Abstract
Using saturation transfer electron paramagnetic resonance, we have detected the rotational motion of a spin label rigidly attached to the sarcoplasmic reticulum Ca2+-ATPase (ATP phosphohydrolase, EC 3.6.1.3). At 4 degrees C, the spectrum indicates an effective rotational correlation time of 60 microsec, determined by comparison with reference spectra obtained from theoretical calculations and from experiments on model systems. This motion appears to correspond to rotation of the enzyme with respect to the membrane, because the motion persists when the membrane fragments are immobilized by sedimentation and the motion stops when the polypeptide chains, but not the membrane vesicles, are crosslinked by glutaraldehyde. The rotational mobility of the enzyme increases with increasing temperature, and this increase becomes more gradual when the temperature exceeds 20 degrees C; the same kind of temperature dependence has been observed previously for lipid fluidity and enzymatic activity.
Full text
PDF
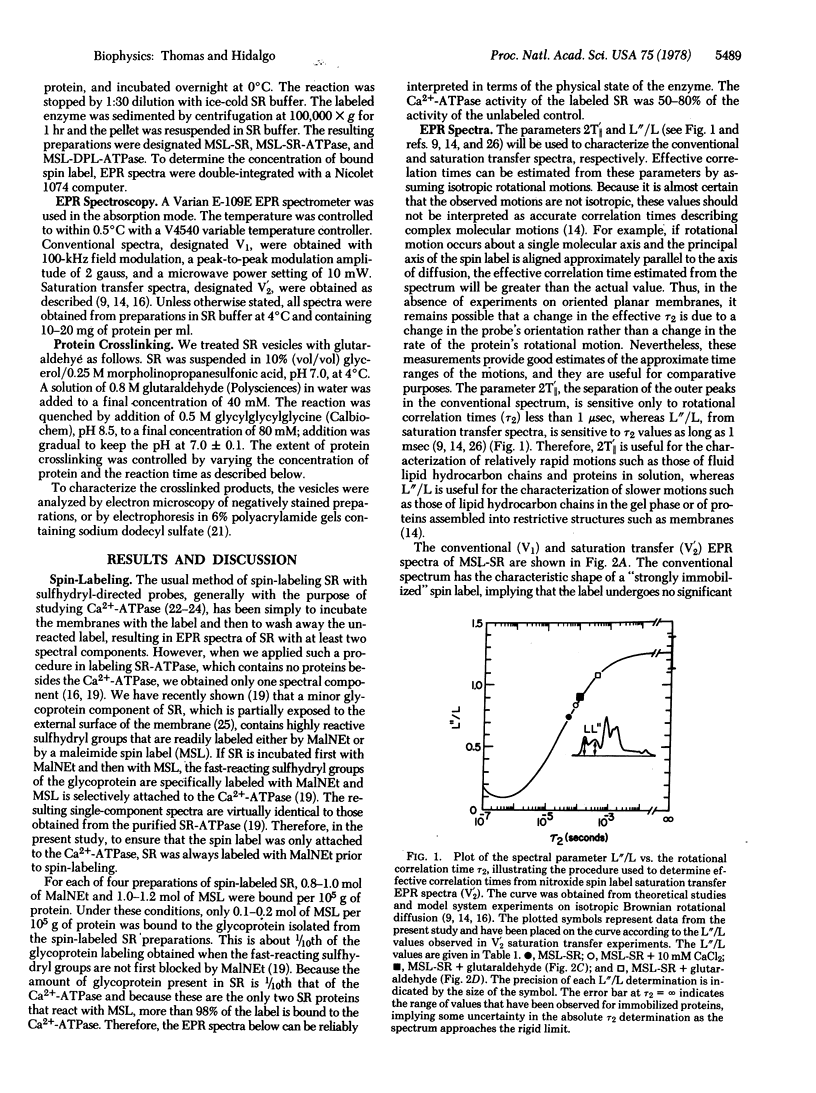
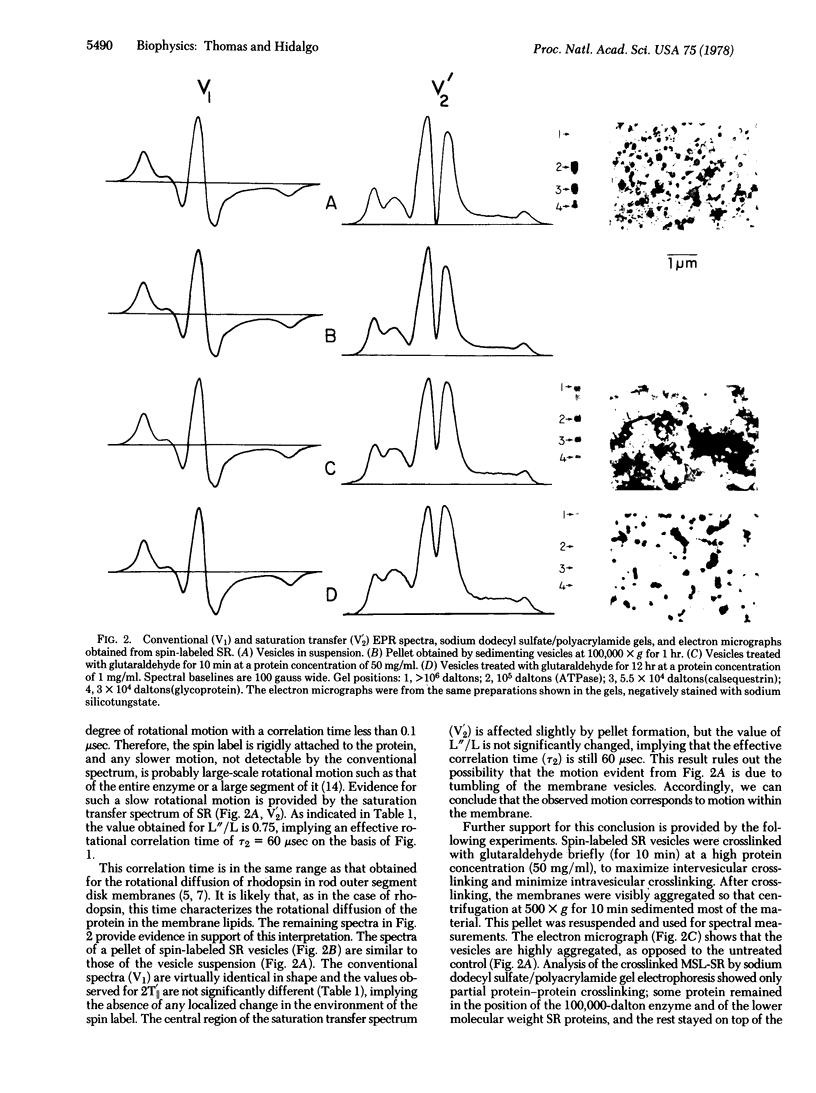
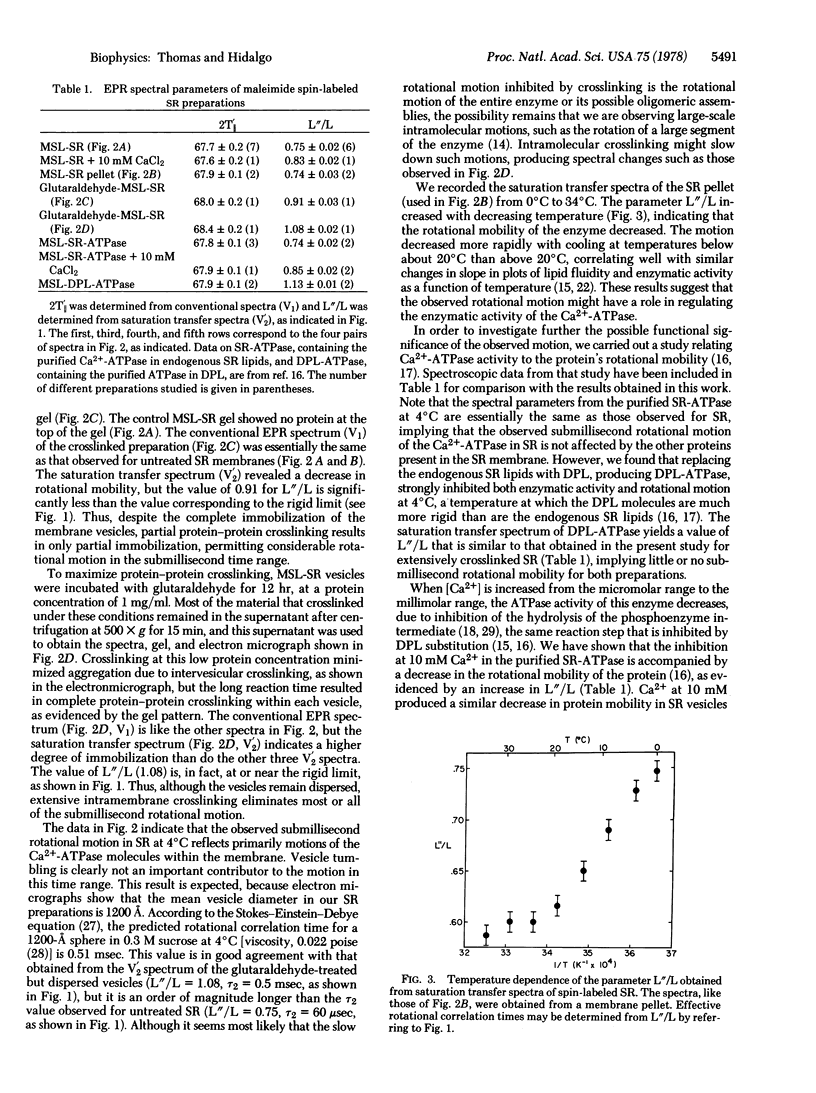

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baroin A., Thomas D. D., Osborne B., Devaux P. F. Saturation transfer electron paramagnetic resonance on membrane-bound proteins. I-Rotational diffusion of rhodopsin in the visual receptor membrane. Biochem Biophys Res Commun. 1977 Sep 9;78(1):442–447. doi: 10.1016/0006-291x(77)91274-8. [DOI] [PubMed] [Google Scholar]
- Cherry R. J., Bürkli A., Busslinger M., Schneider G., Parish G. R. Rotational diffusion of band 3 proteins in the human erythrocyte membrane. Nature. 1976 Sep 30;263(5576):389–393. doi: 10.1038/263389a0. [DOI] [PubMed] [Google Scholar]
- Coan C. R., Inesi G. Ca2+-dependent effect of ATP on spin-labeled sarcoplasmic reticulum. J Biol Chem. 1977 May 10;252(9):3044–3049. [PubMed] [Google Scholar]
- Cone R. A. Rotational diffusion of rhodopsin in the visual receptor membrane. Nat New Biol. 1972 Mar 15;236(63):39–43. doi: 10.1038/newbio236039a0. [DOI] [PubMed] [Google Scholar]
- Dutton A., Rees E. D., Singer S. J. An experiment eliminating the rotating carrier mechanism for the active transport of Ca ion in sarcoplasmic reticulum membranes. Proc Natl Acad Sci U S A. 1976 May;73(5):1532–1536. doi: 10.1073/pnas.73.5.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffney B. J., McNamee C. M. Spin-label measurements in membranes. With appendix: a use of computers in EPR spectroscopy. Methods Enzymol. 1974;32:161–198. [PubMed] [Google Scholar]
- Hidalgo C., Ikemoto N. Disposition of proteins and aminophospholipids in the sarcoplasmic reticulum membrane. J Biol Chem. 1977 Dec 10;252(23):8446–8454. [PubMed] [Google Scholar]
- Hidalgo C., Ikemoto N., Gergely J. Role of phospholipids in the calcium-dependent ATPase of the sarcoplasmic reticulum. Enzymatic and ESR studies with phospholipid-replaced membranes. J Biol Chem. 1976 Jul 25;251(14):4224–4232. [PubMed] [Google Scholar]
- Hidalgo C., Thomas D. D. Heterogeneity of SH groups in sarcoplasmic reticulum. Biochem Biophys Res Commun. 1977 Oct 24;78(4):1175–1182. doi: 10.1016/0006-291x(77)91417-6. [DOI] [PubMed] [Google Scholar]
- Hyde J. S. Saturation-transfer spectroscopy. Methods Enzymol. 1978;49:480–511. doi: 10.1016/s0076-6879(78)49021-4. [DOI] [PubMed] [Google Scholar]
- Ikemoto N. Transport and inhibitory Ca2+ binding sites on the ATPase enzyme isolated from the sarcoplasmic reticulum. J Biol Chem. 1975 Sep 25;250(18):7219–7224. [PubMed] [Google Scholar]
- Inesi G., Millman M., Eletr S. Temperature-induced transitions of function and structure in sarcoplasmic reticulum membranes. J Mol Biol. 1973 Dec 25;81(4):483–504. doi: 10.1016/0022-2836(73)90518-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Martonosi A., Fortier F. The effect of anti-ATPase antibodies upon the Ca++ transport of sarcoplasmic reticulum. Biochem Biophys Res Commun. 1974 Sep 9;60(1):382–389. doi: 10.1016/0006-291x(74)90216-2. [DOI] [PubMed] [Google Scholar]
- Poo M., Cone R. A. Lateral diffusion of rhodopsin in the photoreceptor membrane. Nature. 1974 Feb 15;247(5441):438–441. doi: 10.1038/247438a0. [DOI] [PubMed] [Google Scholar]
- Schlessinger J., Koppel D. E., Axelrod D., Jacobson K., Webb W. W., Elson E. L. Lateral transport on cell membranes: mobility of concanavalin A receptors on myoblasts. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2409–2413. doi: 10.1073/pnas.73.7.2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. D., Seidel J. C., Hyde J. S., Gergely J. Motion of subfragment-1 in myosin and its supramolecular complexes: saturation transfer electron paramagnetic resonance. Proc Natl Acad Sci U S A. 1975 May;72(5):1729–1733. doi: 10.1073/pnas.72.5.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonomura Y., Morales M. F. Change in state of spin labels bound to sarcoplasmic reticulum with change in enzymic state, as deduced from ascorbate-quenching studies. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3687–3691. doi: 10.1073/pnas.71.9.3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Yamada S., Tonomura Y. Reaction mechanism of the Ca 2+ -dependent ATPase of sarcoplasmic reticulum from skeletal muscle. VII. Recognition and release of Ca 2+ ions. J Biochem. 1972 Aug;72(2):417–425. doi: 10.1093/oxfordjournals.jbchem.a129917. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Tonomura Y. Chemical modification of the Ca2+ -dependent ATPase of sarcoplasmic reticulum from skeletal muscle. II. Use of 2, 4, 6-trinitrobenzenesulfonate to show functional movements of the ATPase molecule. J Biochem. 1976 Apr;79(4):693–707. doi: 10.1093/oxfordjournals.jbchem.a131121. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Tonomura Y. Chemical modification of the Ca2+-dependent ATPase of sarcoplasmic reticulum from skeletal muscle. III. Changes in the distribution of exposed lysine residues among subfragments with change in enzymatic state. J Biochem. 1977 Sep;82(3):653–660. doi: 10.1093/oxfordjournals.jbchem.a131740. [DOI] [PubMed] [Google Scholar]