Abstract
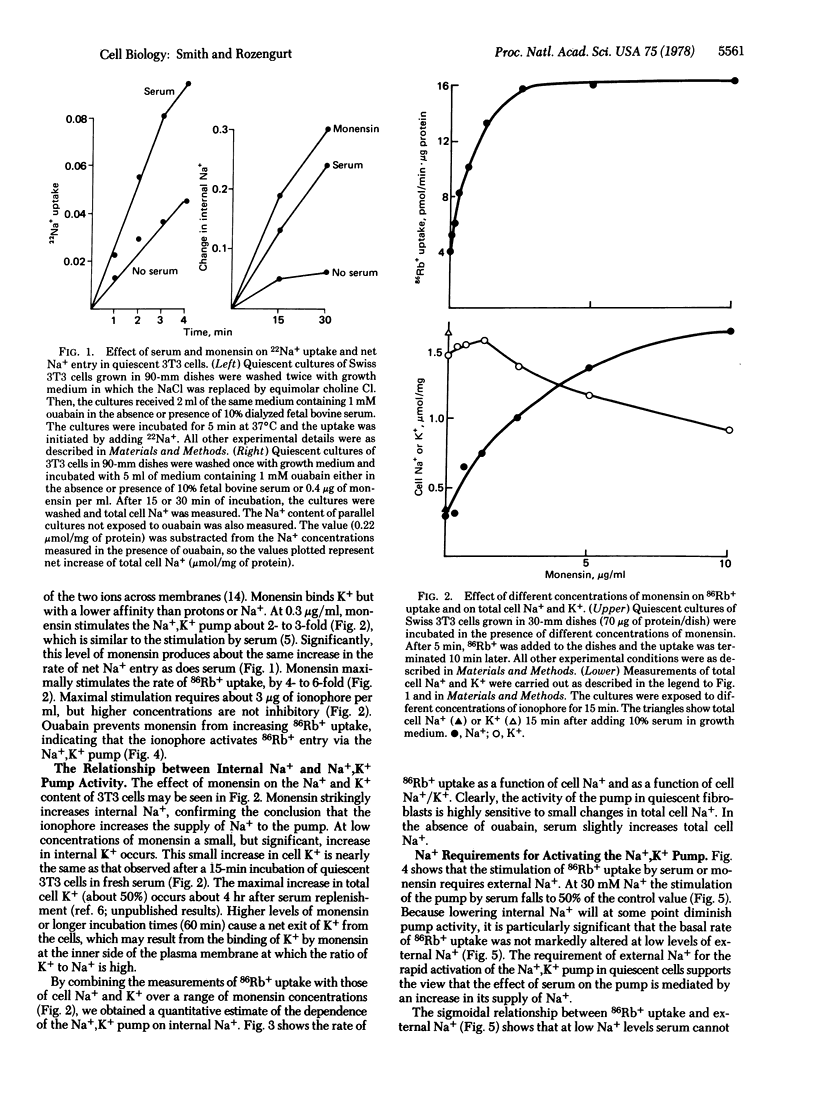
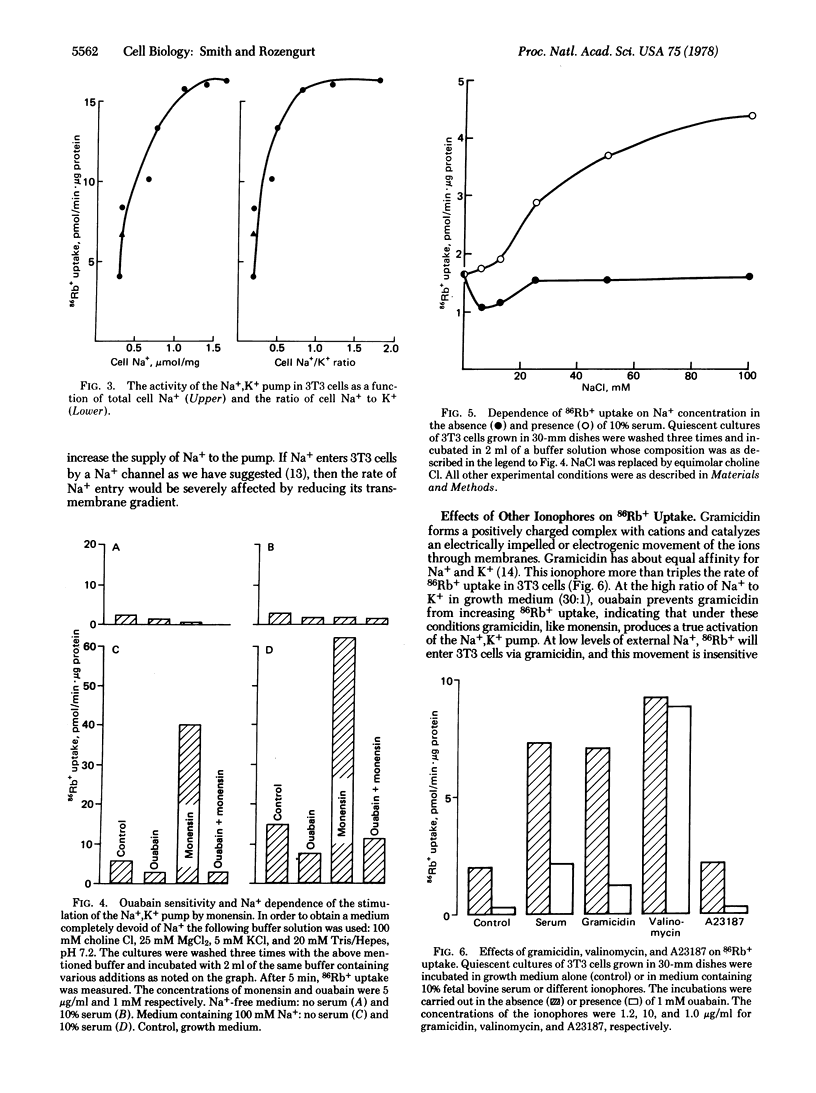
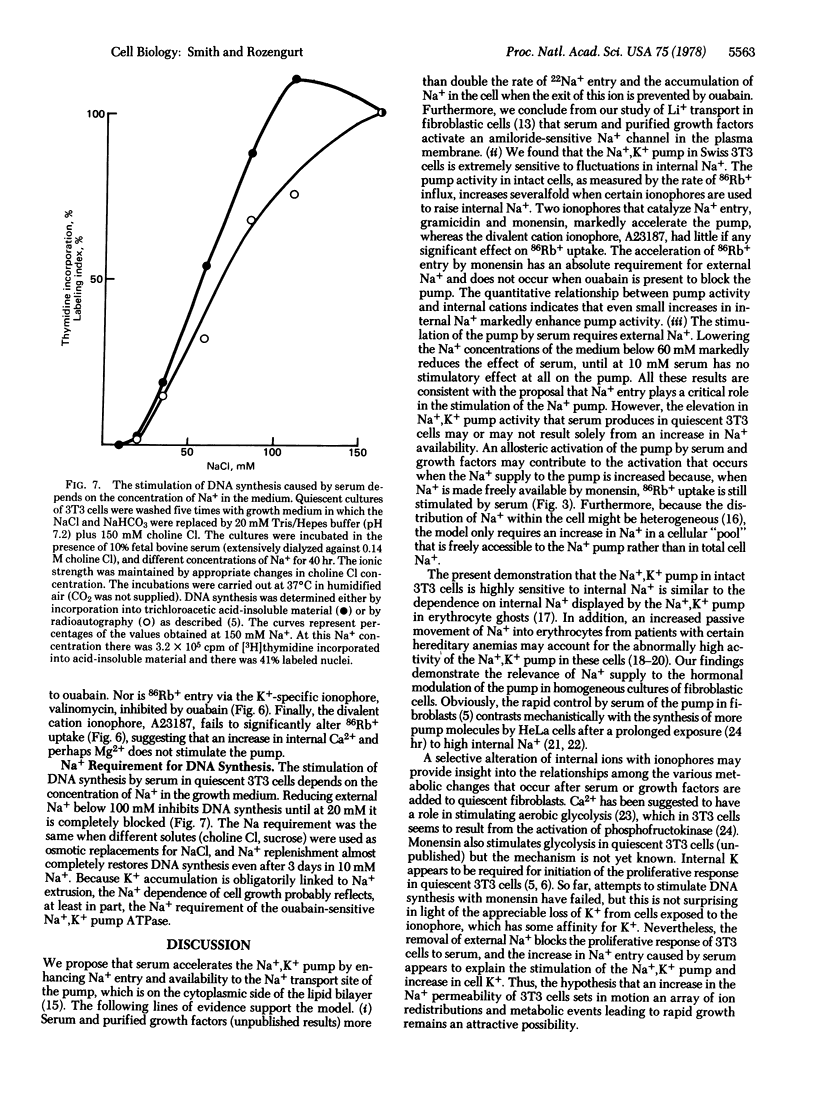
Two ionophores (monensin and gramicidin) that carry Na+ into 3T3 cells markedly enhance the rate of 86Rb+ uptake. Ouabain prevents both ionophores from increasing 86Rb+ uptake, indicating that the ionophores activate the Na+,K+ pump. Measurements of 86Rb+ uptake and cell Na+ and K+ over a range of monensin concentrations show that the activity of the Na+,K+ pump in 3T3 cells is limited by the supply of internal Na+ and is extremely sensitive to small changes in internal Na+. Serum rapidly enhances the rate of 22Na+ uptake and net Na+ entry when Na+ exit is inhibited by ouabain. At 0.3 microgram/ml, monensin increases the rate of net Na+ entry and activates the Na+,K+ pump by the same degree as serum. The stimulation of 86Rb+ uptake by serum or the ionophores has an absolute requirement for external Na+. Thus, serum appears to stimulate the Na+,K+ pump in quiescent 3T3 cells by increasing its supply of Na+.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERTLES J. F. Sodium transport across the surface membrane of red blood cells in hereditary spherocytosis. J Clin Invest. 1957 Jun;36(6 Pt 1):816–824. doi: 10.1172/JCI103487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S. P., Bosmann H. B. Rubidium transport and ouabain binding in normal and virally transformed mouse fibroblasts. Exp Cell Res. 1976 Jun;100(1):153–158. doi: 10.1016/0014-4827(76)90337-2. [DOI] [PubMed] [Google Scholar]
- Boardman L., Huett M., Lamb J. F., Newton J. P., Polson J. M. Evidence for the genetic control of the sodium pump density in HeLa cells. J Physiol. 1974 Sep;241(3):771–794. doi: 10.1113/jphysiol.1974.sp010684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne H. R., Rozengurt E. An 18,000 molecular weight polypeptide induces early events and stimulates DNA synthesis in cultured cells. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4555–4559. doi: 10.1073/pnas.73.12.4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook J. S., Vaughan G. L., Proctor W. R., Brake E. T. Interaction of two mechanisms regulating alkali cations in HeLa cells. J Cell Physiol. 1975 Aug;86(1):59–70. doi: 10.1002/jcp.1040860108. [DOI] [PubMed] [Google Scholar]
- Diamond I., Legg A., Schneider J. A., Rozengurt E. Glycolysis in quiescent cultures of 3T3 cells. Stimulation by serum, epidermal growth factor, and insulin in intact cells and persistence of the stimulation after cell homogenization. J Biol Chem. 1978 Feb 10;253(3):866–871. [PubMed] [Google Scholar]
- Garay R. P., Garrahan P. J. The interaction of sodium and potassium with the sodium pump in red cells. J Physiol. 1973 Jun;231(2):297–325. doi: 10.1113/jphysiol.1973.sp010234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holley R. W. Control of growth of mammalian cells in cell culture. Nature. 1975 Dec 11;258(5535):487–490. doi: 10.1038/258487a0. [DOI] [PubMed] [Google Scholar]
- Ledbetter M. L., Lubin M. Control of protein synthesis in human fibroblasts by intracellular potassium. Exp Cell Res. 1977 Mar 15;105(2):223–236. doi: 10.1016/0014-4827(77)90120-3. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Heppel L. A. Serum rapidly stimulates ouabain-sensitive 86-RB+ influx in quiescent 3T3 cells. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4492–4495. doi: 10.1073/pnas.72.11.4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider J. A., Diamond I., Rozengurt E. Glycolysis of quiescent cultures of 3T3 cells. Addition of serum, epidermal growth factor, and insulin increases the activity of phosphofructokinase in a protein synthesis-independent manner. J Biol Chem. 1978 Feb 10;253(3):872–877. [PubMed] [Google Scholar]
- Smith G. L. Increased ouabain-sensitive 86Rubidium uptake after mitogenic stimulation of quiescent chicken embryo fibroblasts with purified multiplication-stimulating activity. J Cell Biol. 1977 Jun;73(3):761–767. doi: 10.1083/jcb.73.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TODARO G. J., GREEN H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol. 1963 May;17:299–313. doi: 10.1083/jcb.17.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tupper J. T. Variation in potassium transport properties of mouse 3T3 cells as a result of subcultivation. J Cell Physiol. 1977 Nov;93(2):303–307. doi: 10.1002/jcp.1040930216. [DOI] [PubMed] [Google Scholar]
- Tupper J. T., Zorgniotti F., Mills B. Potassium transport and content during G1 and S phase following serum stimulation of 3T3 cells. J Cell Physiol. 1977 Jun;91(3):429–440. doi: 10.1002/jcp.1040910313. [DOI] [PubMed] [Google Scholar]
- Wiley J. S. Inheritance of an increased sodium pump in human red cells. Nature. 1969 Mar 29;221(5187):1222–1224. doi: 10.1038/2211222a0. [DOI] [PubMed] [Google Scholar]
- Zarkowsky H. S., Oski F. A., Sha'afi R., Shohet S. B., Nathan D. G. Congenital hemolytic anemia with high sodium, low potassium red cells. I. Studies of membrane permeability. N Engl J Med. 1968 Mar 14;278(11):573–581. doi: 10.1056/NEJM196803142781101. [DOI] [PubMed] [Google Scholar]


