Abstract
The prevalence of allergy is rising globally at an alarming rate, which is currently at 20-40% of individuals in westernized nations. In the eye, allergic conditions can take on the acute form such as in seasonal and perennial allergic conjunctivitis, or a more severe and debilitating chronic form such as in vernal and atopic keratoconjunctivitis. Indeed, some key aspects of allergic eye disease pathophysiology are understood, such as the chief role of mast cells in the acute allergic reaction, and the contribution of eosinophils in late onset and chronic allergy. However, recent developments in animal models and clinical studies have uncovered new and important roles for previously underappreciated players, including chemokine receptors on ocular surface dendritic cells such as CCR7, histamine and leukotriene receptors on conjunctival goblet cells, and a role for mast cells in late-onset manifestations. Furthermore, recent work in animal models has now delineated the contribution of IL-4 in the increased incidence of corneal graft rejection seen in perioperative allergic conjunctivitis. Recent studies such as these mean that conventional paradigms and concepts should therefore be revisited. The aim of this review is to highlight the most recent advances and insights on newly appreciated players in the pathogenesis of allergic eye disease.
Ocular allergy describes a spectrum of clinical conditions, ranging from the common conditions of seasonal allergic conjunctivitis (SAC), to the clinically more severe and chronic diseases, vernal keratoconjunctivitis (VKC) and atopic keratoconjunctivitis (AKC) [1,2]. Each form of conjunctival inflammation involves different cellular and molecular pathways, including a role for conjunctival epithelial and goblet cells in the inflammatory process.
During SAC the response is predominantly mast cell-mediated, and mast cells and their secreted molecules have been targets for therapeutic intervention, resulting in the development of several combination anti-allergic drugs for topical use. In contrast, in VKC and AKC, the inflammation is chronic, allergen-independent, the conjunctival cellular infiltrate comprises T cells, eosinophils and neutrophils which infiltrate the epithelium and stroma. Hence mast cell-targeted therapy alone is ineffective. In these two chronic forms of disease corneal involvement can occur, leading to impairment of vision. Hence it is important to treat VKC and AKC with immunosuppressive therapy, usually steroids and/or cyclosporin A given topically or, in some cases, systemically. Unfortunately, whilst effective in dampening the immune response, these immunosuppressive drugs often have serious side effects if used long term. Further advancement of our understanding of ocular allergy pathophysiology is therefore imperative in the development of novel strategies and effective to treatment strategies.
To this end, recent studies have shed considerable new light on pathophysiological mechanisms of ocular allergy. Work out of Virginia Calder's and others have led to greater attention being placed on the immunologic contribution of conjunctival epithelium in allergic eye disease. Relatedly, Darlene Dartt's lab has provided strong evidence to suggest that conjunctival goblet cells directly contribute to the pathology of allergic conjunctivitis via histamine (and leukotrienes) ligation. Furthermore, recent findings out of Santa Ono's lab has indicated a contribution of histamine released by mast cells to late-phase responses, and thereby targeting phosphorylation-dependent vimentin disassembly of activated mast cells may have a therapeutic value.
Novel insights into adaptive immune responses in allergic eye disease have also been recently highlighted. Work out of Daniel Saban's lab has identified a key chemokine receptor CCR7 that mediates the dendritic cell-T cell interaction, and that blocking CCR7 at the ocular surface has a significant therapeutic effect on the mouse model of allergic conjunctivitis. Relatedly, mouse modeling work out of Jerry Niederkorn's lab, has delineated the chief T cell secreted cytokine in allergy, IL-4, responsible for increased incidence of corneal immune rejection in perioperative allergic conjunctivitis. This work offers glimmers of hope by way of anti-IL-4 blocking antibody as an anti-rejection therapy for the high-risk atopic host needing a corneal transplant.
This review will discuss such recent findings, with a particular focus on: 1) the immunomodulatory role of the conjunctival epithelium; 2) the contribution of conjunctival goblet cells to allergic conjunctivitis; 3) costimulation of mast cells in ocular allergy biology, the role of CCR7 expression by dendritic cells, and the function of IL-4 in increased incidence of corneal allograft rejection seen in allergic conjunctivitis.
I. Epithelial Cell Biology
a. Immunomodulatory Role of Human Conjunctival Epithelial Cells in Allergic Eye Disease
Epithelial cells of the ocular surface, respiratory and intestinal tracts form part of the mucosal immune system, and are the first to encounter external antigens including microorganisms, airborne allergens and pollutants. In addition to providing a physical barrier against infection, they function as innate effector cells, and their expression of pattern recognition molecules (eg Toll-like Receptors; TLRs) allows them to mediate a range of responses. Upon recognition of a pathogen, epithelial cells release antimicrobial peptides, cytokines and chemokines which, in turn, immobilise and lyse microorganisms, and attract other innate and adaptive cells to effectively respond to the infection.
As a consequence of chronic inflammation, in particular in AKC, the conjunctival epithelium can become thinner and there is evidence of activation, with an upregulation of HLA-DR expression and ICAM-13.
What is the cytokine microenvironment at the ocular surface?
Studies of VKC conjunctival tissue specimens have found increased numbers of activated CD4+ T cells, mainly localized to the subepithelial layer of the affected tissue, with increases in HLA-DR expression, Langerhans’ cells and activated macrophages (CD68+) [3]. CD4+T cell lines and clones derived from VKC conjunctival biopsy specimens were Th2 [4-6], whilst VKC tears-derived T cells expressed IL-4 intracellularly [7]. In contrast, in AKC conjunctival tissues increases in IL-2 mRNA and IFN-g expressing T cells were also detected, suggesting a Th1-cell infiltrate [6]. Comparing conjunctival tissues from VKC with controls, increased expression levels of CCL2, CCL5, CCL7, and CCL11 were detected [8] with expression of CXCR3 being specifically localized to T cells [9], and CXCL9 was highly expressed [10].
Conjunctival epithelial cell receptors
Mucosal epithelia during inflammation have altered expression profiles of TLRs, and, it has been found in vivo and in vitro that MUC-1, a membrane-tethered mucin expressed by mucosal epithelial cells, associates with TLR-5 to downregulate MyD88 signaling events in airway epithelia, thus protecting airways from inflammation [11]. Although it is unclear what conjunctival epithelial signaling pathways are involved, MUC-5AC and MUC-16 are expressed at the ocular surface and levels are altered during AKC [12]. In VKC, an increase in TLR-4 expression by conjunctival and corneal epithelium was observed [13,14] and in AKC, an increase in TLR-2 was reported [15].
The IOBA-NHC human epithelial cell line has been found to express TLRs, and to respond to stimulation via TLR-2, -3 and -4 by secreting IL-8, and upregulating expression of ICAM-1, CD44 and IL-17 receptors(R)-A, -C and -D which could be modulated following stimulation via TLRs (Figure 1; Offiah et al, unpublished data). A similar approach with primary human conjunctival epithelial cells via TLR-3 ligands using oligonucleotide microarrays found an elevation of transcripts and production of several cytokines and chemokines including thymic stromal lymphopoietin (TSLP) [16]. Conjunctival epithelial cell responses to proinflammatory cytokines
Figure 1.
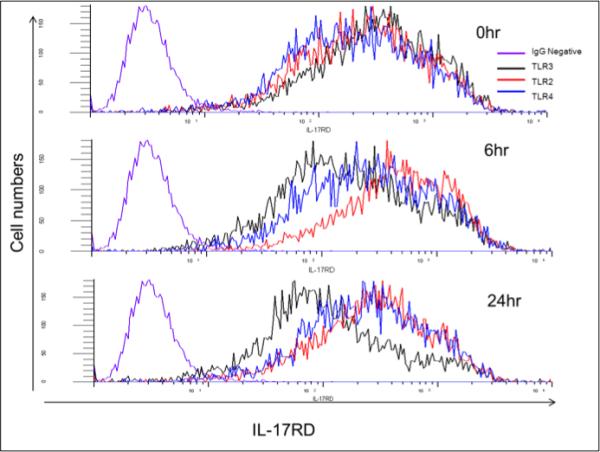
Conjunctival epithelial cell line (IOBA) expression of IL-17RD following exposure to TLR-2, -3, or -4 ligands (zymosan, Poly I:C and LPS, respectively), at 2, 6 and 24hr post treatment.
In addition to Th1 and Th2 cells, Th17 cells have also been detected within the conjunctival epithelium during conjunctival inflammatory disease [17], and the response to cytokines secreted by infiltrating Th1-, Th2- and Th17 cells has been investigated in vitro. The IOBA-NHC line responded to proinflammatory cytokines, with TNF-a inducing production of IL-12p40, and IFN-g upregulating secretion of IP-10 and RANTES, whilst IL-4 and IL-13 increased eotaxin-1 [18]. Primary cultures of human conjunctival epithelial cells and a cell line (ChWK) express ICAM-1 and HLA-DR which were significantly upregulated following exposure to IFN-g [19]. In the same study ChWK cells expressed CD80 and CD86 and augmented T cell proliferation to alloantigens.
Conclusion
Conjunctival epithelial cells play a role in ocular allergy. They have receptors for, and respond to, proinflammatory cytokines and, via production of cytokines, could also regulate inflammation at the ocular surface. A recent review has highlighted epithelial cell-derived IL-33 and TSLP as potential targets for treating asthma [20], and future studies will investigate these mechanisms in allergic eye disease.
b. Goblet Cells Play a Direct Role in Pathogenesis of Allergic Conjunctivitis
In the lung, an increase in mucin secretion by the airway goblet cells is well known to play a role in allergic responses such as asthma [21]. In contrast to the lung, the role of conjunctival goblet cells in allergic conjunctivitis is little investigated. Most types of allergic conjunctivitis are accompanied by an increase in ocular mucus, although the characteristics of ocular mucus can vary between types of allergic conjunctivitis. However, a direct role for conjunctival goblet cell mucous secretion in allergic conjunctivitis had not been demonstrated. With our ability to culture conjunctival goblet cells from rat and human [22-25], we can determine if allergic mediators directly stimulate mucous secretion from these cells.
Multiple allergic mediators are produced during ocular inflammation and their type can vary depending upon the form of allergic conjunctivitis [26]. Examples of allergic mediators produced include histamine, leukotrienes, prostaglandins, and cytokines. In the present study we report on the effect of histamine and the cysteinyl leukotrienes (cysLT) LTC4, LTD4, and LTE4 on conjunctival goblet cell secretion and identify the receptors used for this function.
Goblet cells were cultured from rat and human conjunctiva as previously described [23-25]. Goblet cells were identified by positive staining for cytokeratin 7 and MUC5AC [24, 25]. Experiments described herein were performed with rat conjunctival goblet cells. Human goblet cells have been used in select experiments and demonstrated that results in rat goblet cells mimicked those in human and that rat goblet cells are an excellent model for human cells [23, 27].
Histamine is a major mediator in allergic conjunctivitis that is produced and released by mast cells resident in the conjunctival stroma when activated by allergen. Histamine acts via G protein-linked receptors of which there are four for histamine, H1, H2, H3, and H4 [22]. A given tissue can have 2-3 types of histamine receptors present [28]. We used western blotting analysis of rat conjunctiva and cultured goblet cells to determine which histamine receptors are present in conjunctival goblet cells. We found that all four histamine receptors were present indicated by a major band in conjunctival tissue and cultured goblet cells (Figure 2A). Bands in the conjunctival tissue and goblet cells were detected at the same molecular weights as in the positive controls (Figure 2A). Using immunofluorescence microscopy, all four histamine receptors were present in both stratified squamous and goblet cells of the conjunctival epithelium as well as in cultured rat conjunctival goblet cells (Figure 2B). Results for the H3 receptor are shown. The location of the other histamine receptors was similar [22]. In addition, use of RT-PCR confirmed the presence of all four histamine receptors in the conjunctiva and its goblet cells [22]. Three different techniques demonstrated that all four histamine receptors are present in conjunctival goblet cells both in vivo and in culture.
Figure 2.
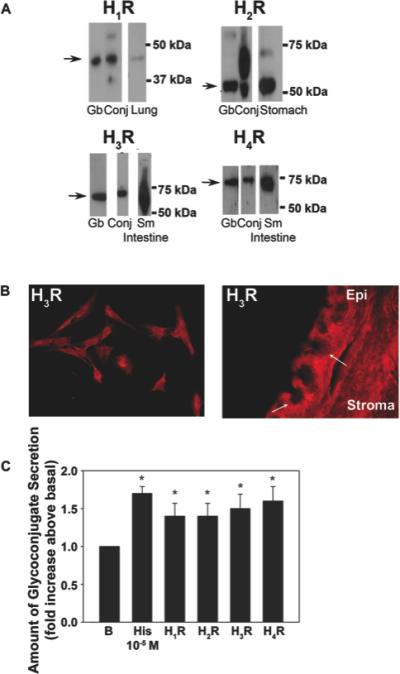
Presence of histamine receptors in rat conjunctiva and cultured goblet cells and stimulation of high molecular weight glycoconjugate secretion from cultured goblet cells. A. Rat conjunctiva and cultured goblet cells were homogenized and western blot analysis performed under denaturing conditions with anti-histamine receptor subtype antibodies. The blot is representative of three individual animals. H1R, histamine receptor subtype 1; H2R, histamine receptor subtype 2; H3R, histamine receptor subtype 3; H4R, histamine receptor subtype 4. B. Rat conjunctiva was removed and fixed. Rat conjunctival goblet cells were grown on glass slides and fixed. Immunofluorescence experiments were performed with antibodies against H3R. Magnification is 200 x. Micrographs are representative of three independent experiments. Arrows indicate the location of goblet cells. EP-epithelium. C. Effect of agonists specific to histamine receptor subtypes on goblet cell glycoconjugate secretion. Cultured goblet cells from rats were incubated for 2 h with the concentration of agonists that caused maximum secretion. The agonists used were histamine (10−5 M), the H1 receptor agonist histamine dimaleate (10−6 M), the H2 receptor agonist amthamine (10−4 M), the H3 receptor agonist α-methylhistamine (10−5 M), and the H4 receptor agonist 4-methylhistamine (10−5 M). Glycoconjugate secretion was measured by enzyme-linked lectin assay. Data are mean±SEM of seven independent experiments. * indicates statistical significance from no addition (B, basal). From Hayashi D et al. Invest Ophthalmol Vis Sci. 2012;53: 2993-3003; DOI:10.1167/iovs. 11-8748.
To determine which histamine receptors are active, we measured secretion of high molecular weight glycoconjugates that include the mucin MUC-5AC from cultured rat conjunctival goblet cells. Secretion was stimulated by histamine or an agonist of the individual histamine receptor subtypes. Histamine and the receptor subtype agonists were all used at the concentration that caused maximal secretion [22]. Histamine at 10−5 M stimulated secretion 1.7 fold compared to basal (Figure 2C). The H1 receptor agonist histamine dimaleate at 10−6 M, the H2 receptor agonist amthamine at 10−4 M, the H3 receptor agonist α-methylhistamine at 10−6 M, and the H4 receptor agonist 4-methylhistamine at 10−5 M each stimulated goblet cell secretion to the same level as histamine. These results suggest that: 1. All four histamine receptors are present and active in conjunctival goblet cells and 2. Conjunctival goblet cells are a direct target of histamine produced during allergic conjunctivitis and these cells respond with mucin secretion.
Leukotrienes are also mediators of allergic responses including allergic conjunctivitis [29]. Leukotrienes are produced from arachidonic acid in response to activation of cytosolic phospholipase A2. One type of leukotrienes, the cysteinyl leukotrienes consist of LTC4 LTD4 and LTE4. The receptors activated by these leukotrienes are the G protein-linked receptors CysLT1 and CysLT2. Using antibodies to CysLT1 and CysLT2 receptors and western blotting analysis, we found that both of these receptors are present in rat conjunctival epithelia and cultured rat conjunctival goblet cells (Figure 3A). CysLT1 was present as a 41 kDa band and CysLT2 as a 35 kDa band. When immunofluorescence microscopy was performed both receptors were located in goblet cells of rat conjunctiva and in cultured rat goblet cells (Figure 3A).
Figure 3.
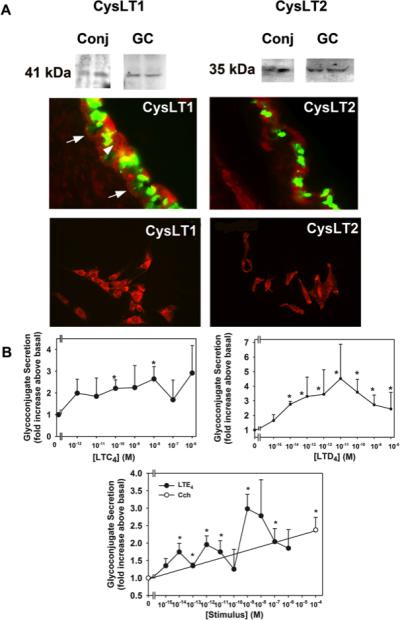
Identification of cysteinyl leukotriene receptors in rat conjunctival epithelium and cultured rat conjunctival goblet cells and stimulation of high molecular weight glycoconjugate secretion from cultured rat goblet cells. A. The presence of CysLT1 and CysLT2 receptors was determined by western blot analysis and is shown in upper panels. Each lane represents a separate animal. Fluorescence micrographs show localization of CysLT1 and CysLT2 receptors in conjunctiva (middle panels) and cultured goblet cells (lower panels). CysLT receptors are shown in red, and UEA-I, which indicates goblet cell secretory granules, is shown in green. Arrows indicate basolateral membranes of goblet cells. Arrowhead indicates stratified squamous cells. Original magnification X200. Micrographs are representative of three separate animals. B. Effect of leukotrienes on glycoconjugate secretion from cultured rat conjunctival goblet cells. Cultured rat conjunctival goblet cells were serum starved for 2 h before stimulation with LTC4 for 2 h (top left panel), LTD4 for 0.5 h (top right panel), or LTE4 for 1 h (bottom panel). The amount of glycoconjugate secretion was determined with ELLA. Data are mean ± SEM from four to five individual experiments. * p<0.05 from no addition, # p<0.01 from no addition (0). Open circle, the effect of carbachol used for 2 h. From: Dartt D et al J Immunol 2011; 186:4455-4466. doi: 10.4049/jimmunol.1000833
We explored whether the cysteinyl leukotriene receptors were active by measuring secretion from rat conjunctival goblet cells in culture. Leukotrienes were added to rat conjunctival goblet cells in culture and secretion of high molecular weight glycoconjugates was measured. LTC4 stimulated goblet cell secretion by a maximum of 2.6 fold at 10−8 M (Figure 3B). LTD4 stimulated goblet cell secretion a maximum of 4.5 fold at 10−11 M. LTE4 used at 10−9 M stimulated goblet cell secretion a maximum of 3.0 fold. Using a CysLT1 receptor antagonist, we demonstrated that LTD4 and to a limited extent LTE4 use this receptor [23]. From these results we suggest that the cysteinyl leukotrienes directly stimulate conjunctival goblet cell secretion and at least CysLT1 receptor is used and provide further evidence that conjunctival goblet cells are a direct target in allergic conjunctivitis.
Conjunctival goblet cells are one of the first lines of defense of the ocular surface and hence the entire eye. These goblet cells secrete a large secretory mucin that along with electrolytes and water covers and protects the cornea and conjunctiva. In addition the mucous layer entraps allergens, debris, pathogens, or other foreign materials and removes them from the ocular surface through the nasolacrimal drainage system. Previous findings from our laboratory demonstrated that goblet cells respond quickly to activation of sensory nerves in the cornea and conjunctiva with secretion of mucin. Conjunctival goblet cells are thus ideally situated and functional to provide rapid protection of the ocular surface from environmental challenges.
We show herein that goblet cells also play a pivotal role in ocular surface pathology, that of allergic conjunctivitis. That several allergic mediators histamine and cysteinyl leukotrienes (Figures 2 and 3) along with LTB4 and prostaglandin (PG) D2 [23] stimulate conjunctival goblet cell secretion, demonstrates that conjunctival goblet cells are direct targets in allergic conjunctivitis. We suggest that the allergic mediators overstimulate goblet cell secretion as these mediators are continuously produced during the course of allergy. We speculate that cytokines or other allergic mediators such as other eicosanoids and prostaglandins, cytokines, other vasoactive amines, nitric oxide, and platelet-activating factor (PAF) could also stimulate goblet cell secretion.
That all four histamine receptor subtypes as well as both cysteinyl leukotriene receptors are present and active in conjunctival goblet cells suggest that there are multiple overlapping mechanisms to activate conjunctival goblet cells and cause mucin secretion onto the ocular surface. As the conjunctiva compared to the cornea does not need to maintain transparency and provide a tight barrier to the external environment, the conjunctiva is not constrained in its responses. Conjunctival goblet cells possess multiple mechanisms to respond to the challenges of the external environment both in health and disease. Thus conjunctival goblet cells, in contrast to corneal epithelial cells have a plethora of cellular mechanisms used to mount a vigorous response to the challenges of the external environment to protect the ocular surface.
We conclude that conjunctival goblet cells have the receptors, cellular signaling pathways and secretory capability to respond to allergic mediators with mucin secretion and thus play a direct role in the pathogenesis of allergic conjunctivitis.
II. Innate immune cells as effectors and stimulators of T helper cells
a. Mast Cell Biology
The allergic events following allergen exposure in a sensitized individual occur in two phases: an early phase and a late phase. During the early-phase response, mast cells release inflammatory mediators such as histamine, tryptases, prostaglandins and leukotrienes in seconds to minutes after cross-linking of the allergen to the allergen-specific IgE bound to the surface of resident mast cells. These mediator molecules cause many of the acute inflammatory symptoms, such as redness, swelling and itching. During the late-phase response, eosinophils, basophils, T cells, neutrophils and macrophages infiltrate into the affected tissue(s) approximately 6-72 hours after allergen exposure. Although the contribution of mast cells to late-phase allergic responses remains controversial [30], mast cell-derived factors likely contribute to the recruitment of the infiltrating immune cells, which cause further tissue damage, irritation and hyper-responsiveness. Mast cells play a critical role in the pathogenesis of ocular allergy, as evidenced by the absence of symptoms in mast cell-deficient mice in an experimental allergic conjunctivitis model [31, 32]. Conjunctival mast cells have been shown to play critical roles both in the early-phase reaction and in late-phase inflammation of IgE-mediated allergic conjunctivitis in vivo [31, 32]. Cross-linking of allergen to IgE bound to the high-affinity IgE receptor, FcεRI, triggers signaling cascades leading to activation of kinases, phosphatases and GTPases, which subsequently induces a variety of events in the activated mast cells, such as degranulation, cytoskeleton rearrangement, increased gene transcription and cytokine/chemokine production [33, 34].
Apart from the classical FcεRI-mediated mechanism, mast cells also express surface receptors for a number of ligands known to be potent mast cell chemoattractants and are activated by chemokines [35, 36]. Chemokines belong to a superfamily of small, structurally related cytokine molecules that induce migration, adhesion, mediator release and hematopoieseis; chemokine receptors are expressed by human mast cell progenitors from umbilical cord blood and by human tissue mast cells [37]. Some chemokines, such as C-C motif chemokine ligand (CCL) 2, CCL3, CCL5 and CCL11, have been reported to activate murine, rat or human mast cells [35, 36, 38, 39]. Conjunctival mast cells have been determined to express C-C motif chemokine receptor (CCR) 1, CCR2, CCR3, CCR5 and C-X-C motif chemokine receptor (CXCR) 3 [36, 40]. Previous studies have shown that chemokine CCL3, also known as macrophage inflammatory protein-1α, is expressed at higher levels in the conjunctiva following antigen exposure [41] and acts as a costimulator for FcεRI-mediated degranulation in vivo in an experimental allergic conjunctivitis murine model and in vitro in a rat basophilic leukemia 2H3 cell line expressing receptor CCR1 (RBL-CCR1) and in murine bone marrow-derived mast cells (BMMCs) [40, 42, 43]. Costimulation of IgE-sensitized cells with recombinant human CCL3 (rCCL3) and antigen results in increased degranulation and arrested chemotaxis of RBL-CCR1 cells, suggesting cross-talk between FcεRI- and CCR1-mediated signaling cascades and its importance for optimal activation in mast cells [42]. Moreover, CCL3 synergistically enhances FcεRI-mediated gene and protein expression of cytokines and chemokines in mast cells [43-45]. CCL2 and CCL7, for example, chemokines with overlapping receptors and transduction pathways, displayed similar gene and protein expression patterns in response to FcεRI and/or CCR1 activation on mast cells [44, 46]. CCL7, also known as monocyte chemotactic protein 3, is expressed at multiple sites of inflammation [47, 48], and is produced by monocytes, fibroblasts, endothelial cells and mast cells [49]. CCL7 acts as a chemoattractant for monocytes, memory T lymphocytes, eosinophils, basophils, dendritic cells and natural killer cells, with activated monocytes exhibiting the strongest response [50]. CCL2, a chemoattractant that induces migration of monocytes, T cells and eosinophils, plays a critical role in activation and accumulation of leukocytes [35, 51]. Increased expression of CCL2 protein in inflammatory tissues of allergic patients has been observed [35, 52-55]. Transcriptional activation of CCL2 production and its release is reported to be dependent on the nuclear factor of activated T cells (NFAT) and mitogen-activated protein (MAP) kinase activation in mast cells [56]. A recent report showed that costimulation of IgE-sensitized cells with rCCL3 and antigen also enhanced phosphorylation of p38 MAP kinase and of extracellular-signal-regulated kinase 1/2 (ERK1/2) and subsequent CCL2 production [57]. Production of this chemokine by costimulated mast cells may help to coordinate allergic responses.
To elucidate the molecular mechanisms for CCL2 production in mast cells, total cell lysates of IgE-sensitized RBL-CCR1 cells, unstimulated or costimulated with rCCL3 and antigenIgE, were analyzed by two-dimensional electrophoresis to identify proteins involved in the cross-talk between FcεRI- and CCR1-mediated signaling pathways. Using matrix assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry, upregulated vimentin (accession number P31000) was identified as the major protein species. Vimentin is a cytoskeletal protein and serves as a major structural component of intermediate filaments, which create cell rigidity and shape [58]. Phosphorylation of vimentin induces disassembly of vimentin fragments and regulates cell morphology, migration and signaling [59-62]. Vimentin has been reported to participate in mast cell degranulation, subsequent to vimentin phosphorylation, by increasing granular mobility [63-65]. FcεRI and CCR1 costimulation in RBL-CCR1 cells was previously shown to synergistically enhance mast cell degranulation in a CCL3-dependent manner [66]; enhance phosphorylation of tyrosine, serine and threonine residues of vimentin; and result in increased levels of vimentin disassembly [57]. While recent evidence shows that there is no association between disassembly of vimentin filaments and mast cell degranulation under costimulation (data unpublished), histochemical analysis shows different patterns of vimentin assembly in costimulated mast cells. Prior to CCL3 plus Ag/IgE costimulation, a core of vimentin filaments is observed in the central axis of mast cells; after costimulation, the cells elongate and have disassembled vimentin fibers, suggesting a potential role for vimentin in mast cell morphology and motility (data unpublished). Vimentin interacts with phosphorylated ERK1/2 in neurons and adipocytes [67-69] as well as mast cells. Coactivation of FcεRI and CCR1 in mast cells induces phosphorylation and subsequent disassembly of vimentin. These disassembled and soluble vimentin fibers further interact with MAP kinase, ERK1/2 and p38 MAP kinase in activated mast cells. Aggregation of vimentin, the converse process of disassembly, reduced the association of vimentin fibers with phosphorylated MAP kinases and the production of CCL2, suggesting that vimentin is a needed component for optimal production of CCL2 in FcεRI- and CCR1-engaged mast cells. Since nuclear translocation of MAP kinases is a known mechanism for regulating gene expression of cytokines and chemokines, disassembled vimentin generated by FcεRI and CCR1 costimulation may act as a shuttle protein for nuclear entry of MAP kinases in mast cells (Fig 4). Thus, targeting the phosphorylation-dependent vimentin disassembly of activated mast cells may have therapeutic value in inhibiting early-phase and late-phase responses of allergic ocular diseases.
Figure 4.
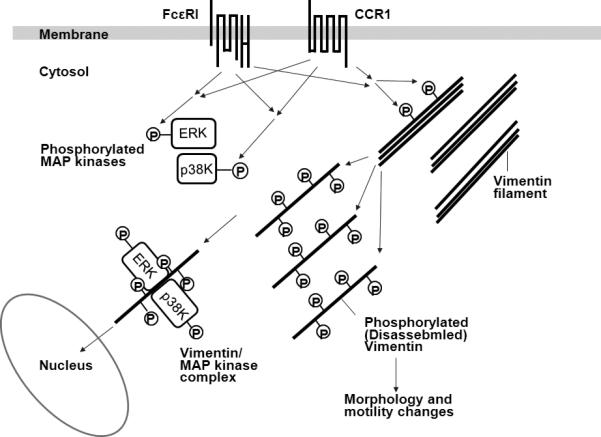
Hypothetical roles of vimentin in FcεRI- and CCR1-mediated signaling pathways in activated mast cells. FcεRI and CCR1 activation synergistically induces phosphorylation of ERK1/2, p38 MAP kinases and vimentin in activated mast cells. Disassembled vimentin filaments, unbundled by phosphorylation, may play a role in morphology and motility changes of activated mast cells. As disassembled vimentin fibers further interact with phosphorylated ERK1/2 and p38 MAPK, vimentin may also serve as a shuttle protein for nuclear entry of MAP kinases in mast cells.
b. Contribution of dendritic cells and CCR7 to allergic eye disease
Known as the most potent population of antigen presenting cells (APC), dendritic cells (DC) are an important leukocyte population in adaptive immunity, such as in allergy, and one that has significant clinical implications [70]. This underscored by the 2011 Nobel Prize in Physiology or Medicine awarded to the late Ralph Steinman for his discovery of the DC and its role in adaptive immunity. The importance of DCs is further underscored by concerted efforts currently being focused on how best to manipulate DC function for potential therapeutic applications in clinical conditions involving transplant rejection, infectious diseases, autoimmunity and cancer.
In allergic conjunctivitis (AC), recent work by Schlereth et al has demonstrated the therapeutic value of inhibiting ocular surface DCs in the mouse model of allergic conjunctivitis (AC) [71]. To appreciate these findings, it is important to understand the fundamental biology of DCs, what is known about DCs of the ocular surface, and how these DCs trigger allergic immune responses in allergic eye disease. These topics will be reviewed in this section.
Ocular surface DCs, their subsets, and role in allergic conjunctivitis
As otherwise innocuous environmental products, why allergens are reacted to by DCs in a manner that triggers pathogenic T cells in allergy (or T helper 2 cells) is not fully understood. Certainly, a chief function of DCs is the priming and activation of T cells. However, evolution has principally developed this activity for protection from invading pathogens, not allergens. Protection by DCs from infection is optimized by their continually patrolling of their environment—seeking ‘danger signals’ via repeated capture and processing of antigens (Ag). This is also in part optimized by their constitutive presence (so-called ‘steady state’ DCs) throughout the body including the ocular surface, as well as the additional infiltration of DCs in inflammation (or so-called ‘inflammatory’ DCs).
The role of DCs in allergic eye disease was recently identified by Schlereth et al, who demonstrated in the mouse model of allergic conjunctivitis (AC) that conjunctival DCs play a key role in triggering Th2 cells. Steady state DC subsets in the conjunctiva previously identified include, langerin+ (C-type lectin) DCs in the epithelium and CD11b expressing DCs in the subepithelial region [72]. Future work is required to further characterize steady-state DCs in the conjunctiva, as well as to identify which subset(s) is responsible for Th2 cell activation in AC.
In the cornea, whether steady state DCs there likewise react to allergen in a manner that leads to Th2 activation is unknown. Corneal DCs that may have the opportunity to do so include those in the epithelium, where the highest density of DCs is normally found—particularly between the paracentral and limbal regions. This includes a small fraction of DCs referred to as Langerhans cells (LC) [73-75]; the highly specialized subset akin to those found in the epidermis. This also includes steady state DCs in the stroma [74], such as langerin expressing DCs described by Hattori et al [73]. Other APC types comprising the cornea may react to allergen in a similar manner as well [76, 77].
DC maturation, Th2 activation, and the allergic reaction
Steady state DCs, such as those in the conjunctiva that lead to allergy [71], patrol tissues in a relatively ‘immature’ state and are thereby focused on antigen capture, rather than T cell activation. Expression of thrombospondin-1 by DCs maintains this immature state [78]. This is in stark contrast to what is seen for example, in inflammation and microbial exposures whereby DCs undergo a series of phenotypic and functional changes referred to as ‘maturation’ following Ag capture. DC maturation similarly occurs to allergen exposure, as seen in individuals that suffer from allergy such AC.
Maturation of DCs makes them potent stimulators of T cells, which in allergy enables DCs to activate Th2 cells. This occurs via upregulation of MHC II molecules (allowing DCs to present Ag to cognate T cells) and costimulatory molecules such as CD80 and CD86 (providing a secondary signal to prime and/or activate cognate T cells). In allergy, mature DC-mediated activation of Th2 in turn triggers a cascade of activities that ultimately result in the culmination of an allergic reaction (Fig 5). For example, IL-4, IL-5, or IL-13 cytokines secreted by Th2 cells promotes B cell differentiation into IgE secreting plasma cells (Fig 5). This is important because binding of allergen to IgE on mast cells cause their release of potent pro-inflammatory mediators such as histamine (Fig 5). Th2 cytokines also destabilize eosinophils to cause a similar response in late phase allergy (Fig 5). This triggers the additional maturation and infiltration of inflammatory DCs, which likewise enter into this feedback loop.
Figure 5.
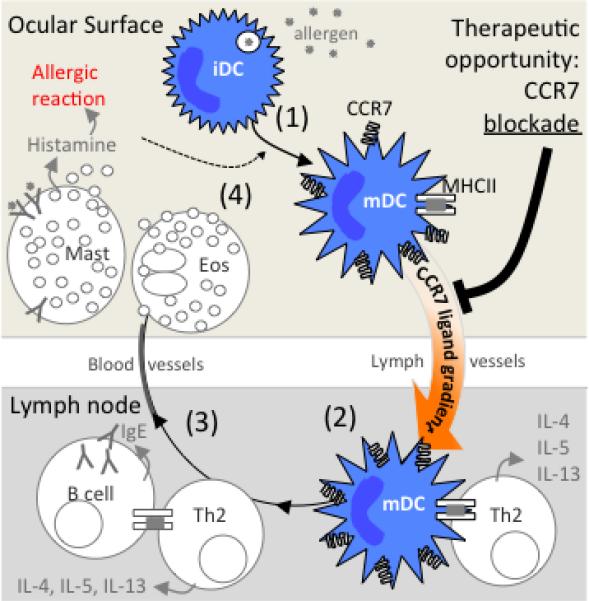
Model proposed explaining the therapeutic effect of topical CCR7 blockade in allergic eye disease. (1) Exposed allergen to the ocular surface is captured by immature steady state DCs, which then begin to up-regulate CCR7 expression as they mature phenotypically (e.g. MHC II). This enables DCs to respond chemotactically to CCR7 ligands CCL19 and CCL21. (2) Increased expression of CCR7 ligands by lymphatic endothelial cells, LN subcapsular sinus, and high endothelial venules, directs DCs via lymphatic vessels to the LN paracortex for activation of Th2. (3) B cells further activate Th2 cells, which lead to secretion of Th2 cytokines (IL-4, IL-5, and IL-13) and consequent differentiation of B cells into IgE secreting plasma cells. (4) Mast cells can now ligate IgE, and their binding of allergen leads to the release of granules comprised of pro-inflammatory mediators such as histamine. Th2 cytokines cause eosinophils to release granules as well. This triggers the allergic reaction, as well maturation and infiltration of inflammatory DCs, which likewise enter into this feedback loop. Thus, the therapeutic effect of topical CCR7 blockade is thought to occur via inhibition DC homing and consequent Th2 activity.
DC expression of CCR7 and lymph node homing
In order to activate Th2 cells, DCs migrate via the lymphatic vessels from allergen-exposed tissues such as the ocular surface, to the lymph node (LN) paracortex—where large pools of T cells are found. Referred to as ‘homing,’ this process is accomplished with the direction of chemotactic or ‘chemokine’ gradients.
Schlereth et al recently identified in the mouse model of AC, that the homing of conjunctival DCs to within the LN for Th2 activation occurs via the CCR7-CCL19/CCL21 chemokine axis. To come to this conclusion, authors derived eGFP+ DCs (CD11c+) ex vivo and injected these cells subconjunctivally into allergy-primed hosts. In addition, hosts were challenged topically with ovalbumin (OVA) allergen tagged with Texas Red fluorescent dye. This approach enabled assessment in the LN of chemokine receptor expression by DCs carrying allergen from the conjunctiva (i.e. CD11c+ eGFP+ Texas Red+). In so doing, authors found that >90% of these DCs had up-regulated their expression of CCR7, verified by both flow cytometry as well as qRT-PCR [71]. These data suggested a role in AC for CCR7-mediated homing of ocular surface DCs to within the LN for Th2 activation.
Schlereth et al also tested the functional relevance of CCR7 by deriving CCR7 knockout (KO) vs. wildtype (WT) DCs for their subconjunctival injection into allergy-primed hosts [71]. Immediately thereafter, hosts were challenged with OVA to induce AC. Authors observed that addition of WT DCs into the conjunctiva led to the augmentation of allergic immune responses in AC—thus, indicating a role for DCs in triggering AC. Strikingly, they also showed that such augmentation is completely lost with the subconjunctival injection instead with CCR7 KO DCs; thus, demonstrating that DCs mediate AC in a CCR7-dependent manner.
Therapeutic opportunity: Blocking CCR7 at the ocular surface
This identification of CCR7-mediated homing of ocular surface DCs to within the LN in Th2 activation revealed CCR7 as a potential therapeutic target in the treatment of allergic eye disease. To test this, Schlereth et al used primed mice and challenged them topically with allergen, along with the instillation of CCR7 blocking antibody (Ab) to the ocular surface. Challenges and CCR7 blockade were administered once a day and clinical scores were subsequently assessed.
Authors found that CCR7 blockade at the ocular surface led to significant decreases in AC clinical signs (Fig 6). Such decreased scores seen post challenge at 20 minutes (i.e. immediate hypersensitivity), 6 and 24 hr (i.e. late phase response) in this model indicates a therapeutic effect of CCR7 blockade on both the early and late phases of AC. This therapeutic effect is due to the inhibition of ocular surface DC homing and consequent Th2 mediated allergic reactivity (Fig 6) [71].
Figure 6.
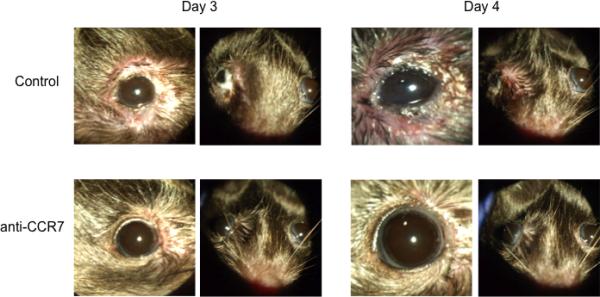
Therapeutic effect of topical CCR7 antibody blockade in the mouse model of AC. Allergen primed mice were challenged topically with allergen once a day for 4 days to induce AC. Topical instillation of CCR7 blocking antibody (or isotype control) was given along with challenges. Representative images are shown from challenges on Day 3 and Day 4. Taken from Schlereth et al, Am J Pathol. 2012 Jun;180(6):2351-60.
Taken together, the understanding of DC biology, and this recent work demonstrating the importance of ocular surface DCs [71], highlights the therapeutic potential for targeting DCs in allergic eye disease. To this end, Schlereth et al has identified a potentially promising target for clinical application in CCR7 expressed by ocular surface DCs. The clinical relevance of this finding is further underscored by the understanding that DCs in humans similarly express CCR7 for LN homing [79-81], and thereby prioritizes the pursuit of targeting CCR7 in allergic eye disease in humans.
III. Allergic Conjunctivitis as a Risk Factor Corneal Allograft Survival
Allergic diseases of the ocular surface are complex inflammatory conditions that are primarily manifested in the conjunctiva, but are often associated with allergic diseases of other tissues such as allergic rhinitis, atopic dermatitis, and allergic asthma. In addition to its disabling effects on the eye, allergic conjunctivitis has been implicated as a risk factor for corneal allograft survival [82-86].
It is widely recognized that the immune rejection of most categories of organ allografts is largely, if not entirely, mediated by CD4+ T lymphocytes. CD4+ T lymphocytes can select one of four basic pathways and differentiate into Th1, Th2, Th17, or T regulatory cells (Tregs). Th1 cells are characterized by their preferential production of the pro-inflammatory cytokine interferon-γ (IFN-γ), while Th2 T cells produce IL-4, IL-5, and IL-13 and are the primary immune cells involved in the development of allergic diseases. Interestingly, the presence of an ongoing Th1 immune response leads to the cross-regulation and silencing of the Th2 arm of the immune response. Likewise, a robust Th2 cell-mediated immune response biases subsequent immune responses to other antigens, including those that would normally evoke a Th1 immune response, to follow a Th2 pathway. The prevailing dogma in the late 1980's and early 1990's proposed that the immune rejection of allografts - including corneal allografts - was mediated by CD4+ Th1 cells. This led some investigators to consider strategies to prevent corneal allograft rejection by creating conditions that favored the generation of Th2 immune responses to the donor histocompatibility antigens [87]. However, subsequent studies revealed that silencing Th1 immune responses by neutralizing the Th1 cytokine, IFN-γ, by systemic administration of antibodies against IFN-γ or by using IFN-γ−/− hosts, did not prevent the rejection of heart, skin, or corneal allografts [88-91]. In fact, some studies reported an exacerbation, rather than a mitigation of corneal allograft rejection in IFN-γ-depleted hosts [90-93]. These observations, along with the aforementioned reported association between atopic diseases and increase risk for corneal allograft rejection prompted us determine the underlying mechanisms that contribute to the exacerbation of corneal allograft rejection in hosts with allergic conjunctivitis.
Allergic conjunctivitis exacerbates corneal allograft rejection
We and others have used a mouse model to evaluate the effect of allergic conjunctivitis on corneal allograft survival and found that mice with allergic conjunctivitis, which was induced with either short ragweed (SRW) pollen or ovalbumin (OVA), experienced a profound increase in the incidence and tempo of corneal allograft rejection [94-96]. Interestingly, the exacerbation of corneal allograft rejection was not simply the local effect of allergic conjunctivitis creating a “hot” inflamed eye. That is, corneal allografts placed onto left eyes of mice with allergic conjunctivitis elicited only in the contralateral right eye, experienced the same increased incidence and tempo of rejection that occurred when grafts were transplanted onto eyes with ongoing allergic conjunctivitis [94]. Moreover, syngeneic corneal allografts placed onto eyes with allergic conjunctivitis remained clear, indicating that the increased incidence and tempo of graft rejection that occurred in allogeneic hosts was immune-mediated and not simply due to the effects of an inflamed conjunctiva or a failure of the corneal grafts to heal.
Allergic airway hyperreactivity exacerbates corneal allograft rejection by perturbing systemic alloimmune responses
The exacerbated rejection of corneal allografts placed onto quiet eyes of mice with ongoing allergic conjunctivitis in the contralateral eye strongly suggested that this was the result of a perturbation in systemic alloimmune responses. A mouse model of allergic asthma was employed to test this hypothesis. Airway hyperreactivity (AHR) was induced with either SRW extract or OVA and its effect on the fate of corneal allografts was ascertained. Interestingly, mice with AHR displayed the same exacerbation of corneal allograft rejection that was observed in mice with allergic conjunctivitis [97]. Thus, allergic diseases, even in organs distant from the eye, rob the corneal allograft of its immune privilege.
Allergic conjunctivitis abolishes immune privilege by disabling T regulatory cell suppression of alloimmune responses
An impressive body of evidence indicates that corneal allograft survival is correlated with the generation of CD4+CD25+ Tregs [92, 93, 98]. That is, CD4+CD25+ Tregs are present in hosts that bear long-term surviving grafts, but are absent in hosts that reject their grafts. In the C57BL/6 → BALB/c donor/host combination 50% of the hosts accept their allografts and display Treg-mediated suppression of anti-C57BL/6 immune responses. With this in mind, we examined the effect of allergic conjunctivitis on the generation and function of CD4+CD25+ Tregs in mice receiving orthotopic corneal allografts (submitted for publication). The results indicated that mice with allergic conjunctivitis developed Tregs that suppressed CD4+ T cells, the primary mediators of corneal allograft rejection. However, CD4+ T cells from mice with allergic conjunctivitis were resistant to Treg-mediated suppression. Further study revealed that the Th2 cytokine IL-4, but not other Th2 cytokines (IL-5 and IL-13), rendered even naïve CD4+ T cells resistant to Treg-mediated suppression (Fig 7). Moreover, CD4+ effector cells from IL-4Rα−/− mice, which are incapable of responding to IL-4, were readily suppressed by Tregs even in the presence of IL-4. Thus, allergic conjunctivitis abolishes the immune privilege of corneal allografts by rendering CD4+ effector cells resistant to Treg-mediated suppression. Importantly, the exacerbation of corneal allograft rejection in mice with allergic conjunctivitis was reversed by administering anti-IL-4 blocking antibody, which offers glimmers of hope as an anti-rejection therapy for the high risk atopic host needing a corneal transplant.
Figure 7.
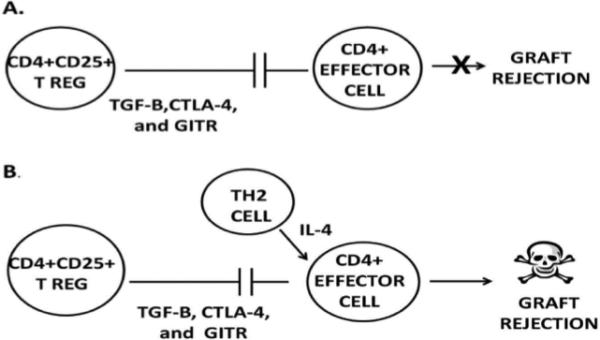
Proposed mechanism whereby allergic conjunctivitis exacerbates corneal allograft rejection. A) CD4+CD25+ Tregs suppress CD4+ effector T cells by a contact-dependent mechanism involving TGF-β, CTLA-4, and glucocorticoid-induced tumor necrosis factor receptor (GITR) and thereby prevent corneal graft rejection. B) IL-4 produced during allergic conjunctivitis renders CD4+ effector T cells resistant to suppression by CD4+CD25+ Tregs.
REFERENCES
- 1.Leonardi A, Motterle L, Bortolotti M. Allergy and the eye. Clin Exp Immunol. 2008;153(Suppl 1):17–21. doi: 10.1111/j.1365-2249.2008.03716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bielory L. Ocular allergy guidelines: a practical treatment algorithm. Drugs. 2002;62:1611–34. doi: 10.2165/00003495-200262110-00004. [DOI] [PubMed] [Google Scholar]
- 3.Metz DP, Bacon AS, Holgate S, Lightman SL. Phenotypic characterization of T cells infiltrating the conjunctiva in chronic allergic eye disease. J Allergy Clin Immunol. 1996;98:686–96. doi: 10.1016/s0091-6749(96)70103-3. [DOI] [PubMed] [Google Scholar]
- 4.Maggi E, Biswas P, Del Prete G, et al. Accumulation of Th-2-like helper T cells in the conjunctiva of patients with vernal conjunctivitis. J Immunol. 1991;146:1169–74. [PubMed] [Google Scholar]
- 5.Calder VL, Jolly G, Hingorani M, et al. Cytokine production and mRNA expression by conjunctival T-cell lines in chronic allergic eye disease. Clin Exp Allergy. 1999;29:1214–22. doi: 10.1046/j.1365-2222.1999.00585.x. [DOI] [PubMed] [Google Scholar]
- 6.Metz DP, Hingorani M, Calder VL, et al. T-cell cytokines in chronic allergic eye disease. J Allergy Clin Immunol. 1997;100:817–24. doi: 10.1016/s0091-6749(97)70279-3. [DOI] [PubMed] [Google Scholar]
- 7.Leonardi A, DeFranchis G, Zancanaro F, et al. Identification of local Th2 and Th0 lymphocytes in vernal conjunctivitis by cytokine flow cytometry. Invest Ophthalmol Vis Sci. 1999;40:3036–40. [PubMed] [Google Scholar]
- 8.Abu El-Asrar AM, Struyf S, Al-Kharashi SA, et al. Chemokines in the limbal form of vernal keratoconjunctivitis. Br J Ophthalmol. 2000;84:1360–6. doi: 10.1136/bjo.84.12.1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abu El-Asrar AM, Struyf S, Al-Mosallam AA, et al. Expression of chemokine receptors in vernal keratoconjunctivitis. Br J Ophthalmol. 2001;85:1357–61. doi: 10.1136/bjo.85.11.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abu El-Asrar AM, Struyf S, Al-Kharashi SA, et al. The T-lymphocyte chemoattractant Mig is highly expressed in vernal keratoconjunctivitis. Am J Ophthalmol. 2003;136:853–60. doi: 10.1016/s0002-9394(03)00446-x. [DOI] [PubMed] [Google Scholar]
- 11.Kato K, Lillehoj EP, Park YS, et al. Membrane-tethered MUC1 mucin is phosphorylated by epidermal growth factor receptor in airway epithelial cells and associates with TLR5 to inhibit recruitment of MyD88. J Immunol. 2012;188:2014–22. doi: 10.4049/jimmunol.1102405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dogru M, Matsumoto Y, Okada N, et al. Alterations of the ocular surface epithelial MUC16 and goblet cell MUC5AC in patients with atopic keratoconjunctivitis. Allergy. 2008;63:1324–34. doi: 10.1111/j.1398-9995.2008.01781.x. [DOI] [PubMed] [Google Scholar]
- 13.Bonini S, Micera A, Iovieno A, Lambiase A, Bonini S. Expression of Toll-like receptors in healthy and allergic conjunctiva. Ophthalmol. 2005;112:1528–1534. doi: 10.1016/j.ophtha.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 14.Lambiase A, Micera A, Sacchetti M, Mantelli F, Bonini S. Toll-like receptors in ocular surface diseases: overview and new findings. Clin Sci (Lond) 2011;120:441–50. doi: 10.1042/CS20100425. [DOI] [PubMed] [Google Scholar]
- 15.Cook EB, Stahl JL, Esnault S, Barney NP, Graziano FM. Toll-like receptor 2 expression on human conjunctival epithelial cells: a pathway for Staphylococcus aureus involvement in chronic ocular proinflammatory responses. Ann Allergy Asthma Immunol. 2005;94:486–97. doi: 10.1016/S1081-1206(10)61120-9. [DOI] [PubMed] [Google Scholar]
- 16.Lambiase A, Micera A, Mantelli F, et al. T-helper 17 lymphocytes in ocular cicatricial pemphigoid. Mol Vis. 2009;15:1449–55. [PMC free article] [PubMed] [Google Scholar]
- 17.Ueta M, Mizushima K, Yokoi N, Naito Y, Kinoshita S. Gene-expression analysis of polyI:C-stimulated primary human conjunctival epithelial cells. Br J Ophthalmol. 2010;94(11):1528–32. doi: 10.1136/bjo.2010.180554. [DOI] [PubMed] [Google Scholar]
- 18.Enríquez-de-Salamanca A, Calder V, Gao J, et al. Cytokine responses by conjunctival epithelial cells: an in vitro model of ocular inflammation. Cytokine. 2008;44:160–7. doi: 10.1016/j.cyto.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 19.Zhan H, Towler HM, Calder VL. The immunomodulatory role of human conjunctival epithelial cells. Invest Ophthalmol Vis Sci. 2003;44:3906–10. doi: 10.1167/iovs.02-0665. [DOI] [PubMed] [Google Scholar]
- 20.Holgate ST. Trials and tribulations in identifying new biologic treatments for asthma. Trends Immunol. 2012;33:238–46. doi: 10.1016/j.it.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 21.Rogers DF. The airway goblet cell. Int J Biochem Cell Biol. 2003;35:1–6. doi: 10.1016/s1357-2725(02)00083-3. [DOI] [PubMed] [Google Scholar]
- 22.Hayashi D, Li D, Hayashi C, Shatos M, Hodges RR, Dartt DA. Role of histamine and its receptor subtypes in stimulation of conjunctival goblet cell secretion. Invest Ophthalmol Vis Sci. 2012;53:2993–3003. doi: 10.1167/iovs.11-8748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dartt DA, Hodges RR, Li D, Shatos MA, Lashkari K, Serhan CN. Conjunctival goblet cell secretion stimulated by leukotrienes is reduced by resolvins D1 and E1 to promote resolution of inflammation. J Immunol. 2011;186:4455–4466. doi: 10.4049/jimmunol.1000833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shatos MA, Rios JD, Horikawa Y, Hodges RR, Chang EL, Bernardino CR, Rubin PA, Dartt DA. Isolation and characterization of cultured human conjunctival goblet cells. Invest Ophthalmol Vis Sci. 2003;44:2477–2486. doi: 10.1167/iovs.02-0550. [DOI] [PubMed] [Google Scholar]
- 25.Shatos MA, Rios JD, Tepavcevic V, Kano H, Hodges R, Dartt DA. Isolation, characterization, and propagation of rat conjunctival goblet cells in vitro. Invest Ophthalmol Vis Sci. 2001;42:1455–1464. [PubMed] [Google Scholar]
- 26.Leonardi A, Motterle L, Bortolotti M. Allergy and the eye. Clin Exp Immunol. 2008;153(Suppl 1):17–21. doi: 10.1111/j.1365-2249.2008.03716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horikawa Y, Shatos MA, Hodges RR, Zoukhri D, Rios JD, Chang EL, Bernardino CR, Rubin PA, Dartt DA. Activation of mitogen-activated protein kinase by cholinergic agonists and EGF in human compared with rat cultured conjunctival goblet cells. Invest Ophthalmol Vis Sci. 2003;44:2535–2544. doi: 10.1167/iovs.02-1117. [DOI] [PubMed] [Google Scholar]
- 28.Thurmond RL, Gelfand EW, Dunford PJ. The role of histamine H1 and H4 receptors in allergic inflammation: the search for new antihistamines. Nat Rev Drug Discov. 2008;7:41–53. doi: 10.1038/nrd2465. [DOI] [PubMed] [Google Scholar]
- 29.Samuelsson B, Dahlen SE, Lindgren JA, Rouzer CA, Serhan CN. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science. 1987;237:1171–1176. doi: 10.1126/science.2820055. [DOI] [PubMed] [Google Scholar]
- 30.Ueta M, Nakamura T, Tanaka S, Kojima K, Kinoshita S. Development of eosinophilic conjunctival inflammation at late-phase reaction in mast cell-deficient mice. J Allergy Clin Immunol. 2007 Aug;120(2):476–8. doi: 10.1016/j.jaci.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 31.Miyazaki D, Tominaga T, Yakura K, Kuo CH, Komatsu N, Inoue Y, et al. Conjunctival mast cell as a mediator of eosinophilic response in ocular allergy. Mol Vis. 2008;14:1525–32. [PMC free article] [PubMed] [Google Scholar]
- 32.Fukuda K, Ohbayashi M, Morohoshi K, Zhang L, Liu FT, Ono SJ. Critical role of IgE-dependent mast cell activation in a murine model of allergic conjunctivitis. J Allergy Clin Immunol. 2009 Oct;124(4):827–33. e2. doi: 10.1016/j.jaci.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 33.Abramson J, Pecht I. Regulation of the mast cell response to the type 1 Fc epsilon receptor. Immunol Rev. 2007 Jun;217:231–54. doi: 10.1111/j.1600-065X.2007.00518.x. [DOI] [PubMed] [Google Scholar]
- 34.Gilfillan AM, Tkaczyk C. Integrated signalling pathways for mast-cell activation. Nat Rev Immunol. 2006 Mar;6(3):218–30. doi: 10.1038/nri1782. [DOI] [PubMed] [Google Scholar]
- 35.Ono SJ, Nakamura T, Miyazaki D, Ohbayashi M, Dawson M, Toda M. Chemokines: roles in leukocyte development, trafficking, and effector function. J Allergy Clin Immunol. 2003 Jun;111(6):1185–99. doi: 10.1067/mai.2003.1594. quiz 200. [DOI] [PubMed] [Google Scholar]
- 36.Beer F, Kuo CH, Morohoshi K, Goodliffe J, Munro P, Aye CC, et al. Role of β-chemokines in mast cell activation and type I hypersensitivity reactions in the conjunctiva: in vivo and in vitro studies. Immunol Rev. 2007 Jun;217:96–104. doi: 10.1111/j.1600-065X.2007.00521.x. [DOI] [PubMed] [Google Scholar]
- 37.Ochi H, Hirani WM, Yuan Q, Friend DS, Austen KF, Boyce JA. T helper cell type 2 cytokine-mediated comitogenic responses and CCR3 expression during differentiation of human mast cells in vitro. J Exp Med. 1999 Jul 19;190(2):267–80. doi: 10.1084/jem.190.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tominaga T, Miyazaki D, Sasaki S, Mihara S, Komatsu N, Yakura K, et al. Blocking mast cell-mediated type I hypersensitivity in experimental allergic conjunctivitis by monocyte chemoattractant protein-1/CCR2. Invest Ophthalmol Vis Sci. 2009 Nov;50(11):5181–8. doi: 10.1167/iovs.09-3637. [DOI] [PubMed] [Google Scholar]
- 39.Campbell EM, Charo IF, Kunkel SL, Strieter RM, Boring L, Gosling J, et al. Monocyte chemoattractant protein-1 mediates cockroach allergen-induced bronchial hyperreactivity in normal but not CCR2−/− mice: the role of mast cells. J Immunol. 1999 Aug 15;163(4):2160–7. [PubMed] [Google Scholar]
- 40.Miyazaki D, Nakamura T, Toda M, Cheung-Chau KW, Richardson RM, Ono SJ. Macrophage inflammatory protein-1α as a costimulatory signal for mast cell-mediated immediate hypersensitivity reactions. J Clin Invest. 2005 Feb;115(2):434–42. doi: 10.1172/JCI18452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Keane-Myers AM, Miyazaki D, Liu G, Dekaris I, Ono S, Dana MR. Prevention of allergic eye disease by treatment with IL-1 receptor antagonist. Invest Ophthalmol Vis Sci. 1999 Nov;40(12):3041–6. [PubMed] [Google Scholar]
- 42.Toda M, Dawson M, Nakamura T, Munro PM, Richardson RM, Bailly M, et al. Impact of engagement of FcεRI and CC chemokine receptor 1 on mast cell activation and motility. J Biol Chem. 2004 Nov 12;279(46):48443–8. doi: 10.1074/jbc.M408725200. [DOI] [PubMed] [Google Scholar]
- 43.Fifadara NH, Aye CC, Raghuwanshi SK, Richardson RM, Ono SJ. CCR1 expression and signal transduction by murine BMMC results in secretion of TNF-alpha, TGFbeta-1 and IL-6. Int Immunol. 2009 Aug;21(8):991–1001. doi: 10.1093/intimm/dxp066. [DOI] [PubMed] [Google Scholar]
- 44.Aye CC, Toda M, Morohoshi K, Ono SJ. Identification of genes and proteins specifically regulated by costimulation of mast cell Fcepsilon Receptor I and chemokine receptor 1. Exp Mol Pathol. 2012 Jun;92(3):267–74. doi: 10.1016/j.yexmp.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 45.Toda M, Nakamura T, Ohbayashi M, Ikeda Y, Dawson M, Richardson RM, et al. Role of CC chemokines and their receptors in multiple aspects of mast cell biology: comparative protein profiling of FcεRI- and/or CCR1-engaged mast cells using protein chip technology. Novartis Found Symp. 2005;271:131–40. discussion 40-51. [PubMed] [Google Scholar]
- 46.Sozzani S, Zhou D, Locati M, Rieppi M, Proost P, Magazin M, et al. Receptors and transduction pathways for monocyte chemotactic protein-2 and monocyte chemotactic protein-3. Similarities and differences with MCP-1. J Immunol. 1994 Apr 1;152(7):3615–22. [PubMed] [Google Scholar]
- 47.Jacobsson B, Holst RM, Andersson B, Hagberg H. Monocyte chemotactic protein-2 and -3 in amniotic fluid: relationship to microbial invasion of the amniotic cavity, intra-amniotic inflammation and preterm delivery. Acta Obstet Gynecol Scand. 2005 Jun;84(6):566–71. doi: 10.1111/j.0001-6349.2005.00830.x. [DOI] [PubMed] [Google Scholar]
- 48.Schenk S, Mal N, Finan A, Zhang M, Kiedrowski M, Popovic Z, et al. Monocyte chemotactic protein-3 is a myocardial mesenchymal stem cell homing factor. Stem Cells. 2007 Jan;25(1):245–51. doi: 10.1634/stemcells.2006-0293. [DOI] [PubMed] [Google Scholar]
- 49.Wakahara S, Fujii Y, Nakao T, Tsuritani K, Hara T, Saito H, et al. Gene expression profiles for FcεRI, cytokines and chemokines upon FcεRI activation in human cultured mast cells derived from peripheral blood. Cytokine. 2001 Nov 21;16(4):143–52. doi: 10.1006/cyto.2001.0958. [DOI] [PubMed] [Google Scholar]
- 50.Dahinden CA, Geiser T, Brunner T, von Tscharner V, Caput D, Ferrara P, et al. Monocyte chemotactic protein 3 is a most effective basophil- and eosinophil-activating chemokine. J Exp Med. 1994 Feb 1;179(2):751–6. doi: 10.1084/jem.179.2.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Toda M, Nakamura T, Ohbayashi M, Ikeda Y, Dawson M, Eye CC, et al. Mechanisms of leukocyte trafficking in allergic diseases: insights into new therapies targeting chemokines and chemokine receptors. Expert Rev Clin Immunol. 2007;3(3):351–64. doi: 10.1586/1744666X.3.3.351. [DOI] [PubMed] [Google Scholar]
- 52.Sousa AR, Lane SJ, Nakhosteen JA, Yoshimura T, Lee TH, Poston RN. Increased expression of the monocyte chemoattractant protein-1 in bronchial tissue from asthmatic subjects. Am J Respir Cell Mol Biol. 1994 Feb;10(2):142–7. doi: 10.1165/ajrcmb.10.2.8110469. [DOI] [PubMed] [Google Scholar]
- 53.Holgate ST, Bodey KS, Janezic A, Frew AJ, Kaplan AP, Teran LM. Release of RANTES, MIP-1 alpha, and MCP-1 into asthmatic airways following endobronchial allergen challenge. Am J Respir Crit Care Med. 1997 Nov;156(5):1377–83. doi: 10.1164/ajrccm.156.5.9610064. [DOI] [PubMed] [Google Scholar]
- 54.Toda M, Nakamura T, Ohbayashi M, Ikeda Y, Dawson M, Aye CC, et al. Mechanisms of leukocyte trafficking in allergic diseases: insights into new therapies targeting chemokines and chemokine receptors. Expert Rev Clin Immunol. 2007 May;3(3):351–64. doi: 10.1586/1744666X.3.3.351. [DOI] [PubMed] [Google Scholar]
- 55.Gaga M, Ong YE, Benyahia F, Aizen M, Barkans J, Kay AB. Skin reactivity and local cell recruitment in human atopic and nonatopic subjects by CCL2/MCP-1 and CCL3/MIP-1alpha. Allergy. 2008 Jun;63(6):703–11. doi: 10.1111/j.1398-9995.2007.01578.x. [DOI] [PubMed] [Google Scholar]
- 56.Teshima R, Onose J, Okunuki H, Sawada J. Effect of Ca(2+) ATPase inhibitors on MCP-1 release from bone marrow-derived mast cells and the involvement of p38 MAP kinase activation. Int Arch Allergy Immunol. 2000 Jan;121(1):34–43. doi: 10.1159/000024295. [DOI] [PubMed] [Google Scholar]
- 57.Toda M, Kuo CH, Borman SK, Richardson RM, Inoko A, Inagaki M, et al. Evidence That Formation of Vimentin{middle dot}Mitogen-activated Protein Kinase (MAPK) Complex Mediates Mast Cell Activation following Fc{epsilon}RI/CC Chemokine Receptor 1 Cross-talk. J Biol Chem. 2012 Jul 13;287(29):24516–24. doi: 10.1074/jbc.M111.319624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chang L, Goldman RD. Intermediate filaments mediate cytoskeletal crosstalk. Nat Rev Mol Cell Biol. 2004 Aug;5(8):601–13. doi: 10.1038/nrm1438. [DOI] [PubMed] [Google Scholar]
- 59.Sihag RK, Inagaki M, Yamaguchi T, Shea TB, Pant HC. Role of phosphorylation on the structural dynamics and function of types III and IV intermediate filaments. Exp Cell Res. 2007 Jun 10;313(10):2098–109. doi: 10.1016/j.yexcr.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li QF, Spinelli AM, Wang R, Anfinogenova Y, Singer HA, Tang DD. Critical role of vimentin phosphorylation at Ser-56 by p21-activated kinase in vimentin cytoskeleton signaling. J Biol Chem. 2006 Nov 10;281(45):34716–24. doi: 10.1074/jbc.M607715200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Helfand BT, Chou YH, Shumaker DK, Goldman RD. Intermediate filament proteins participate in signal transduction. Trends Cell Biol. 2005 Nov;15(11):568–70. doi: 10.1016/j.tcb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 62.Ivaska J, Pallari HM, Nevo J, Eriksson JE. Novel functions of vimentin in cell adhesion, migration, and signaling. Exp Cell Res. 2007 Jun 10;313(10):2050–62. doi: 10.1016/j.yexcr.2007.03.040. [DOI] [PubMed] [Google Scholar]
- 63.Nahm DH, Tkaczyk C, Fukuishi N, Colucci-Guyon E, Gilfillan AM, Metcalfe DD. Identification of Fyn-binding proteins in MC/9 mast cells using mass spectrometry. Biochem Biophys Res Commun. 2003 Oct 10;310(1):202–8. doi: 10.1016/j.bbrc.2003.08.132. [DOI] [PubMed] [Google Scholar]
- 64.Izushi K, Fujiwara Y, Tasaka K. Identification of vimentin in rat peritoneal mast cells and its phosphorylation in association with histamine release. Immunopharmacology. 1992 May-Jun;23(3):153–61. doi: 10.1016/0162-3109(92)90021-4. [DOI] [PubMed] [Google Scholar]
- 65.Sahara N, Siraganian RP, Oliver C. Morphological changes induced by the calcium ionophore A23187 in rat basophilic leukemia (2H3) cells. J Histochem Cytochem. 1990 Jul;38(7):975–83. doi: 10.1177/38.7.1693935. [DOI] [PubMed] [Google Scholar]
- 66.Toda M, Dawson M, Nakamura T, Munro PM, Richardson RM, Bailly M, et al. Impact of engagement of FcepsilonRI and CC chemokine receptor 1 on mast cell activation and motility. J Biol Chem. 2004 Nov 12;279(46):48443–8. doi: 10.1074/jbc.M408725200. [DOI] [PubMed] [Google Scholar]
- 67.Perlson E, Hanz S, Ben-Yaakov K, Segal-Ruder Y, Seger R, Fainzilber M. Vimentin-dependent spatial translocation of an activated MAP kinase in injured nerve. Neuron. 2005 Mar 3;45(5):715–26. doi: 10.1016/j.neuron.2005.01.023. [DOI] [PubMed] [Google Scholar]
- 68.Perlson E, Michaelevski I, Kowalsman N, Ben-Yaakov K, Shaked M, Seger R, et al. Vimentin binding to phosphorylated Erk sterically hinders enzymatic dephosphorylation of the kinase. J Mol Biol. 2006 Dec 15;364(5):938–44. doi: 10.1016/j.jmb.2006.09.056. [DOI] [PubMed] [Google Scholar]
- 69.Kumar N, Robidoux J, Daniel KW, Guzman G, Floering LM, Collins S. Requirement of vimentin filament assembly for beta3-adrenergic receptor activation of ERK MAP kinase and lipolysis. J Biol Chem. 2007 Mar 23;282(12):9244–50. doi: 10.1074/jbc.M605571200. [DOI] [PubMed] [Google Scholar]
- 70.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007 Sep 27;449(7161):419–26. doi: 10.1038/nature06175. Review. PubMed PMID: 17898760. [DOI] [PubMed] [Google Scholar]
- 71.Schlereth S, Lee HS, Khandelwal P, Saban DR. Blocking CCR7 at the ocular surface impairs the pathogenic contribution of dendritic cells in allergic conjunctivitis. Am J Pathol. 2012 Jun;180(6):2351–60. doi: 10.1016/j.ajpath.2012.02.015. Epub 2012 Apr 14. PubMed PMID: 22507838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ohbayashi M, Manzouri B, Flynn T, Toda M, Ikeda Y, Nakamura T, Ono SJ. Dynamic changes in conjunctival dendritic cell numbers, anatomical position and phenotype during experimental allergic conjunctivitis. Exp Mol Pathol. 2007 Oct;83(2):216–23. doi: 10.1016/j.yexmp.2007.04.007. Epub 2007 May 10. PubMed PMID: 17560570. [DOI] [PubMed] [Google Scholar]
- 73.Hattori T, Chauhan SK, Lee H, Ueno H, Dana R, Kaplan DH, Saban DR. Characterization of Langerin-expressing dendritic cell subsets in the normal cornea. Invest Ophthalmol Vis Sci. 2011 Jun 28;52(7):4598–604. doi: 10.1167/iovs.10-6741. doi: 10.1167/iovs.10-6741. Print 2011 Jun. PubMed PMID: 21482644; PubMed Central PMCID: PMC3175952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hamrah P, Huq SO, Liu Y, Zhang Q, Dana MR. Corneal immunity is mediated by heterogeneous population of antigen-presenting cells. J Leukoc Biol. 2003 Aug;74(2):172–8. doi: 10.1189/jlb.1102544. Review. PubMed PMID: 12885933. [DOI] [PubMed] [Google Scholar]
- 75.Mayer WJ, Irschick UM, Moser P, Wurm M, Huemer HP, Romani N, Irschick EU. Characterization of antigen-presenting cells in fresh and cultured human corneas using novel dendritic cell markers. Invest Ophthalmol Vis Sci. 2007 Oct;48(10):4459–67. doi: 10.1167/iovs.06-1184. PubMed PMID: 17898266. [DOI] [PubMed] [Google Scholar]
- 76.Brissette-Storkus CS, Reynolds SM, Lepisto AJ, Hendricks RL. Identification of a novel macrophage population in the normal mouse corneal stroma. Invest Ophthalmol Vis Sci. 2002 Jul;43(7):2264–71. PubMed PMID: 12091426; PubMed Central PMCID: PMC3253392. [PMC free article] [PubMed] [Google Scholar]
- 77.Sosnová M, Bradl M, Forrester JV. CD34+ corneal stromal cells are bone marrow-derived and express hemopoietic stem cell markers. Stem Cells. 2005 Apr;23(4):507–15. doi: 10.1634/stemcells.2004-0291. PubMed PMID: 15790772. [DOI] [PubMed] [Google Scholar]
- 78.Saban DR, Bock F, Chauhan SK, Masli S, Dana R. Thrombospondin-1 derived from APCs regulates their capacity for allosensitization. J Immunol. 2010 Oct 15;185(8):4691–7. doi: 10.4049/jimmunol.1001133. Epub 2010 Sep 15. PubMed PMID: 20844200; PubMed Central PMCID: PMC3090006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yoshida R, Imai T, Hieshima K, Kusuda J, Baba M, Kitaura M, Nishimura M, Kakizaki M, Nomiyama H, Yoshie O. Molecular cloning of a novel human CC chemokine EBI1-ligand chemokine that is a specific functional ligand for EBI1, CCR7. J Biol Chem. 1997 May 23;272(21):13803–9. doi: 10.1074/jbc.272.21.13803. PubMed PMID: 9153236. [DOI] [PubMed] [Google Scholar]
- 80.Yoshie O, Imai T, Nomiyama H. Novel lymphocyte-specific CC chemokines and their receptors. J Leukoc Biol. 1997 Nov;62(5):634–44. doi: 10.1002/jlb.62.5.634. Review. PubMed PMID: 9365118. [DOI] [PubMed] [Google Scholar]
- 81.Dieu MC, Vanbervliet B, Vicari A, Bridon JM, Oldham E, Aït-Yahia S, Brière F, Zlotnik A, Lebecque S, Caux C. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J Exp Med. 1998 Jul 20;188(2):373–86. doi: 10.1084/jem.188.2.373. PubMed PMID: 9670049; PubMed Central PMCID: PMC2212459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Easty D, Entwistle C, Funk A, Witcher J. Herpes simplex keratitis and keratoconus in the atopic patient. A clinical and immunological study. Trans Ophthalmol Soc U K. 1975;95:267–276. [PubMed] [Google Scholar]
- 83.Hargrave S, Chu Y, Mendelblatt D, Mayhew E, Niederkorn J. Preliminary findings in corneal allograft rejection in patients with keratoconus. Am J Ophthalmol. 2003;135:452–460. doi: 10.1016/s0002-9394(02)02055-x. [DOI] [PubMed] [Google Scholar]
- 84.Kuchle M, Cursiefen C, Nguyen NX, et al. Risk factors for corneal allograft rejection: intermediate results of a prospective normal-risk keratoplasty study. Graefes Arch Clin Exp Ophthalmol. 2002;240:580–584. doi: 10.1007/s00417-002-0496-5. [DOI] [PubMed] [Google Scholar]
- 85.Lyons CJ, Dart JK, Aclimandos WA, Lightman S, Buckley RJ. Sclerokeratitis after keratoplasty in atopy. Ophthalmology. 1990;97:729–733. doi: 10.1016/s0161-6420(90)32523-x. [DOI] [PubMed] [Google Scholar]
- 86.Reinhard T, Moller M, Sundmacher R. Penetrating keratoplasty in patients with atopic dermatitis with and without systemic cyclosporin A. Cornea. 1999;18:645–651. doi: 10.1097/00003226-199911000-00003. [DOI] [PubMed] [Google Scholar]
- 87.Yamada J, Yoshida M, Taylor AW, Streilein JW. Mice with Th2-biased immune systems accept orthotopic corneal allografts placed in “high risk” eyes. J Immunol. 1999;162:5247–5255. [PubMed] [Google Scholar]
- 88.Bishop DK, Chan Wood S, Eichwald EJ, Orosz CG. Immunobiology of allograft rejection in the absence of IFN-gamma: CD8+ effector cells develop independently of CD4+ cells and CD40-CD40 ligand interactions. J Immunol. 2001;166:3248–3255. doi: 10.4049/jimmunol.166.5.3248. [DOI] [PubMed] [Google Scholar]
- 89.Goldman M, Le Moine A, Braun M, Flamand V, Abramowicz D. A role for eosinophils in transplant rejection. Trends Immunol. 2001;22:247–251. doi: 10.1016/s1471-4906(01)01893-2. [DOI] [PubMed] [Google Scholar]
- 90.Hargrave SL, Hay C, Mellon J, Mayhew E, Niederkorn JY. Fate of MHC-matched corneal allografts in Th1-deficient hosts. Invest Ophthalmol Vis Sci. 2004;45:1188–1193. doi: 10.1167/iovs.03-0515. [DOI] [PubMed] [Google Scholar]
- 91.Yamada J, Hamuro J, Fukushima A, et al. MHC-matched corneal allograft rejection in an IFN-gamma/IL-17-independent manner in C57BL/6 mice. Invest Ophthalmol Vis Sci. 2009;50:2139–2146. doi: 10.1167/iovs.08-2993. [DOI] [PubMed] [Google Scholar]
- 92.Cunnusamy K, Chen PW, Niederkorn JY. IL-17 promotes immune privilege of corneal allografts. J Immunol. 2010;185:4651–4658. doi: 10.4049/jimmunol.1001576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cunnusamy K, Paunicka K, Reyes N, Yang W, Chen PW, Niederkorn JY. Two different regulatory T cell populations that promote corneal allograft survival. Invest Ophthalmol Vis Sci. 2010;51:6566–6574. doi: 10.1167/iovs.10-6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Beauregard C, Stevens C, Mayhew E, Niederkorn JY. Cutting edge: atopy promotes Th2 responses to alloantigens and increases the incidence and tempo of corneal allograft rejection. J Immunol. 2005;174:6577–6581. doi: 10.4049/jimmunol.174.11.6577. [DOI] [PubMed] [Google Scholar]
- 95.Flynn TH, Ohbayashi M, Ikeda Y, Ono SJ, Larkin DF. Effect of allergic conjunctival inflammation on the allogeneic response to donor cornea. Invest Ophthalmol Vis Sci. 2007;48:4044–4049. doi: 10.1167/iovs.06-0973. [DOI] [PubMed] [Google Scholar]
- 96.Niederkorn JY, Chen PW, Mellon J, Stevens C, Mayhew E. Allergic conjunctivitis exacerbates corneal allograft rejection by activating Th1 and th2 alloimmune responses. J Immunol. 2010;184:6076–6083. doi: 10.4049/jimmunol.0902300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Niederkorn JY, Chen PW, Mellon J, Stevens C, Mayhew E. Allergic airway hyperreactivity increases the risk for corneal allograft rejection. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2009;9:1017–1026. doi: 10.1111/j.1600-6143.2009.02603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chauhan SK, Saban DR, Lee HK, Dana R. Levels of Foxp3 in regulatory T cells reflect their functional status in transplantation. J Immunol. 2009;182:148–153. doi: 10.4049/jimmunol.182.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]


