Abstract
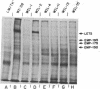
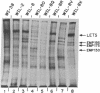
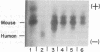
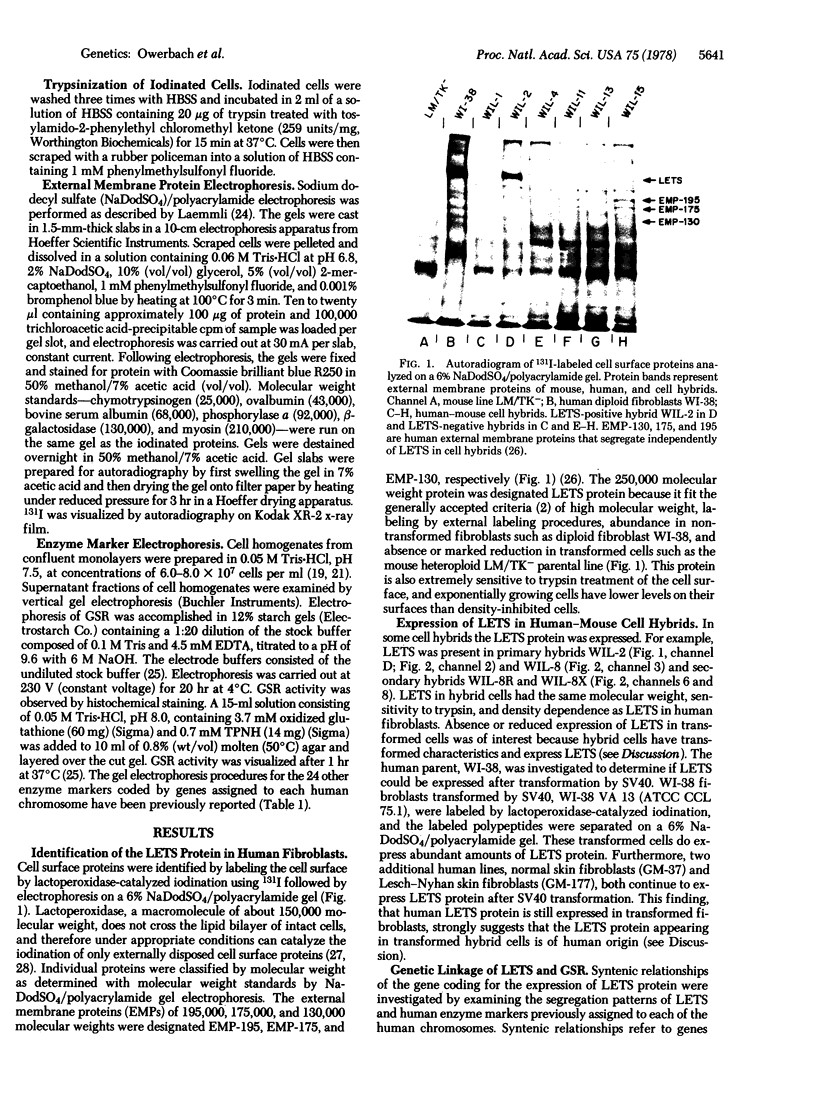
Techniques have been developed to analyze the genetics of the large, external, transformation-sensitive (LETS) protein (fibronectin). External membrane proteins of human-mouse somatic cell hybrids with reduced numbers of human but not mouse chromosomes were labeled by lactoperoxidase-catalyzed iodination. Cell surface proteins were identified after sodium dodecyl sulfate/polyacrylamide gel electrophoresis by autoradiography of the dried gel. The LETS protein was identified in parental human cells, and LETS segregated in human-mouse cell hybrids formed from human WI-38 fibroblasts and a mouse L-cell line not expressing LETS. The LETS protein segregated concordantly with the chromosome 8 enzyme marker glutathione reductase (EC 1.6.4.2) and human chromosome 8. These findings demonstrate that a gene, LETS, encoded on chromosome 8, is responsible for the LETS protein expression in humans. Because LETS has been implicated in tumorigenicity and cellular transformation, it is of interest that rearrangement or modifications in the number of chromosome 8 have been associated with certain forms of cancer.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams S. L., Sobel M. E., Howard B. H., Olden K., Yamada K. M., de Crombrugghe B., Pastan I. Levels of translatable mRNAs for cell surface protein, collagen precursors, and two membrane proteins are altered in Rous sarcoma virus-transformed chick embryo fibroblasts. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3399–3403. doi: 10.1073/pnas.74.8.3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L. B., Gallimore P. H., McDougall J. K. Correlation between tumor induction and the large external transformation sensitive protein on the cell surface. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3570–3574. doi: 10.1073/pnas.73.10.3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahmberg C. G., Hakomori S. I. Altered growth behavior of malignant cells associated with changes in externally labeled glycoprotein and glycolipid. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3329–3333. doi: 10.1073/pnas.70.12.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallimore P. H., McDougall J. K., Chen L. B. In vitro traits of adenovirus-transformed cell lines and their relevance to tumorigenicity in nude mice. Cell. 1977 Apr;10(4):669–678. doi: 10.1016/0092-8674(77)90100-3. [DOI] [PubMed] [Google Scholar]
- Hogg N. M. A comparison of membrane proteins of normal and transformed cells by lactoperoxidase labeling. Proc Natl Acad Sci U S A. 1974 Feb;71(2):489–492. doi: 10.1073/pnas.71.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard A. L., Cohn Z. A. Externally disposed plasma membrane proteins. I. Enzymatic iodination of mouse L cells. J Cell Biol. 1975 Feb;64(2):438–460. doi: 10.1083/jcb.64.2.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard A. L., Cohn Z. A. The enzymatic iodination of the red cell membrane. J Cell Biol. 1972 Nov;55(2):390–405. doi: 10.1083/jcb.55.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O. Alteration of cell-surface proteins by viral transformation and by proteolysis. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3170–3174. doi: 10.1073/pnas.70.11.3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O. Cell surface proteins and malignant transformation. Biochim Biophys Acta. 1976 Apr 30;458(1):73–107. doi: 10.1016/0304-419x(76)90015-9. [DOI] [PubMed] [Google Scholar]
- Kaplan J. C., Beutler E. Electrophoretic study of glutathione reductase in human erythrocytes and leucocytes. Nature. 1968 Jan 20;217(5125):256–258. doi: 10.1038/217256a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lalley P. A., Rattazzi M. C., Shows T. B. Human beta-D-N-acetylhexosaminidases A and B: expression and linkage relationships in somatic cell hybrids. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1569–1573. doi: 10.1073/pnas.71.4.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitelman F., Levan G. Clustering of aberrations to specific chromosomes in human neoplasms. II. A survey of 287 neoplasms. Hereditas. 1976 Jun 14;82(2):167–174. doi: 10.1111/j.1601-5223.1976.tb01553.x. [DOI] [PubMed] [Google Scholar]
- Olden K., Yamada K. M. Mechanism of the decrease in the major cell surface protein of chick embryo fibroblasts after transformation. Cell. 1977 Aug;11(4):957–969. doi: 10.1016/0092-8674(77)90307-5. [DOI] [PubMed] [Google Scholar]
- Phillips D. R., Morrison M. The arrangement of proteins in the human erythrocyte membrane. Biochem Biophys Res Commun. 1970 Jul 27;40(2):284–289. doi: 10.1016/0006-291x(70)91007-7. [DOI] [PubMed] [Google Scholar]
- Rowley J. D. Chromosomes in leukemia and lymphoma. Semin Hematol. 1978 Jul;15(3):301–319. [PubMed] [Google Scholar]
- Rowley J. D. Identificaton of a translocation with quinacrine fluorescence in a patient with acute leukemia. Ann Genet. 1973 Jun;16(2):109–112. [PubMed] [Google Scholar]
- Shows T. B., Brown J. A., Eddy R. L., Byers M. G., Haley L. L., Cooper E. S., Goggin A. P. Assignment of peptidase S (PEPS) to chromosome 4 in man using somatic cell hybrids. Hum Genet. 1978 Aug 31;43(2):119–125. doi: 10.1007/BF00293588. [DOI] [PubMed] [Google Scholar]
- Shows T. B., Brown J. A. Human X-Linked genes regionally mapped utilizing X-autosome translocations and somatic cell hybrids. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2125–2129. doi: 10.1073/pnas.72.6.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shows T. B., Brown J. A. Mapping AK1, ACONs, and AK3 to chromosome 9 in man employing and X/9 translocation and somatic cell hybrids. Cytogenet Cell Genet. 1977;19(1):26–37. doi: 10.1159/000130791. [DOI] [PubMed] [Google Scholar]
- Shows T. B. Genetics of human-mouse somatic cell hybrids: linkage of human genes for isocitrate dehydrogenase and malate dehydrogenase. Biochem Genet. 1972 Dec;7(3):193–204. doi: 10.1007/BF00484817. [DOI] [PubMed] [Google Scholar]
- Shows T. B. Genetics of human-mouse somatic cell hybrids: linkage of human genes for lactate dehydrogenase-A and esterase-A 4 . Proc Natl Acad Sci U S A. 1972 Feb;69(2):348–352. doi: 10.1073/pnas.69.2.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles C. D., Desmond W., Jr, Sato G., Saier M. H., Jr Failure of human cells transformed by simian virus 40 to form tumors in athymic nude mice. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4971–4975. doi: 10.1073/pnas.72.12.4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone K. R., Smith R. E., Joklik W. K. Changes in membrane polypeptides that occur when chick embryo fibroblasts and NRK cells are transformed with avian sarcoma viruses. Virology. 1974 Mar;58(1):86–100. doi: 10.1016/0042-6822(74)90143-3. [DOI] [PubMed] [Google Scholar]
- Tweto J., Friedman E., Doyle D. Proteins of the hepatoma tissue culture cell plasma membrane. J Supramol Struct. 1976;4(2):141–159. doi: 10.1002/jss.400040202. [DOI] [PubMed] [Google Scholar]
- Vaheri A., Ruoslahti E. Disappearance of a major cell-type specific surface glycoprotein antigen (SF) after transformation of fibroblasts by Rous sarcoma virus. Int J Cancer. 1974 May 15;13(5):579–586. doi: 10.1002/ijc.2910130502. [DOI] [PubMed] [Google Scholar]
- Willingham M. C., Yamada K. M., Yamada S. S., Pouysségur J., Pastan I. Microfilament bundles and cell shape are related to adhesiveness to substratum and are dissociable from growth control in cultured fibroblasts. Cell. 1977 Mar;10(3):375–380. doi: 10.1016/0092-8674(77)90024-1. [DOI] [PubMed] [Google Scholar]
- Yamada K. M., Ohanian S. H., Pastan I. Cell surface protein decreases microvilli and ruffles on transformed mouse and chick cells. Cell. 1976 Oct;9(2):241–245. doi: 10.1016/0092-8674(76)90115-x. [DOI] [PubMed] [Google Scholar]
- Yamada K. M., Weston J. A. Isolation of a major cell surface glycoprotein from fibroblasts. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3492–3496. doi: 10.1073/pnas.71.9.3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K. M., Yamada S. S., Pastan I. Cell surface protein partially restores morphology, adhesiveness, and contact inhibition of movement to transformed fibroblasts. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1217–1221. doi: 10.1073/pnas.73.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K. M., Yamada S. S., Pastan I. Quantitation of a transformation-sensitive, adhesive cell surface glycoprotein. Decrease of several untransformed permanent cell lines. J Cell Biol. 1977 Aug;74(2):649–654. doi: 10.1083/jcb.74.2.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K. M., Yamada S. S., Pastan I. The major cell surface glycoprotein of chick embryo fibroblasts is an agglutinin. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3158–3162. doi: 10.1073/pnas.72.8.3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zech L., Haglund U., Nilsson K., Klein G. Characteristic chromosomal abnormalities in biopsies and lymphoid-cell lines from patients with Burkitt and non-Burkitt lymphomas. Int J Cancer. 1976 Jan 15;17(1):47–56. doi: 10.1002/ijc.2910170108. [DOI] [PubMed] [Google Scholar]