Abstract
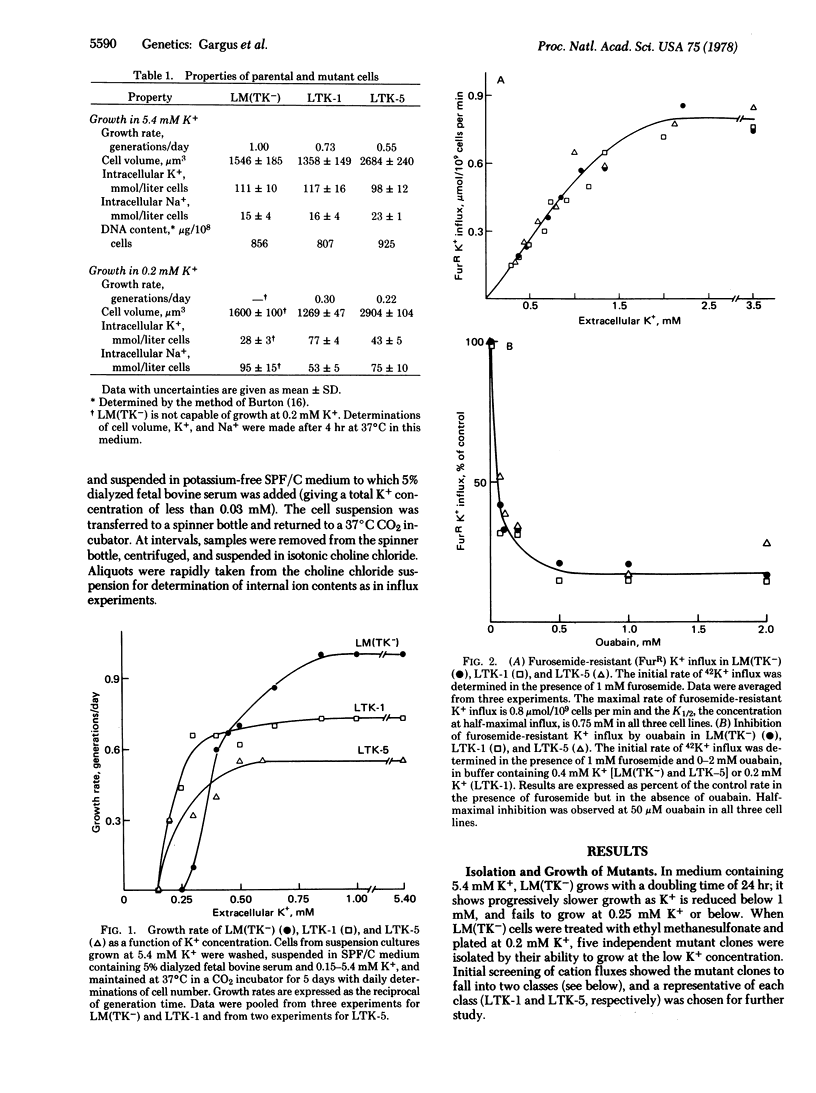
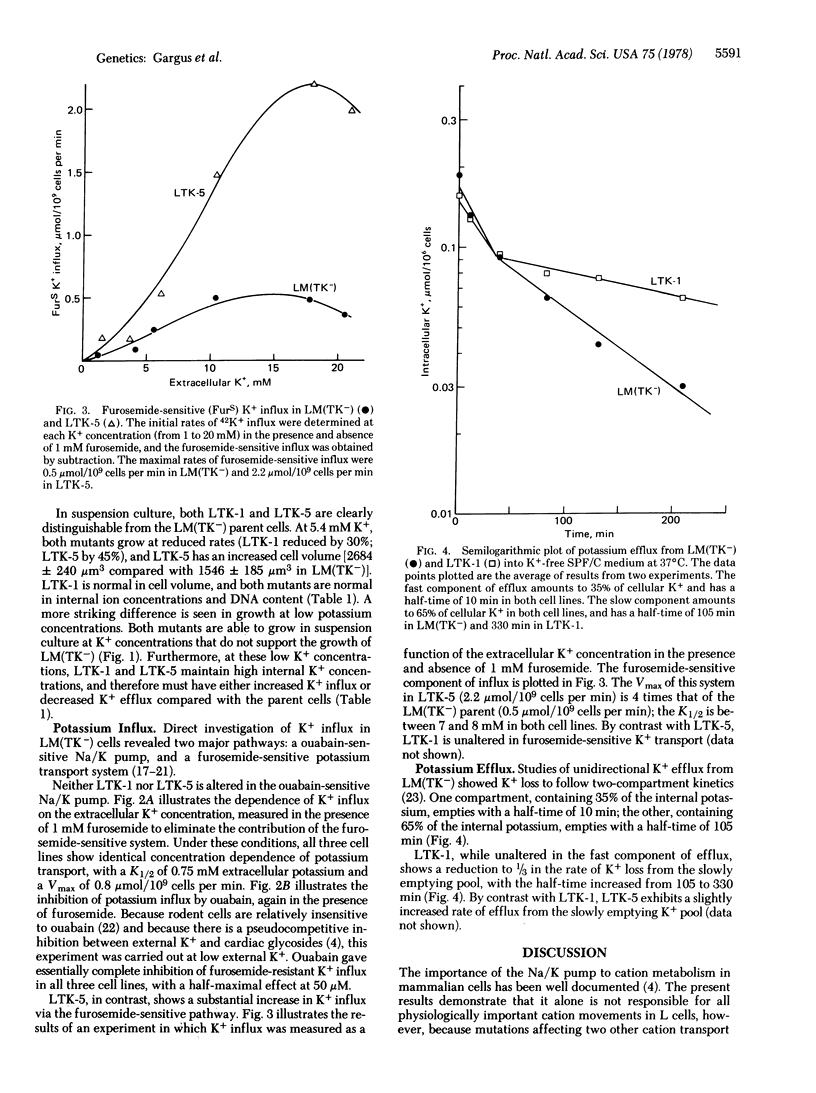
Starting with mutagenized cultures of the mouse fibroblastic cell line LM(TK-), we have selected mutant clones by their ability to grow at 0.2 micrometer K+, a concentration unable to support the growth of the parent cell. The mutants fall into two classes on the basis of their potassium transport properties. Both classes maintain a high intracellular K+ concentration when growing in low-potassium medium, and both are unaltered in the ouabain-sensitive Na/K pump. One class shows an increased activity of a ouabain-resistant, furosemide-sensitive K+ transport system; the other class shows a decreased activity of a specific component of K+ efflux.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Control of cell division: a unifying hypothesis. J Cyclic Nucleotide Res. 1975;1(5):305–320. [PubMed] [Google Scholar]
- Finkelstein M. C., Adelberg E. A. Neutral amino acid transport in an established mouse lymphocytic cell line. J Biol Chem. 1977 Oct 25;252(20):7101–7108. [PubMed] [Google Scholar]
- Glynn I. M., Karlish S. J. The sodium pump. Annu Rev Physiol. 1975;37:13–55. doi: 10.1146/annurev.ph.37.030175.000305. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M. Ion currents and physiological functions in microorganisms. Annu Rev Microbiol. 1977;31:181–203. doi: 10.1146/annurev.mi.31.100177.001145. [DOI] [PubMed] [Google Scholar]
- Hoffman J. F., Kregenow F. M. The characterization of new energy dependent cation transport processes in red blood cells. Ann N Y Acad Sci. 1966 Jul 14;137(2):566–576. doi: 10.1111/j.1749-6632.1966.tb50182.x. [DOI] [PubMed] [Google Scholar]
- Kaplan J. G. Membrane cation transport and the control of proliferation of mammalian cells. Annu Rev Physiol. 1978;40:19–41. doi: 10.1146/annurev.ph.40.030178.000315. [DOI] [PubMed] [Google Scholar]
- Kregenow F. M. Functional separation of the Na-K exchange pump from the volume controlling mechanism in enlarged duck red cells. J Gen Physiol. 1974 Oct;64(4):393–412. doi: 10.1085/jgp.64.4.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kregenow F. M. The response of duck erythrocytes to hypertonic media. Further evidence for a volume-controlling mechanism. J Gen Physiol. 1971 Oct;58(4):396–412. doi: 10.1085/jgp.58.4.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kregenow F. M. The response of duck erythrocytes to nonhemolytic hypotonic media. Evidence for a volume-controlling mechanism. J Gen Physiol. 1971 Oct;58(4):372–395. doi: 10.1085/jgp.58.4.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kregenow F. M. The response of duck erythrocytes to norepinephrine and an elevated extracellular potassium. Volume regulation in isotonic media. J Gen Physiol. 1973 Apr;61(4):509–527. doi: 10.1085/jgp.61.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb J. F., Lindsay R. Transient effects of ethacrynic acid on Na and K movements in cultured cells. Q J Exp Physiol Cogn Med Sci. 1973 Oct;58(4):345–355. doi: 10.1113/expphysiol.1973.sp002228. [DOI] [PubMed] [Google Scholar]
- Lamb J. F., MacKinnon M. G. Effect of ouabain and metabolic inhibitors on the Na and K movements and nucleotide contents of L cells. J Physiol. 1971 Mar;213(3):665–682. doi: 10.1113/jphysiol.1971.sp009407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb J. F., MacKinnon M. G. The membrane potential and permeabilities of the L cell membrane to Na, K and chloride. J Physiol. 1971 Mar;213(3):683–689. doi: 10.1113/jphysiol.1971.sp009408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meech R. W. Calcium-dependent potassium activation in nervous tissues. Annu Rev Biophys Bioeng. 1978;7:1–18. doi: 10.1146/annurev.bb.07.060178.000245. [DOI] [PubMed] [Google Scholar]
- Mills B., Tupper J. T. Cation permeability and ouabain-insensitive cation flux in the Ehrlich ascites tumor cell. J Membr Biol. 1975;20(1-2):75–97. doi: 10.1007/BF01870629. [DOI] [PubMed] [Google Scholar]
- Mills B., Tupper J. T. Cell cycle dependent changes in potassium transport. J Cell Physiol. 1976 Sep;89(1):123–132. doi: 10.1002/jcp.1040890112. [DOI] [PubMed] [Google Scholar]
- Nelson P. G., Peacock J. H. Acetylcholine responses in L cells. Science. 1972 Sep 15;177(4053):1005–1007. doi: 10.1126/science.177.4053.1005. [DOI] [PubMed] [Google Scholar]
- Nelson P. G., Peacock J. H. Transmission on an active electrical response between fibroblasts (L cells) in cell culture. J Gen Physiol. 1973 Jul;62(1):25–36. doi: 10.1085/jgp.62.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y., Doida Y., Roy G., Tsuchiya W., Inouye K., Inouye A. Oscillations of membrane potential in L cells. I. Basic characteristics. J Membr Biol. 1977 Aug 4;35(4):319–335. doi: 10.1007/BF01869957. [DOI] [PubMed] [Google Scholar]
- Okada Y., Roy G., Tsuchiya W., Doida Y., Inouye A. Oscillations of membrane potential in L cells. II. Effect of monovalent ion concentrations and conductance changes associated with oscillations. J Membr Biol. 1977 Aug 4;35(4):337–350. doi: 10.1007/BF01869958. [DOI] [PubMed] [Google Scholar]
- Ross E. M., Gilman A. G. Reconstitution of catecholamine-sensitive adenylate cyclase activity: interactions of solubilized components with receptor-replete membranes. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3715–3719. doi: 10.1073/pnas.74.9.3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roti Roti L. W., Rothstein A. Adaptation of mouse leukemic cells (L5178Y) to anisotonic media. I. Cell volume regulation. Exp Cell Res. 1973 Jun;79(2):295–310. doi: 10.1016/0014-4827(73)90448-5. [DOI] [PubMed] [Google Scholar]
- Roy G., Okada Y. Oscillation of membrane potential in L cells: III K + current-voltage curves. J Membr Biol. 1978 Feb 3;38(4):347–357. doi: 10.1007/BF01870151. [DOI] [PubMed] [Google Scholar]
- SANFORD K. K., EARLE W. R., LIKELY G. D. The growth in vitro of single isolated tissue cells. J Natl Cancer Inst. 1948 Dec;9(3):229–246. [PubMed] [Google Scholar]
- Sachs J. R. Ouabain-insensitive sodium movements in the human red blood cell. J Gen Physiol. 1971 Mar;57(3):259–282. doi: 10.1085/jgp.57.3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt W. F., 3rd, McManus T. J. Ouabain-insensitive salt and water movements in duck red cells. I. Kinetics of cation transport under hypertonic conditions. J Gen Physiol. 1977 Jul;70(1):59–79. doi: 10.1085/jgp.70.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt W. F., 3rd, McManus T. J. Ouabain-insensitive salt and water movements in duck red cells. II. Norepinephrine stimulation of sodium plus potassium cotransport. J Gen Physiol. 1977 Jul;70(1):81–97. doi: 10.1085/jgp.70.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt W. F., 3rd, McManus T. J. Ouabain-insensitive salt and water movements in duck red cells. III. The role of chloride in the volume response. J Gen Physiol. 1977 Jul;70(1):99–121. doi: 10.1085/jgp.70.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz S. G., Curran P. F. Coupled transport of sodium and organic solutes. Physiol Rev. 1970 Oct;50(4):637–718. doi: 10.1152/physrev.1970.50.4.637. [DOI] [PubMed] [Google Scholar]
- Spaggiare S., Wallach M. J., Tupper J. T. Potassium transport in normal and transformed mouse 3T3 cells. J Cell Physiol. 1976 Nov;89(3):403–416. doi: 10.1002/jcp.1040890306. [DOI] [PubMed] [Google Scholar]
- Stanners C. P., Eliceiri G. L., Green H. Two types of ribosome in mouse-hamster hybrid cells. Nat New Biol. 1971 Mar 10;230(10):52–54. doi: 10.1038/newbio230052a0. [DOI] [PubMed] [Google Scholar]
- TOSTESON D. C., HOFFMAN J. F. Regulation of cell volume by active cation transport in high and low potassium sheep red cells. J Gen Physiol. 1960 Sep;44:169–194. doi: 10.1085/jgp.44.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tupper J. T. Cation flux in the ehrlich ascites tumor cell. Evidence for Na+-for-Na+ and K+-for-K+ exchange diffusion. Biochim Biophys Acta. 1975 Jul 18;394(4):586–596. doi: 10.1016/0005-2736(75)90144-3. [DOI] [PubMed] [Google Scholar]
- Tupper J. T., Zorgniotti F., Mills B. Potassium transport and content during G1 and S phase following serum stimulation of 3T3 cells. J Cell Physiol. 1977 Jun;91(3):429–440. doi: 10.1002/jcp.1040910313. [DOI] [PubMed] [Google Scholar]


