density of 2×106 cells/mL of alginate solution. Microsphere formation was accomplished by adding the MSC-alginate mixture dropwise to 100 mM CaCl2 solution. The resulting microspheres were incubated at 37°C for 45 min to complete cross-linking and then washed three times in non-supplemented DMEM. RGD-coupled alginate hydrogel without cells (and not loaded with TGF- β3) was used as the negative control in this study.
2.3. Live/dead staining
Following 14 days of incubation in culture medium, the cell viability of the encapsulated MSCs was measured as described previously [28, 29] using calcein AM to stain live cells and ethidium bromide homodimer-1 to stain dead cells (Invitrogen). The percentage of live cells was measured using NIH ImageJ software (NIH, Bethesda, MD). The effect of the presence of RGD tripeptide on the viability of the encapsulated MSCs was also evaluated by encapsulating dental-derived MSCs or hBMMSCs in alginate microspheres without RGD and performing a live/dead staining assay after 14 days of culturing.
2.4. Scanning electron microscopy (SEM)
The morphology and structure of the microspheres were characterized using scanning electron microscopy (SEM) (JEOL 5300, Peabody, MA). On day 14 of culture in regular medium, the MSC-alginate microspheres were rinsed with 2 ml of PBS and fixed with 1% glutaraldehyde overnight. Samples were dehydrated using graded alcohol solution, sputter coated with gold, and observed using SEM.
2.5. Confocal laser scanning microscopy (CLSM)
The MSC-alginate microspheres were further characterized using CLSM (Fluoview FV10i, Olympus Corp, Tokyo, Japan). On day 14 of culturing, encapsulated cells were fixed in paraformaldehyde (4% in PBS) for 30 min, washed with PBS (pH = 7.4), and incubated in 0.1% Triton X-100 for 5 min at room temperature. MSCs were stained with anti-human CD146-FITC conjugated and anti-human CD105- PE conjugated antibodies (Abcam) as positive MSC markers. Additionally, specimens were stained with anti-CD34 PE conjugated antibody (Santa Cruz Biotechnology Inc, Santa Cruz, CA) as a negative hematopoietic stem cell marker. All the specimens were counterstained with DAPI for nucleus staining.
2.6. In vitro release profile characterization
In order to characterize the release profile of TGF-β3, RGD-coupled alginate microspheres were loaded with TGF-β3 at three different concentrations (10, 50, and 100 μg/mL) and incubated in 500 μL of high-glucose DMEM supplemented with 100 U/mL penicillin and 100 μg/mL streptomycin in 48-well plates on a rotational shaker at 37°C for one week. At each selected time interval (1, 3, 5, 7, 10, 12, and 14 days), the medium was collected and analyzed for released TGF- β3 using a TGF-β3 ELISA Kit (R&D Systems Inc. Minneapolis, MN). At the end of release study, the remaining TGF-β3 was extracted from the microspheres by dissolving the alginate scaffolds in 50 mM sodium citrate dehydrate solution (Sigma Aldrich, St Louis, MO) in distilled water and the percentage of cumulative released TGF-β3 was measured. Finally, the percentage of released TGF-β3 was measured based on the ratio of the amount released after two weeks to the amount that was initially loaded [49].
2.7. In vitro culture and immunofluorescence staining
To induce tenogenic differentiation, encapsulated PDLSCs and GMSCs as well as hBMMSCs (2×106 cells in 1 mL of TGF-β3-loaded alginate microspheres) were cultured in a tenogenic medium containing DMEM with 15% FBS, 2 mM L-glutamine, 100 nM Dex, 100 3M ascorbic acid, 2 mM sodium pyruvate (R&D Systems Inc), 100 U/mL penicillin, and 100 μg/mL streptomycin [26]. Cell-free RGD-coupled alginate microspheres without TGF-β3 were used as the negative control.
Four weeks after induction, the samples were fixed with 4% PFA, and paraffin sections were made. Sections were immunolabeled using antibodies against Tenomodulin (Tnmd), Eya 1, Eya2 (from Santa Cruz Biotechnology, Inc, Dallas, TX), and Scleraxis (Abcam) at 4°C overnight, detected using Alexa fluor conjugated secondary antibody (1:200 dilution; Invitrogen), and counterstained with DAPI.
2.8. RNA isolation, reverse transcription and real time PCR
RNA was extracted from the encapsulated cells after 2 weeks of culturing following published methods [21]. Briefly, 10 alginate microspheres were collected and dissolved by gentle stirring in a sterile depolymerization buffer consisting of 50 mM sodium citrate dehydrate and 80 mM sodium chloride (Sigma Aldrich) for 15–20 min. Following dissolution, the decapsulated cells were centrifuged at 9400 g for 10 min and the pellet was washed with PBS and centrifuged again at 9400 g for 3 min. Total RNA was extracted using Trizol reagent (Invitrogen) according to the manufacturer’s recommendations. Single-stranded cDNA synthesis was performed with 100 ng total RNA using a Superscript III cDNA synthesis kit (Invitrogen). Data were analyzed by the 2-ΔΔCt method, with normalization to the Ct of the housekeeping gene GAPDH (glyceraldehyde 3-phosphate dehydrogenase). Primer and probe sequences are described in Table 1.
Table 1.
Oligonucleotide primers used in RT-PCR analysis.
| Gene | Sequence | Amplication (bp) |
|---|---|---|
| Scleraxis (Scx) | 5’-AACACGGCCTTCACTGCGCTG-3’ (forward) 5’-CAGTAGCACGTTGCCCAGGTG-3’(reverse) |
123 |
| Decorin (Dcn) | 5’-ATGATTGTCATAGAACTGGGC-3’ (forward) 5’-TTGTTGTTATGAAGGTAGAC-3’ (reverse) |
382 |
| Biglycan (Bgn) | 5’-CTCAACTACCTGCGCATCTCAG (forward) 5’-GATGGCCTGGATTTTGTTGTG (reverse) |
105 |
| Tenomodulin (Tnmd) | 5’-CCATGCTGGATGAGAGAGGTTAC-3’ (forward) 5’-CACAGACCCTGCGGCAGTA-3’ (reverse) |
72 |
| Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) | Sense: 5’-AGCCGCATCTTCTTTTGCGTC-3’: Antisense: 5’-TCATATTTGGCAGGTTTTT CT-3’ |
418 |
2.9. Western blot analysis
After four weeks in culture, the differentiation of stem cells was analyzed using Western blot analysis. Briefly, stem cell-alginate constructs were dissolved in citrate buffer (6% w/v, pH 7.4). Cell pellets were obtained by centrifugation, washed twice with PBS, and lysed with protein extraction buffer (Bio-Rad, Irvine, CA) for 30 sec. The supernatant was cleared by centrifugation and the protein concentration was determined using a BCA assay (Pierce, Rockford, IL) by extrapolation to a known BSA standard concentration by serial dilution across one order of magnitude. Equal amounts of protein extracts were fractionated to size by electroporation in 10% sodium dodecyl sulfate-polyacrylamide gels (PAGE), and the size-resolved proteins were electrophoretically transferred to a nitrocellulose membrane (Bio-Rad). The nitrocellulose membrane was incubated with rabbit antibody directed against Tenomodulin (Tnmd) (Santa Cruz Biotechnology, Inc). Immune-protein complexes were detected using a secondary antibody at 1:500 (polyclonal goat anti-rabbit IgG/HRP, EMD Millipore, Billerica, MA). The membranes were stripped and re-probed with an antibody directed against the housekeeping gene β-actin (Abcam) to ensure that equal mass was loaded to each lane. The chemiluminescent reagent (Amersham Life Science, Pittsburgh, PA) was added to the membrane for 1 min and exposed to x-ray film for variable periods (Thermo Scientific, Rockford, IL) to produce images.
2.10. Tendon tissue regeneration in animal model
All animals in our in vivo experiments were treated according to the Guidelines and Regulations for the Use and Care of Animals at the University of Southern California. PDLSCs, GMSCs, and hBMMSCs (approximately 4 × 106 cells) were encapsulated in alginate microspheres loaded with TGF-β3 and 10 microspheres (500 μl) were transplanted subcutaneously into the dorsal surface of 5-month-old Beige nude XID III (NU/NU) mice (Harlan, Livermore, CA; N=4 for each group). After 8 weeks the mice were sacrificed, and the microspheres and the surrounding tissue were surgically removed and analyzed using histological and immunohistochemical staining. Cell-free RGD-coupled alginate microspheres without TGF-β3 were used as the negative control group.
2.11. Histological and immunohistochemical analyses
For histological examination, harvested specimens were fixed in 10% formalin solution, dehydrated in an ascending series of ethanol, and embedded in paraffin. Six-micrometer sections were cut using a microtome and mounted on glass slides. Four randomly selected cross-sections from each implant were stained with Hematoxylin & Eosin (H&E) and Masson’s Trichrome. Furthermore, histological sections were observed under a polarized light microscope to evaluate collagen fiber formation.
For immunohistochemical analysis, de-paraffinized sections were washed, and non-specific endogenous peroxidase activity was quenched by immersing in 3% H2O2/methanol for 15 min. Sections were incubated with primary antibody (1:200–1:300 dilution) for 1 h. Immunohistochemistry examination was performed on sections using anti-Tenomodulin (Santa Cruz Biotechnology, Inc, 1:100 dilution), anti-Eya 1, anti-Eya2 (Santa Cruz Biotechnology, Inc, 1:100 dilution), anti- Scleraxis (Abcam, 1:100 dilution), and counterstaining with hematoxylin.
The effect of RGD tripeptide on the tendon regeneration capacity of encapsulated MSCs was also evaluated by comparison with MSCs encapsulated in non-RGD containing alginate microspheres. Microspheres were transplanted as described above and histomorphometric analysis was performed on slides stained with Masson’s Trichrome staining. The positive staining was determined for 4 independent samples for each experimental group. Five areas were randomly selected from each sample, and then the positive area in each field was calculated with NIH Image-J software and shown as a percentage of the total field area.
2.12. Statistical Analysis
The Kruskal–Wallis rank sum test was utilized to analyze the data at a significance level of α = 0.05. Also, a two-tailed Student’s t-test was utilized for pairwise comparisons whenever needed. Quantitative data are expressed as mean ± standard deviation (SD).
3. Results
3.1. Biomaterial fabrication and characterization
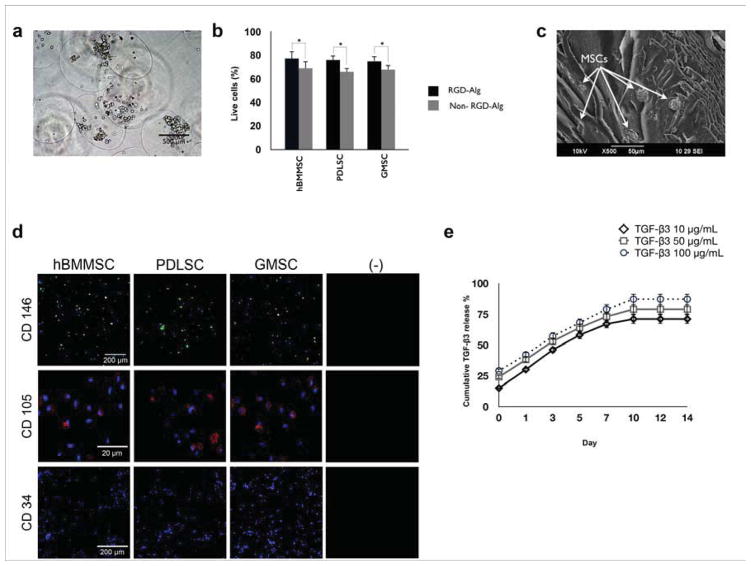
In this study, an RGD-coupled alginate encapsulation system was developed in order to investigate the capacity of PDLSCs and GMCSs to contribute to tendon tissue differentiation and regeneration. We utilized hBMMSCs encapsulated in alginate microspheres as the positive control, whereas our negative control consisted of alginate microspheres alone. In addition, the ability of RGD-containing alginate to contribute to MSC viability and differentiation was examined. Alginate microspheres with an average diameter of 1 ± 0.1 mm were fabricated. Microscopic images of the stem cell-loaded microcapsules showed that the microcapsules were of uniform size and exhibited even cell distribution (Fig. 1a). Live/dead assays confirmed that all the encapsulated MSC types had high viability in culture for up to two weeks (Fig. 1b). No significant difference was observed between the percentages of live cells in the PDLSC, GMSC and hBMMSC groups (P>0.05). In addition, cell viability remained high well after the initial two-week culturing period (data not shown). Furthermore, in comparison to MSCs encapsulated in non-RGD coupled alginate microspheres, MSCs encapsulated in RGD-containing alginate microspheres showed significantly higher degrees of viability at all the tested time intervals (Fig. 1b). Additionally, the effects of presence of TGF- β3 on the viability of encapsulated MSCs were analyzed. Our data showed that the presence of RGD tripeptide played an important role in promoting MSC viability, while the presence of TGF-β3 ligands did not exhibit any significant effects on MSC viability (Supplementary Fig. 1S).
Figure 1. Development and characterization of MSC encapsulation system based on TGF-β3-loaded RGD coupled-alginate hydrogel.
(a) Bright field image of translucent alginate microspheres showing their spherical shape with a uniform cell distribution (average microsphere diameter 1 ± 0.1 mm). (b) Viability of the encapsulated MSCs measured as a percentage of live cells in either RGD-coupled alginate or non-RGD- coupled alginate microspheres after two weeks of culturing in regular media. (c) SEM image of MSCs encapsulated within alginate microspheres showing the porous morphology of the alginate hydrogel and the presence of stem cells after two weeks of culturing in regular media. (d) Expression of stem cell surface marker CD 146 (green) and CD 105 (red) by periodontal ligament stem cells (PDLSCs), gingival mesenchymal stem cells (GMSCs), and human bone marrow mesenchymal stem cells (hBMMSCs) as determined by confocal laser scanning microcopy (CLSM) analysis. CLSM images confirmed that neither of the experimental groups (PDLSCs and GMSCs) nor the negative control (hBMMSCs) expressed the negative hematopoietic lineage marker CD 34. (e) Sustained release of TGF-β3 from the alginate microspheres. Characterization of the in vitro release profile of TGF-β3-loaded alginate microspheres showing sustained release of TGF-β3. Cumulative release is calculated from the total amount of TGF-β3 released after 14 days. Faster TGF-β3 release was observed at higher initial concentrations. *P <0.05.
SEM analysis showed that the prepared scaffolds had a porous morphology and confirmed the presence of the encapsulated MSCs (Fig. 1c). Further characterization of encapsulated dental-derived MSCs using CLSM demonstrated that the cells expressed the specific MSC markers CD146 and CD105, but not the hematopoietic lineage marker CD34, after two weeks of culturing. These results confirmed the suitability of RGD-coupled alginate as the encapsulating biomaterial for PDLSCs and GMSCs, as well as hBMMSCs (Fig. 1d).
In the next step, alginate microspheres with three different concentrations of TGF-β3 (10, 50 and 100 μg/mL) were prepared and the release profile of TGF-β3 from the RGD-coupled alginate scaffold was characterized for two weeks. Higher initial concentrations of TGF-β3 were observed to produce a faster release profile (Fig. 1e). The cumulative release profile confirmed the sustained release of TGF-β3 for up to 14 days. Additionally, the effect of initial concentration of TGF-β3 on the percentage of released ligand was evaluated and our data (not shown) confirmed that 50 μg/mL TGF-β3 exhibited the most favorable release profile. Therefore, this concentration was selected for the rest of the experiments in this study.
3.2. Tenogenic differentiation of dental MSCs in vitro
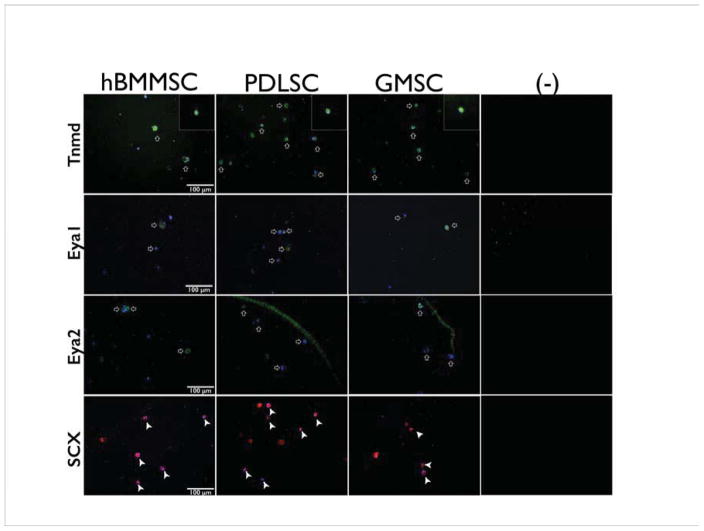
After four weeks of tenogenic differentiation in vitro, tenogenic differentiation of PDLSCs, GMSCs, and hBMMSCs was confirmed by positive immunostaining with antibodies against Tenomodulin, Eya1, Eya2, and Scleraxis (Fig. 2). Tenomodulin (Tnmd) is a type II transmembrane glycoprotein that is predominantly expressed in tissues such as tendons and ligaments [36]. It has been shown that early Scleraxis-expressing progenitor cells lead to the eventual formation of tendon tissue [36]. Additionally, Eya1 and Eya 2 genes are expressed during limb tendon development and encode a transcriptional activation function. [47] These transcription factors are involved in a number of cellular and developmental processes including the development of tendons and ligaments [38].
Figure 2. Tenogenic differentiation of dental MSCs in vitro.
Immunofluorescence staining against Tenomodulin (Tnmd), Eya 1, Eya2, and Scleraxis (Scx) antibodies after four weeks of tenogenic differentiation in vitro. Top panel: Tnmd: the inserts show MSCs positioned inside alginate microspheres at higher magnification (40x) positively immunostained with antibodies against Tenomodulin; Two middle panels: Eya1 and Eya2, respectively; Lower panel: Scx. Results confirmed that PDLSCs and GMSCs were positively stained for tendon markers (white arrows), while the negative control (-) unseeded alginate microsphere, without TGF-β3, immunostaining failed to show any of these markers.
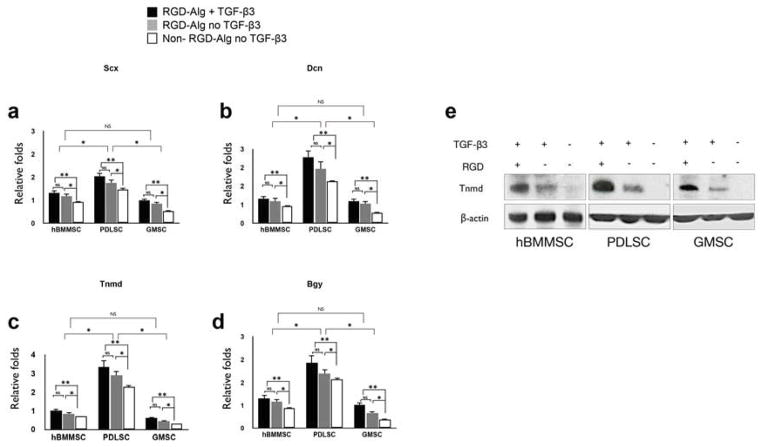
Next, the molecular mechanism underlying the tendon regeneration in the TGF-β3-loaded alginate microspheres was analyzed. Gene expression analyses were performed using several markers that are associated with tendon differentiation, including a transcription factor Scleraxis (Scx), an extracellular matrix protein Decorin (Dcn), and a surface marker Tenomodulin (Tnmd) [36–39], as well as Biglycan (Bgy), which is a class I small leucine-rich proteoglycan (SLRP) involved in the regulation of collagen fibrillogenesis [48]. The expression levels of these genes were confirmed and compared via RT-PCR. The results showed that all of the MSC groups expressed abundant Scx, DCn, Tnmd, and Bgy (Figs. 3a-d). However, PDLSCs showed significantly higher expression levels for all the tested genes than GMSCs or hBMMSCs (p<0.05). No significant differences in the expression levels of Scx, DCn, Tnmd, or Bgy were found between hBMMSCs and GMSCs (P>0.05). However, for all the tested genes, hBMMSCs showed higher levels of expression than GMSCs did. As expected, MSCs encapsulated in RGD-coupled alginate microspheres exhibited significantly higher levels of tendon-specific gene expression than did their non-RGD coupled counterparts (P<0.05) (Figs. 3a-d). In addition, further analysis clearly confirmed that TGF-β3 plays a more important role than RGD tripeptide in the tenogenic differentiation of MSCs. Moreover, the expression levels of Dcn and Tnmd were evaluated in hBMMSCs, PDLSCs, and GMSCs without the scaffold after two weeks of tenogenic differentiation in vitro in the presence of TGF-β3 ligand. Interestingly, the same trend reported for the encapsulated MSCs (Figs. 3a-d) was observed for scaffold-free specimens (Supplementary Fig. S2). However, in comparison to MSCs without scaffolds, the encapsulated MSCs expressed higher levels (p>0.05) of expression for the tendon markers that were examined, confirming the important role of the encapsulation biomaterial (Supplementary Fig. S2).
Figure 3. Specific gene expression and underlying molecular pathway for tendon regeneration in vitro.
Expression level (in fold changes) of (a) Scx, (b) Dcn, (c) Tnmd, and (d) Bgy genes for each encapsulated stem cell population after 4 weeks of culturing in induction media in vitro evaluated by RT-PCR. Data were normalized by the Ct of the housekeeping gene GAPDH and expressed relative to the expression level for the same gene at day 1. (e) Western blot analysis showing changes in the levels of expression of regulators of tenogenesis of MSCs. The level of Tnmd is elevated in the encapsulated MSCs in RGD-containing alginate microspheres in the presence of TGF-β3. MSCs encapsulated in non-RGD-coupled alginate microspheres in the presence of TGF-β3 exhibited very modest levels of Tnmd expression, while specimens in the absence of TGF-β3, encapsulated in non-RGD-coupled alginate microspheres failed to express Tnmd. *P <0.05, NS= not significant.
The results of Western blot analysis correlated well with the data from the immunostaining and PCR analyses. Increased expression levels of the tenogenic-specific molecule, Tnmd, were detected in specimens encapsulated in TGF-β3-loaded RGD-containing alginate microspheres (Fig. 3e). In contrast, MSCs encapsulated in alginate microspheres without RGD but still loaded with TGF-β3 exhibited very modest levels of Tnmd expression, while encapsulated specimens in the absence of TGF-β3 and RGD failed to express Tnmd (Fig. 3f). PDLSCs encapsulated in TGF-β3-loaded RGD-containing alginate microspheres showed higher levels of Tnmd expression than either hBMMSCs or GMSCs.
3.3. Ectopic tendon regeneration in vivo
The ability of PDLSCs and GMSCs encapsulated in RGD-coupled alginate microspheres loaded with TGF-β3 to regenerate tendon tissue was evaluated in an ectopic site after subcutaneous transplantation into immunocompromised mice. Histological sections stained with H&E and Masson’s Trichrome dyes revealed the presence of wave-like aligned fibrils with tendon-like structure. However, a more organized structure, with more extracellular matrix and collagen, was observed with PDLSCs in comparison to GMSCs and hBMMSCs (Fig. 4a). Trichrome staining further confirmed the presence and regeneration of tendon-like tissue in the experimental and positive control groups after 8 weeks of transplantation, but no histological evidence for regeneration was observed in the negative control, cell-free transplant group. In addition, to evaluate the deposition level of collagen, histological sections were observed under polarized light microscopy (Fig.4b). Collagen fibers with a yellow color were more easily observed in the PDLSC group than in the hBMMSC and GMSC groups. Our histomorphometric analysis confirmed that MSCs encapsulated in RGD-containing alginate microspheres exhibited significantly higher amounts of tendon tissue regeneration (by Masson’s Trichrome staining) than their counterparts without RGD (Fig. 4c).
Figure 4. Characterization of the fate of encapsulated MSCs after transplantation.
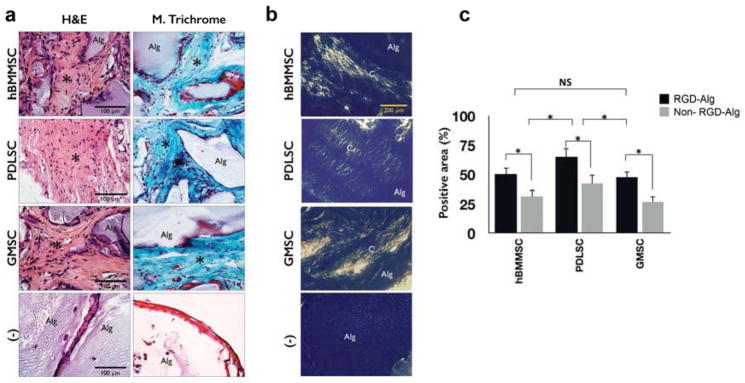
Tendon tissue formation in TGF-β3-loaded alginate microspheres was evaluated after subcutaneous transplantation into immunocompromised mice. MSC-alginate transplants were removed after 8 weeks, sectioned, and stained with (a) hematoxylin and eosine and Masson’s Trichrome. The negative control (−) was an unseeded alginate scaffold. Histological evaluation confirmed partial regeneration with typical tendon sinusoidal morphology (indicated by asterisk). (b) Polarized light microscopy images of specimens where C indicates collagen fibrils and A indicates unresorbed alginate scaffold. (c) The percentage of positive stained area (by Masson’s Trichrome staining) for MSCs encapsulated in RGD-coupled alginate microspheres is shown in comparison to non-RGD-coupled counterparts. *P <0.05, NS: not significant.
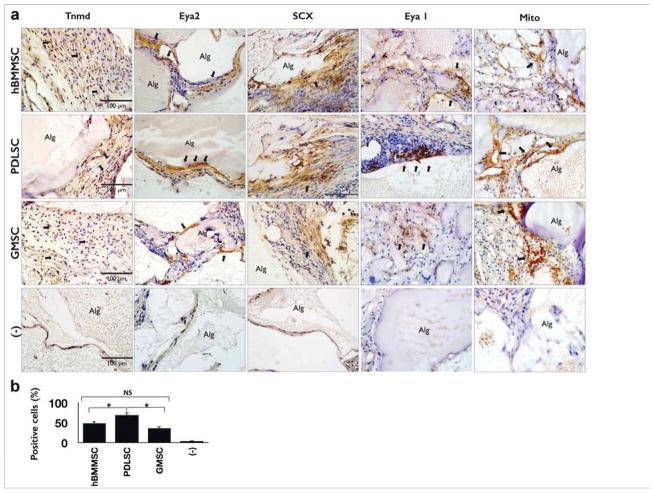
To further characterize dental-derived MSC-mediated tendon regeneration in vivo, immunohistochemical detection was utilized to detect proteins suggested by the gene expression analysis. Immunohistochemical staining for Scleraxis, Tenomodulin, EYA 1, and EYA2 antigens after 8 weeks of transplantation revealed extensive production and deposition of these markers within the regenerated tendon tissues (Fig. 5a). As expected, the amount of tendon-like tissue generated was more pronounced in the PDLSC group than in the GMSC or hBMMSC groups (P<0.05) (Fig. 5b), while GMSCs and hBMMSCs showed the same moderate potential for tendon regeneration (P<0.05), as confirmed by immunohistochemical detection of Scx protein. A considerably higher percentage of cells were positive for anti-Scx antibody staining in the PDLSC group than in the GMSC or hBMMSC groups (Fig. 5b). In addition, the human origin of the newly-formed tendon tissue was confirmed via immunohistochemical staining (Fig. 5a). No evidence of cells positive for anti-Scx antibody was observed in the cell-free RGD-coupled alginate negative control group.
Figure 5. Immunohistochemical characterization of the fate of encapsulated MSCs and quantitative evaluation of in vivo tendon regeneration capacity of encapsulated MSCs.
(a) Immunohistochemical staining using antibodies against Tenomodulin (Tnmd), Eya 1, Eya2, and Scleraxis (Scx) confirmed the regeneration capacity of encapsulated MSCs. Positive staining appears brown (black arrows) for anti-Tnmd, EYA2, and Scx antibody staining, while negative control (−), cell free RGD coupled alginates without TGF-β3, immunohistochemical staining results failed to identify the antigen. (b) Semi-quantitative analysis of the percentage of MSCs positive for anti-Scx antibodies via immunohistochemical staining images in 6a. *P <0.05, NS: not significant.
4. Discussion
Tendon injuries due to age-related degeneration or rigorous physical activity are a common clinical problem. Damaged tendon tissue heals very slowly and rarely attains the structural integrity or mechanical strength of normal, undamaged tendon [36]. A limited understanding of basic tendon biology has hampered the development of new treatment options for injured tendons [40, 41]. Local delivery of growth factors in combination with MSCs shows promise as a modality of treatment for tendon regeneration. Several animal studies have confirmed that the application of MSCs can lead to significant improvement in tendon repair and regeneration [42, 43]. Due to the tendon-like structure of collagen bundles in the periodontal ligament, we hypothesized that PDLSCs could provide a uniquely well-suited source of MSCs for tendon regeneration. Moreover, GMSCs, and to some extent PDLSCs, are easily harvested from the oral cavity or can often be obtained from discarded biological samples. Therefore, these MSC sources can be considered ideal for stem cell banking purposes provided they show promise in MSC-based tissue regeneration. However, to our knowledge, the capacity of PDLSCs and GMSCs to regenerate tendon tissue has previously not been reported. Therefore, in this study alginate microspheres were coupled with the RGD tripeptide and loaded with TGF-β3 in a strategy to optimize the microenvironment for dental-derived MSCs to differentiate into tendon tissue while also assessing the capacity of stem cells from the periodontal ligament and the gingiva, when encapsulated in these alginate microspheres, to participate in tendon regeneration.
Through our in vitro and in vivo studies, we provide evidence that our encapsulation system supported the viability of MSCs and their differentiation into tendon-like tissue. We also confirmed the important role of the microenvironment on the viability of encapsulated MSCs. Additionally, in the current study we utilized two unique, easily accessible and abundant sources of dental-derived MSCs (PDLSCs and GMSCs) for this purpose, and showed them to be promising candidate sources for tendon tissue regeneration. From a practical perspective, PDLSCs and GMSCs are superior sources of stem cells in comparison to hBMMSCs, considering their accessibility and suitability for autologous transplantation. As a result, human PDLSC- or GMSC-mediated tissue regeneration shows promise as a cell-based treatment for tendon tissue engineering. Dental-derived MSCs encapsulated in alginate microspheres in the presence of a suitable signaling molecule (in this study, TGF-β3) could effectively induce patterns of gene expression suitable to regenerate tendon-like tissue.
In the current study, we found that MSCs derived from the periodontal ligament have significantly higher levels of tendon markers mRNA expression than do hBMMSCs. Stem cells derived from gingival tissues showed expression levels of tendon-specific markers comparable to the levels seen in hBMMSCs. In addition, in our in vivo study, histological analysis of regenerated tissues confirmed the formation of tendon-like organization with typical characteristics of tendon tissue, including sinusoidal wave-like patterning of the nuclei and cytoplasm of the cells. We confirmed our hypothesis that PDLSCs would show greater capacity for regenerating tendon tissue than either GMSCs or hBMMSCs. Interestingly, GMSCs showed tendon regeneration capacity comparable to that of hBMMSCs. However, both PDLSCs and GMSCs have a unique advantage over hBMMSCs, namely, their ready availability and high capacity for tenogenesis, which stand in marked contrast to the more involved harvesting and osteoegenic proclivity of hBMMSCs. This tendency toward osteogenesis is a major concern because the generation of bony tissue is an outcome which could result in serious adverse effects for clinical tendon regenerative applications. In contrast, no osteogenic differentiation was observed at the transplantation sites of PDLSCs and GMSCs, as confirmed by H&E and Trichrome staining. Additionally, in vivo culture of PDLSCs and GMSCs in the presence of TGF-β3 induction medium showed no increase in the expression level of osteogenic markers such as OCN and ALP, as evaluated by qPCR (data not shown), while an almost three-fold increase was observed in the expression levels of Scx, Dcn, Tnmd, and Bgy.
It has been shown that the cell delivery vehicle plays a critical role in the effectiveness of MSC-based regenerative therapies [44–46]. In this study, we utilized RGD-coupled alginate microspheres as a delivery vehicle for dental-derived MSCs. This encapsulation system provides a unique 3D cell delivery scaffold for tendon tissue engineering. As SEM analysis showed, the alginate microspheres exhibit a porous structure, which enables the diffusion of oxygen, nutrients and signaling molecules throughout the alginate hydrogel. This structural characteristic also enables growth factors, such as TGF-β3, to penetrate the alginate microspheres and participate in regulating the proliferation and differentiation of the encapsulated MSCs. This unique structural property, combined with the presence of RGD tripeptide, is designed to facilitate MSC-alginate interactions, leading to enhanced MSC adhesion and availability of oxygen, nutrients and desirable growth factors. These properties make alginate hydrogel a highly suitable scaffold biomaterial for tendon tissue engineering.
One particularly promising aspect of the current study is the fact that the MSCs were not required to be cultured in tendon tissue induction media prior to their participation in in vivo studies or subcutaneous transplantation. We showed that providing a favorable microenvironment and an initiating signal from TGF-β3 can be sufficient to promote tendon tissue formation. The results of the current study confirmed the importance of the delivery of appropriate cytokines, specifically TGF-β3, to support tendon tissue regeneration by the encapsulated dental-derived MSCs. These results highlight the vital role played by the microenvironment, as well as the value of presenting inductive signals necessary to support the viability and differentiation of MSCs along a desired phenotype.
Here, we sought to establish dental-derived MSCs as a population of MSCs that can be used in applications where the regeneration of tendon tissue is desired. Therefore, one of our primary objectives was to determine the plasticity of these MSCs in this regenerative mode at an ectopic site. In our future work, the combination of these dental-derived MSCs and our RGD-coupled alginate encapsulation system will be used in sites where tendon tissue regeneration is likely to find clinical applications.
5. Conclusions
In the current study, we developed an alternative treatment modality for tendon regeneration based on encapsulated dental-derived stem cells, namely PDLSCs and GMSCs, using an injectable and biodegradable RGD-coupled alginate hydrogel scaffold to support their differentiation. The MSC and alginate constructs were shown to effectively differentiate and organize their extracellular matrix into tendon tissue. Our findings demonstrate the important role of the microenvironment as well as the presentation of inductive signals (TGF-β3) for the viability and differentiation of dental MSCs into tendon-like tissue.
Supplementary Material
Figure s1. Quantitative live/dead staining of encapsulated MSCs in either RGD-coupled alginate or non-RGD-coupled alginate microspheres, in the presence or absence of TGF-β3, after 14 days of culturing in regular culture media. In comparison to MSCs encapsulated in non-RGD-coupled alginate microspheres, MSCs encapsulated in RGD-containing alginate microspheres showed significantly higher degrees of viability at all the tested time intervals, while the presence of TGF-β3 ligands did not have any significant effects on MSC viability. *P <0.05, NS= not significant.
Figure s2. The expression levels of Dcn and Tnmd in hBMMSCs, PDLSCs, and GMSCs without the scaffold after two weeks of tenogenic differentiation in vitro in the presence of TGF-β3 ligand. In comparison to MSCs without scaffolds, the encapsulated MSCs expressed higher levels (p>0.05) of tendon markers, confirming the important role of the encapsulation biomaterial. *P <0.05, NS= not significant.
Acknowledgments
This work was supported by grants from the National Institute of Dental and Craniofacial Research (R01 DE019932 and R01 DE017449 to S.S.). An author (AM) was supported by Provost’s Postdoctoral Scholar Research Grant by the USC Office of Postdoctoral Affairs, while another (MLS) was supported by R01 DE013045. The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sharma P, Maffulli N. Biology of tendon injury: healing, modeling and remodeling. J Musculoskelet Neuronal Interact. 2006;6:181–190. [PubMed] [Google Scholar]
- 2.Hunziker EB. Articular cartilage repair: basic science and clinical progress. a review of the current status and prospects. Osteoarthritis Cartilage. 2002;10:432–63. doi: 10.1053/joca.2002.0801. [DOI] [PubMed] [Google Scholar]
- 3.Beredjiklian PK, Favata M, Cartmell JS, Flanagan CL, Crombleholme TM, Soslowsky LJ. Regenerative versus reparative healing in tendon: a study of biomechanical and histological properties in fetal sheep. Ann Biomed Eng. 2003;31:1143–1152. doi: 10.1114/1.1616931. [DOI] [PubMed] [Google Scholar]
- 4.Boileau P, Brassart N, Watkinson DJ, Carles M, Hatzidakis AM, Krishnan SG. Arthroscopic repair of full-thickness tears of the supra- spinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87:1229–1240. doi: 10.2106/JBJS.D.02035. [DOI] [PubMed] [Google Scholar]
- 5.Aspenberg P. Stimulation of tendon repair: mechanical loading, GDFs and platelets. A mini-review Int Orthop. 2007;31:783–9. doi: 10.1007/s00264-007-0398-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.James R, Kumbar SG, Laurencin CT, Balian G, Chhabra AB. Tendon tissue engineering: adipose-derived stem cell and GDF-5 mediated regeneration using electrospun matrix systems. Biomed Mater. 2011;6:1–13. doi: 10.1088/1748-6041/6/2/025011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Juncosa-Melvin N, Boivin GP, Gooch C, Galloway MT, West JR, Dunn MG, et al. The effect of autologous mesenchymal stem cells on the biomechanics and histology of gel-collagen sponge constructs used for rabbit patellar tendon repair. Tissue Eng Part A. 2006;12:369–79. doi: 10.1089/ten.2006.12.369. [DOI] [PubMed] [Google Scholar]
- 8.Nourissat G, Diop A, Maurel N, Salvat C, Dumont S, Pigenet A, et al. Mesenchymal stem cell therapy regenerates the native bone-tendon junction after surgical repair in a degenerative rat model. PLoS One. 2010;18:e12248. doi: 10.1371/journal.pone.0012248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bi Y, Ehirchiou D, Kilts TM, Inkson CA, Embree MC, Sonoyama W, et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med. 2007;13:1219–27. doi: 10.1038/nm1630. [DOI] [PubMed] [Google Scholar]
- 10.Tan Q, Lui PP, Rui YF, Wong YM. Comparison of potentials of stem cells isolated from tendon and bone marrow for musculoskeletal tissue engineering. Tissue Eng Part A. 2012;18:840–851. doi: 10.1089/ten.tea.2011.0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: stem cells and their niche. Cell. 2004;116:769–778. doi: 10.1016/s0092-8674(04)00255-7. [DOI] [PubMed] [Google Scholar]
- 12.Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. PNAS. 2000;97:13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, et al. SHED: Stem cells from human exfoliated deciduous teeth. PNAS. 2003;100:5807–5812. doi: 10.1073/pnas.0937635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iwata T, Yamato M, Zhang Z, Mukobata S, Washio K, Ando T, et al. Validation of human periodontal ligament-derived cells as a reliable source for cytotherapeutic use. J Clin Periodontol. 2010;37:1088–1099. doi: 10.1111/j.1600-051X.2010.01597.x. [DOI] [PubMed] [Google Scholar]
- 15.Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–1155. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Q, Shi S, Liu Y, Uyanne J, Shi Y, Shi S, et al. Mesenchymal stem cells derived from human gingiva are capable of immunomodulatory functions and ameliorate inflammation-related tissue destruction in experimental colitis. J Immunol. 2009;183:7787–7798. doi: 10.4049/jimmunol.0902318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mensing N, Gasse H, Hambruch N, Haeger JD, Pfarrer C, Staszyk C. Isolation and characterization of multipotent mesenchymal stromal cells from the gingiva and the periodontal ligament of the horse. BMC Vet Res. 2011;7:42–55. doi: 10.1186/1746-6148-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu J, Sato C, Cerletti M, Wagers A. Notch signaling in the regulation of stem cell self-renewal and differentiation. Curr Top Dev Biol. 2010;92:367–409. doi: 10.1016/S0070-2153(10)92012-7. [DOI] [PubMed] [Google Scholar]
- 19.Kratchmarova I, Blagoev B, Haack-Sorensen M, Kassem M, Mann M. Mechanism of divergent growth factor effects in mesenchymal stem cell differentiation. Science. 2005;308:1472–1477. doi: 10.1126/science.1107627. [DOI] [PubMed] [Google Scholar]
- 20.Czyz J, Wobus AM. Embryonic stem cell differentiation: the role of extracellular factors. Differentiation. 2001;68:167–174. doi: 10.1046/j.1432-0436.2001.680404.x. [DOI] [PubMed] [Google Scholar]
- 21.Pham QP, Kasper FK, Scott Baggett L, Raphael RM, Jansen JA, Mikos AG. The influence of an in vitro generated bone-like extracellular matrix on osteoblastic gene expression of marrow stromal cells. Biomaterials. 2008;29:2729–2739. doi: 10.1016/j.biomaterials.2008.02.025. [DOI] [PubMed] [Google Scholar]
- 22.Jorgensen HG, McLellan SD, Crossan JF, Curtis AS. Neutralisation of TGF beta or binding of VL A-4 to fibronectin prevents rat tendon adhesion following transection. Cytokine. 2005;30:195–202. doi: 10.1016/j.cyto.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 23.Klass BR, Rolfe KJ, Grobbelaar AO. In vitro flexor tendon cell response to TGF-beta1: a gene expression study. J Hand Surg Am. 2009;34:495–503. doi: 10.1016/j.jhsa.2008.10.032. [DOI] [PubMed] [Google Scholar]
- 24.Chen X, Yin Z, Chen JL, Shen WL, Liu HH, Tang QM, et al. Force and scleraxis synergistically promote the commitment of human ES cells derived MSCs to tenocytes. Sci Rep. 2012;2:977. doi: 10.1038/srep00977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pryce BA, Watson SS, Murchison ND, Staverosky JA, Dünker N, Schweitzer R. Recruitment and maintenance of tendon progenitors by TGF beta signaling are essential for tendon formation. Development. 2009;136:1351–61. doi: 10.1242/dev.027342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang K, Wang Z, Du Q, Yu J, Wang A, Xiong Y. A new TGF-β3 controlled-released chitosan scaffold for tissue engineering synovial sheath. J Biomed Mater Res A. 2013 doi: 10.1002/jbm.a.34742. [DOI] [PubMed] [Google Scholar]
- 27.Barsby T, Guest D. Transforming Growth Factor Beta3 Promotes Tendon Differentiation of Equine Embryo-Derived Stem Cells. Tissue Eng Part A. 2013 doi: 10.1089/ten.tea.2012.0372. [DOI] [PubMed] [Google Scholar]
- 28.Moshaverinia A, Chen C, Akiyama K, Ansari S, Xu X, Chee WW, et al. Alginate hydrogel as a promising scaffold for dental-derived stem cells: an in vitro study. J Mater Sci: Mater Med. 2012;23:3041–3051. doi: 10.1007/s10856-012-4759-3. [DOI] [PubMed] [Google Scholar]
- 29.Moshaverinia A, Chen C, Akiyama K, Xu X, Chee WW, Schricker SR, et al. Encapsulated dental-derived stem cells in an injectable and biodegradable scaffold for applications in bone tissue engineering. J Biomed Mater Res Part A. 2013;101:3285–94. doi: 10.1002/jbm.a.34546. [DOI] [PubMed] [Google Scholar]
- 30.Moshaverinia A, Ansari S, Chen C, Xu X, Akiyama K, Snead SL, et al. Co-encapsulation of anti-BMP2 monoclonal antibody and mesenchymal stem cells in alginate microspheres for bone tissue engineering. Biomaterials. 2013;34:6572–9. doi: 10.1016/j.biomaterials.2013.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rowley JA, Madlambayan G, Mooney DJ. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials. 1999;20:45–51. doi: 10.1016/s0142-9612(98)00107-0. [DOI] [PubMed] [Google Scholar]
- 32.Evangelista MB, Hsiong SX, Fernandes R, Sampaio P, Kong H, Barrias CC, et al. Upregulation of bone cell differentiation through immobilization within a synthetic extracellular matrix. Biomaterials. 2007;28:3644–3655. doi: 10.1016/j.biomaterials.2007.04.028. [DOI] [PubMed] [Google Scholar]
- 33.Re’em T, Tsur-Gang O, Cohen S. The effect of immobilized RGD peptide in macroporous alginate scaffolds on TGFb1-induced chondrogenesis of human mesenchymal stem cells. Biomaterials. 2010;31:6746–6755. doi: 10.1016/j.biomaterials.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 34.Moshaverinia A, Xu X, Chen C, Akiyama K, Snead ML, Shi S. Dental mesenchymal stem cells encapsulated in an alginate hydrogel co-delivery microencapsulation system for cartilage regeneration. Acta Biomater. 2013;9:9343–50. doi: 10.1016/j.actbio.2013.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bian L, Zhai DY, Tous E, Rai R, Mauck RL, Burdick JA. Enhanced MSC chondrogenesis following delivery of TGF-b3 from alginate microspheres within hyaluronic acid hydrogels in vitro and in vivo. Biomaterials. 2011;32:6425–6434. doi: 10.1016/j.biomaterials.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Docheva D, Hunziker EB, Fässler R, Brandau O. Tenomodulin Is Necessary for Tenocyte Proliferation and Tendon Maturation. Mol Cell Biol. 2005;25:699–705. doi: 10.1128/MCB.25.2.699-705.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cserjesi P, Brown D, Ligon KL, Lyons GE, Copeland NG, Gilbert DJ, et al. Scleraxis: a basic helix-loop-helix protein that prefigures skeletal formation during mouse embryogenesis. Development. 1995;121:1099–110. doi: 10.1242/dev.121.4.1099. [DOI] [PubMed] [Google Scholar]
- 38.Lee SH, Yang DK, Choi BY, Lee YH, Kim SY, Jeong D, et al. The transcription factor Eya2 prevents pressure overload-induced adverse cardiac remodeling. J Mol Cell Cardiol. 2009;46:596–605. doi: 10.1016/j.yjmcc.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 39.Robinson PS, Huang TF, Kazam E, Iozzo RV, Birk DE, Soslowsky LJ. Influence of decorin and biglycan on mechanical properties of multiple tendons in knockout mice. J Biomech Eng. 2005;127:181–5. doi: 10.1115/1.1835363. [DOI] [PubMed] [Google Scholar]
- 40.Sharma P, Maffulli N. Biology of tendon injury: healing, modeling and remodeling. J Musculoskelet Neuronal Interact. 2006;6:181–190. [PubMed] [Google Scholar]
- 41.Kannus P. Structure of the tendon connective tissue. Scand J Med Sci Sports. 2000;10:312–320. doi: 10.1034/j.1600-0838.2000.010006312.x. [DOI] [PubMed] [Google Scholar]
- 42.Awad HA, Butler DL, Boivin BP, Smith FN, Malaviya P, Huibregtse B, et al. Autologous mesenchymal stem cell-mediated repair of tendon. Tissue Engineering. 1999;5:267–277. doi: 10.1089/ten.1999.5.267. [DOI] [PubMed] [Google Scholar]
- 43.Chong AK, Chang J, Go JC. Mesenchymal stem cells and tendon healing. Frontiers in Bioscience. 2009;14:4598– 4605. doi: 10.2741/3552. [DOI] [PubMed] [Google Scholar]
- 44.Cantu DA, Hematti P, Kao WJ. Cell encapsulating biomaterial regulates mesenchymal stromal/stem cell differentiation and macrophage immunophenotype. Stem Cell Transl Med. 2012;1:740–9. doi: 10.5966/sctm.2012-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang W, Ma N, Kratz K, Xu X, Li Z, Roch T, et al. The influence of polymer scaffolds on cellular behaviour of bone marrow derived human mesenchymal stem cells. Clin Hemorheol Microcirc. 2012;52:357–73. doi: 10.3233/CH-2012-1611. [DOI] [PubMed] [Google Scholar]
- 46.Alsberg A, Anderson KW, Albeiruti A, Franceschi RT, Mooney DJ. Cell- interactive alginate hydrogels for bone tissue engineering. J Dent Res. 2001;80:2025–2029. doi: 10.1177/00220345010800111501. [DOI] [PubMed] [Google Scholar]
- 47.Xu PX, Cheng J, Epstein JA, Maas RL. Mouse Eya genes are expressed during limb tendon development and encode a transcriptional activation function. Proc Natl Acad Sci USA. 1997;94:11974–11979. doi: 10.1073/pnas.94.22.11974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dourte LM, Pathmanathan L, Mienaltowski MJ, Jawad AF, Birk DE, Soslowsky LJ. Mechanical, Compositional, and Structural Properties of the Mouse Patellar Tendon with Changes in Biglycan Gene Expression. J Orthop Res. 2013;31:1430–1437. doi: 10.1002/jor.22372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu HH, Vo JM, Chin HS, Lin J, Cozin M, Tsay R, et al. Controlled delivery of platelet-rich plasma derived growth factors for bone formation. J Biomed Mater Res A. 2008;86:1128–36. doi: 10.1002/jbm.a.31740. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure s1. Quantitative live/dead staining of encapsulated MSCs in either RGD-coupled alginate or non-RGD-coupled alginate microspheres, in the presence or absence of TGF-β3, after 14 days of culturing in regular culture media. In comparison to MSCs encapsulated in non-RGD-coupled alginate microspheres, MSCs encapsulated in RGD-containing alginate microspheres showed significantly higher degrees of viability at all the tested time intervals, while the presence of TGF-β3 ligands did not have any significant effects on MSC viability. *P <0.05, NS= not significant.
Figure s2. The expression levels of Dcn and Tnmd in hBMMSCs, PDLSCs, and GMSCs without the scaffold after two weeks of tenogenic differentiation in vitro in the presence of TGF-β3 ligand. In comparison to MSCs without scaffolds, the encapsulated MSCs expressed higher levels (p>0.05) of tendon markers, confirming the important role of the encapsulation biomaterial. *P <0.05, NS= not significant.