Abstract
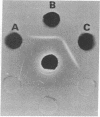
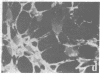
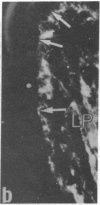
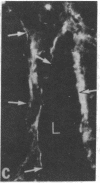
Synthesis of fibronectin in an epithelial cell line (IEC-6) established from rat small intestine was demonstrated by using immunofluorescence, radioimmunoassay, and collagen-binding. Internally labeled radioactive fibronectin isolated from the IEC-6 cells gave a single main band in sodium dodecyl sulfate/polyacrylamide gel electrophoresis under reducing conditions. Fibronectin isolated from rat plasma gave two closely spaced bands. The slower one had the same mobility as the epithelial cell fibronectin. The distribution of fibronectin in IEC-6 cells as detected by immunofluorescence was different from that described for fibroblasts and other cell types; fibronectin was present exclusively in regions of cell-to-cell contact. No fluorescence was detected on the surface membrane facing the culture medium or underneath the cells. This suggests that fibronectin may not be involved in the adhesion of the epithelial cells to the growth surface but could mediate cell-to-cell contacts. In microscopic sections of the small intestine, immunofluorescent staining with antifibronectin serum was strong in the basement membrane underlying the epithelial cells in the crypts. The in vitro synthesis of fibronectin by the crypt cells and its abundant presence in the basement membrane underlying the same cells in vivo suggests that fibronectin is a structural component of the basement membrane, and that it may be, at least in part, synthesized and deposited by the intestinal epithelium.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali I. U., Hynes R. O. Effects of cytochalasin B and colchicine on attachment of a major surface protein of fibroblasts. Biochim Biophys Acta. 1977 Nov 15;471(1):16–24. doi: 10.1016/0005-2736(77)90388-1. [DOI] [PubMed] [Google Scholar]
- Ali I. U., Mautner V., Lanza R., Hynes R. O. Restoration of normal morphology, adhesion and cytoskeleton in transformed cells by addition of a transformation-sensitive surface protein. Cell. 1977 May;11(1):115–126. doi: 10.1016/0092-8674(77)90322-1. [DOI] [PubMed] [Google Scholar]
- Birdwell C. R., Gospodarowicz D., Nicolson G. L. Identification, localization, and role of fibronectin in cultured bovine endothelial cells. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3273–3277. doi: 10.1073/pnas.75.7.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L. B. Alteration in cell surface LETS protein during myogenesis. Cell. 1977 Mar;10(3):393–400. doi: 10.1016/0092-8674(77)90026-5. [DOI] [PubMed] [Google Scholar]
- Chen L. B., Gallimore P. H., McDougall J. K. Correlation between tumor induction and the large external transformation sensitive protein on the cell surface. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3570–3574. doi: 10.1073/pnas.73.10.3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L. B., Maitland N., Gallimore P. H., McDougall J. K. Detection of the large external transformation-sensitive protein on some epithelial cells. Exp Cell Res. 1977 Apr;106(1):39–46. doi: 10.1016/0014-4827(77)90238-5. [DOI] [PubMed] [Google Scholar]
- Dessau W., Adelmann B. C., Timpl R. Identification of the sites in collagen alpha-chains that bind serum anti-gelatin factor (cold-insoluble globulin). Biochem J. 1978 Jan 1;169(1):55–59. doi: 10.1042/bj1690055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engvall E., Ruoslahti E. Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int J Cancer. 1977 Jul 15;20(1):1–5. doi: 10.1002/ijc.2910200102. [DOI] [PubMed] [Google Scholar]
- Engvall E., Ruoslahti E., Miller E. J. Affinity of fibronectin to collagens of different genetic types and to fibrinogen. J Exp Med. 1978 Jun 1;147(6):1584–1595. doi: 10.1084/jem.147.6.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furcht L. T., Mosher D. F., Wendelschafer-Crabb G. Immunocytochemical localization of fibronectin (LETS proteins) on the surface of L6 myoblasts: light and electron microscopic studies. Cell. 1978 Feb;13(2):263–271. doi: 10.1016/0092-8674(78)90195-2. [DOI] [PubMed] [Google Scholar]
- Gahmberg C. G., Hakomori S. I. Altered growth behavior of malignant cells associated with changes in externally labeled glycoprotein and glycolipid. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3329–3333. doi: 10.1073/pnas.70.12.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell F., Hays D. G., Minter D. Cell adhesion and spreading factor. Partial purification and properties. Exp Cell Res. 1977 Nov;110(1):175–190. doi: 10.1016/0014-4827(77)90284-1. [DOI] [PubMed] [Google Scholar]
- Hedman K., Vaheri A., Wartiovaara J. External fibronectin of cultured human fibroblasts is predominantly a matrix protein. J Cell Biol. 1978 Mar;76(3):748–760. doi: 10.1083/jcb.76.3.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg N. M. A comparison of membrane proteins of normal and transformed cells by lactoperoxidase labeling. Proc Natl Acad Sci U S A. 1974 Feb;71(2):489–492. doi: 10.1073/pnas.71.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O. Alteration of cell-surface proteins by viral transformation and by proteolysis. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3170–3174. doi: 10.1073/pnas.70.11.3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O., Humphryes K. C. Characterization of the external proteins of hamster fibroblasts. J Cell Biol. 1974 Aug;62(2):438–448. doi: 10.1083/jcb.62.2.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O., Martin G. S., Shearer M., Critchley D. R., Epstein C. J. Viral transformation of rat myoblasts: effects on fusion and surface properties. Dev Biol. 1976 Jan;48(1):35–46. doi: 10.1016/0012-1606(76)90043-9. [DOI] [PubMed] [Google Scholar]
- Hök M., Rubin K., Oldberg A., Obrink B., Vaheri A. Cold-insoluble globulin mediates the adhesion of rat liver cells to plastic Petri dishes. Biochem Biophys Res Commun. 1977 Dec 7;79(3):726–733. doi: 10.1016/0006-291x(77)91172-x. [DOI] [PubMed] [Google Scholar]
- Jaffe E. A., Mosher D. F. Synthesis of fibronectin by cultured human endothelial cells. Ann N Y Acad Sci. 1978 Jun 20;312:122–131. doi: 10.1111/j.1749-6632.1978.tb16797.x. [DOI] [PubMed] [Google Scholar]
- Klebe R. J. Isolation of a collagen-dependent cell attachment factor. Nature. 1974 Jul 19;250(463):248–251. doi: 10.1038/250248a0. [DOI] [PubMed] [Google Scholar]
- Kleinman H. K., McGoodwin E. B. Localization of the cell attachment region in types I and II collagens. Biochem Biophys Res Commun. 1976 Sep 20;72(2):426–432. doi: 10.1016/s0006-291x(76)80060-5. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Linder E., Vaheri A., Ruoslahti E., Wartiovaara J. Distribution of fibroblast surface antigen in the developing chick embryo. J Exp Med. 1975 Jul 1;142(1):41–49. doi: 10.1084/jem.142.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh M. N., Trier J. S. Morphology and cell proliferation of subepithelial fibroblasts in adult mouse jejunum. I. Structural features. Gastroenterology. 1974 Oct;67(4):622–635. [PubMed] [Google Scholar]
- Mautner V., Hynes R. O. Surface distribution of LETS protein in relation to the cytoskeleton of normal and transformed cells. J Cell Biol. 1977 Dec;75(3):743–768. doi: 10.1083/jcb.75.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher D. F. Labeling of a major fibroblast surface protein (fibronectin) catalyzed by blood coagulation factor XIIa. Biochim Biophys Acta. 1977 Mar 28;491(1):205–210. doi: 10.1016/0005-2795(77)90056-3. [DOI] [PubMed] [Google Scholar]
- Mosher D. F., Saksela O., Keski-Oja J., Vaheri A. Distribution of a major surface-associated glycoprotein, fibronectin, in cultures of adherent cells. J Supramol Struct. 1977;6(4):551–557. doi: 10.1002/jss.400060408. [DOI] [PubMed] [Google Scholar]
- Pearlstein E. Plasma membrane glycoprotein which mediates adhesion of fibroblasts to collagen. Nature. 1976 Aug 5;262(5568):497–500. doi: 10.1038/262497a0. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Vaheri A. Interaction of soluble fibroblast surface antigen with fribrinogen and fibrin. J Exp Med. 1975 Feb 1;141(2):497–501. doi: 10.1084/jem.141.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E., Vaheri A., Kuusela P., Linder E. Fibroblast surface antigen: a new serum protein. Biochim Biophys Acta. 1973 Oct 18;322(2):352–358. doi: 10.1016/0005-2795(73)90310-3. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Vuento M., Engvall E. Interaction of fibronectin with antibodies and collagen in radioimmunoassay. Biochim Biophys Acta. 1978 Jun 21;534(2):210–218. doi: 10.1016/0005-2795(78)90003-x. [DOI] [PubMed] [Google Scholar]
- Stenman S., Vaheri A. Distribution of a major connective tissue protein, fibronectin, in normal human tissues. J Exp Med. 1978 Apr 1;147(4):1054–1064. doi: 10.1084/jem.147.4.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone K. R., Smith R. E., Joklik W. K. Changes in membrane polypeptides that occur when chick embryo fibroblasts and NRK cells are transformed with avian sarcoma viruses. Virology. 1974 Mar;58(1):86–100. doi: 10.1016/0042-6822(74)90143-3. [DOI] [PubMed] [Google Scholar]
- Vaheri A., Ruoslahti E. Disappearance of a major cell-type specific surface glycoprotein antigen (SF) after transformation of fibroblasts by Rous sarcoma virus. Int J Cancer. 1974 May 15;13(5):579–586. doi: 10.1002/ijc.2910130502. [DOI] [PubMed] [Google Scholar]
- Vaheri A., Ruoslahti E., Westermark B., Ponten J. A common cell-type specific surface antigen in cultured human glial cells and fibroblasts: loss in malignant cells. J Exp Med. 1976 Jan 1;143(1):64–72. doi: 10.1084/jem.143.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuento M., Wrann M., Ruoslahti E. Similarity of fibronectins isolated from human plasma and spent fibroblast culture medium. FEBS Lett. 1977 Oct 15;82(2):227–231. doi: 10.1016/0014-5793(77)80590-5. [DOI] [PubMed] [Google Scholar]
- Wartiovaara J., Linder E., Ruoslahti E., Vaheri A. Distribution of fibroblast surface antigen: association with fibrillar structures of normal cells and loss upon viral transformation. J Exp Med. 1974 Dec 1;140(6):1522–1533. doi: 10.1084/jem.140.6.1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser M. M. Intestinal epithelial cell surface membrane glycoprotein synthesis. I. An indicator of cellular differentiation. J Biol Chem. 1973 Apr 10;248(7):2536–2541. [PubMed] [Google Scholar]
- Yamada K. M., Yamada S. S., Pastan I. Cell surface protein partially restores morphology, adhesiveness, and contact inhibition of movement to transformed fibroblasts. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1217–1221. doi: 10.1073/pnas.73.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]