Abstract
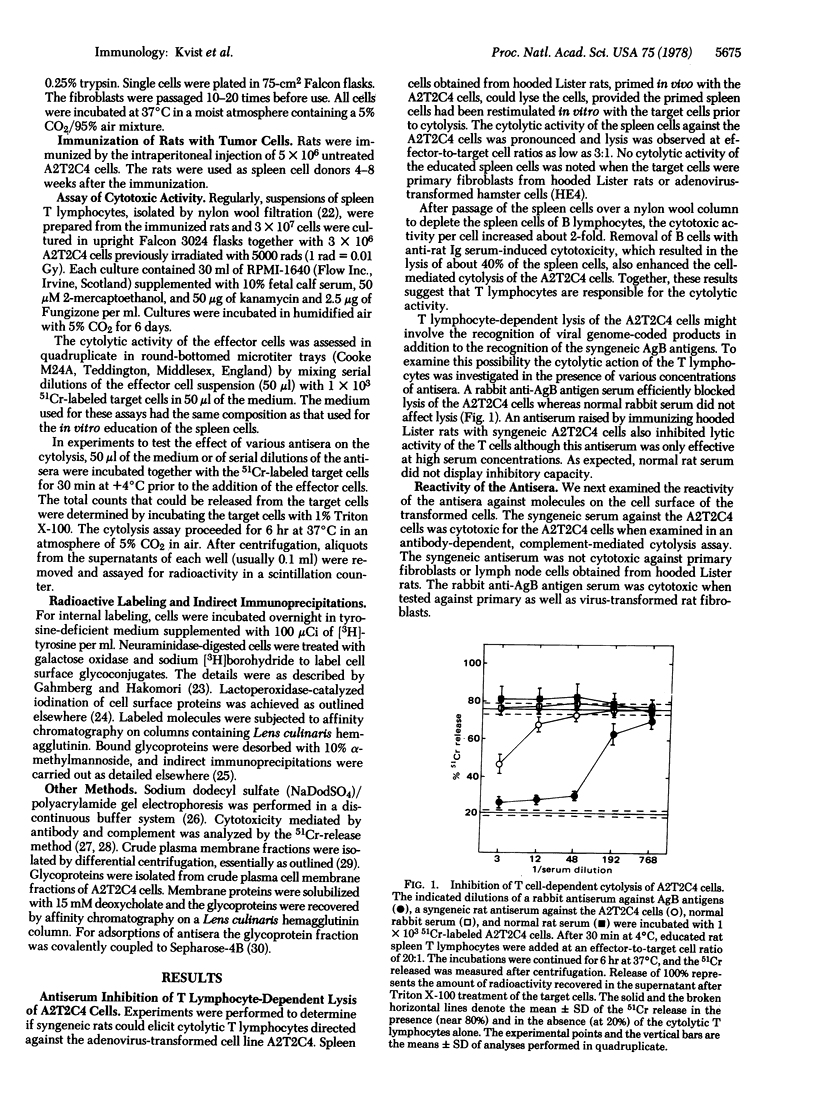
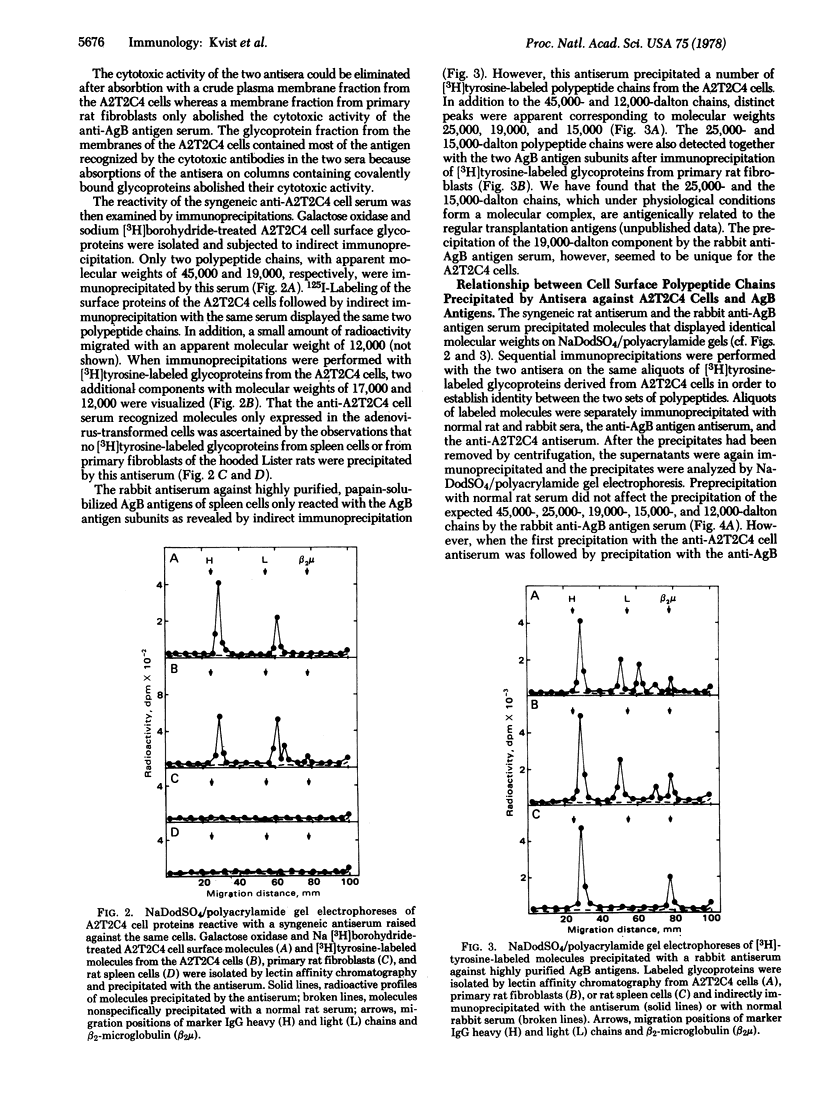
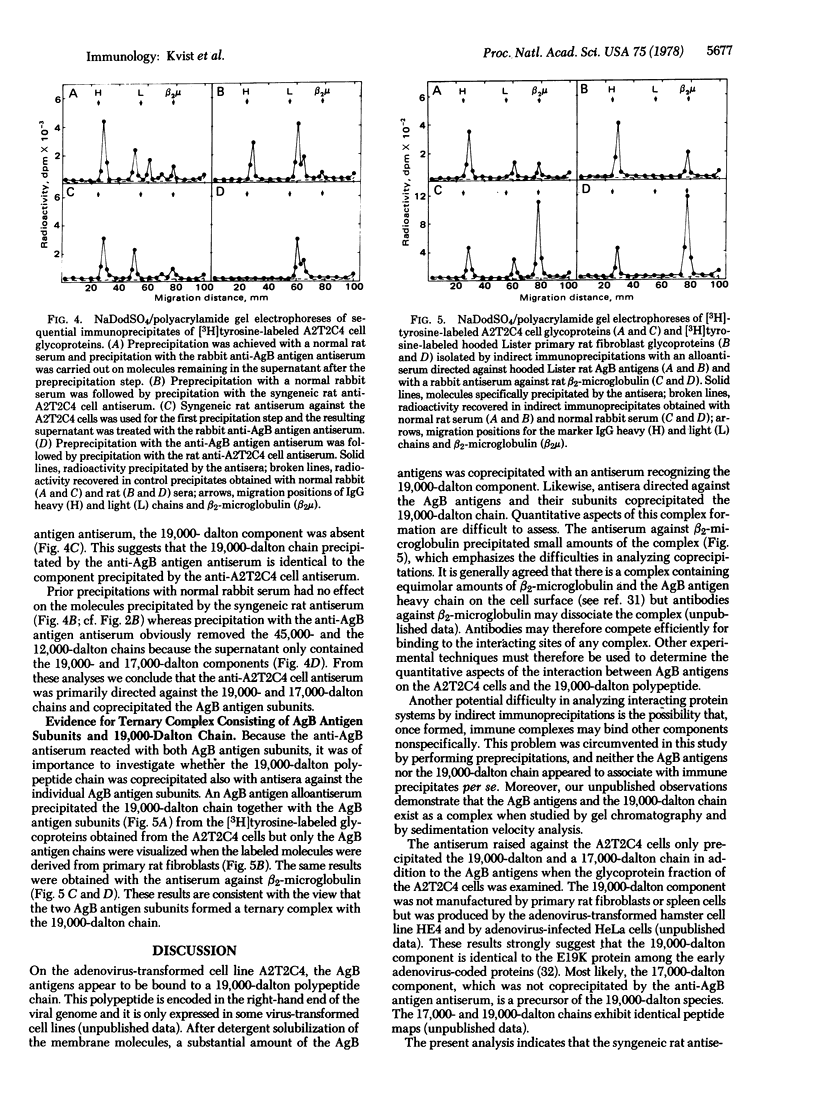
A rat cell line (A2T2C4) transformed with adenovirus type 2 elicited cytotoxic T lymphocytes in syngeneic rats. Cytotoxicity was abolished by a rabbit antiserum directed against the major histocompatibility (AgB) antigens and by a syngeneic rat antiserum raised against the virus-transformed cell line. The syngeneic antiserum immunoprecipitated surface proteins with apparent molecular weights of 45,000, 19,000, 17,000, and 12,000 from the A2T2C4 cells but it displayed no reactivity against primary rat fibroblasts and spleen cells. The rabbit antiserum against AgB antigens precipitated a 19,000-dalton component from the A2T2C4 cells which was not observed in primary rat fibroblasts. Sequential immunoprecipitation revealed identity between the major polypeptides recognized by the two antisera. Because the rabbit anti-AgB antigen serum was specific for the transplantation antigen subunits and because the syngeneic rat antiserum against the A2T2C4 cells failed to react with the AgB antigens in normal cells, it is concluded that the 19,000-dalton component is coprecipitated with the AgB antigens. Antisera directed specifically against beta2-microglobulin and the alloantigenic AgB antigen subunit also coprecipitated the 19,000-dalton component. These results indicate that the AgB antigen subunits form a ternary complex with a virus-coded protein on the surface of the virus-transformed A2T2C4 cells. This molecular complex may be recognized by the cytoloytic T lymphocytes
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bevan M. J. The major histocompatibility complex determines susceptibility to cytotoxic T cells directed against minor histocompatibility antigens. J Exp Med. 1975 Dec 1;142(6):1349–1364. doi: 10.1084/jem.142.6.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourguignon L. Y., Hyman R., Trowbridge I., Singer S. J. Participation of histocompatibility antigens in capping of molecularly independent cell surface components by their specific antibodies. Proc Natl Acad Sci U S A. 1978 May;75(5):2406–2410. doi: 10.1073/pnas.75.5.2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubbers J. E., Chen S., Lilly F. Nonrandom inclusion of H-2K and H-2D antigens in Friend virus particles from mice of various strains. J Exp Med. 1978 Feb 1;147(2):340–351. doi: 10.1084/jem.147.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- Doherty P. C., Blanden R. V., Zinkernagel R. M. Specificity of virus-immune effector T cells for H-2K or H-2D compatible interactions: implications for H-antigen diversity. Transplant Rev. 1976;29:89–124. doi: 10.1111/j.1600-065x.1976.tb00198.x. [DOI] [PubMed] [Google Scholar]
- Forman J., Vitetta E. S. Absence of H-2 antigens capable of reacting with cytotoxic T cells on a teratoma line expressing a T/t locus antigen. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3661–3665. doi: 10.1073/pnas.72.9.3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahmberg C. G., Hakomori S. I. External labeling of cell surface galactose and galactosamine in glycolipid and glycoprotein of human erythrocytes. J Biol Chem. 1973 Jun 25;248(12):4311–4317. [PubMed] [Google Scholar]
- Gallimore P. H. Viral DNA in transformed cells. II. A study of the sequences of adenovirus 2 DNA IN NINE LINES OF TRANSFORMED RAT CELLS USING SPECIFIC FRAGMENTS OF THE VIRAL GENOME;. J Mol Biol. 1974 Oct 15;89(1):49–72. doi: 10.1016/0022-2836(74)90162-4. [DOI] [PubMed] [Google Scholar]
- Germain R. N., Dorf M. E., Benacerraf B. Inhibition of T-lymphocyte-mediated tumor-specific lysis by alloantisera directed against the H-2 serological specificities of the tumor. J Exp Med. 1975 Oct 1;142(4):1023–1028. doi: 10.1084/jem.142.4.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., Abrahams P. J., Mulder C., Heijneker H. L., Warnaar S. O., De Vries F. A., Fiers W., Van Der Eb A. J. Studies on in vitro transformation by DNA and DNA fragments of human adenoviruses and simian virus 40. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):637–650. doi: 10.1101/sqb.1974.039.01.077. [DOI] [PubMed] [Google Scholar]
- HUEBNER R. J., ROWE W. P., TURNER H. C., LANE W. T. SPECIFIC ADENOVIRUS COMPLEMENT-FIXING ANTIGENS IN VIRUS-FREE HAMSTER AND RAT TUMORS. Proc Natl Acad Sci U S A. 1963 Aug;50:379–389. doi: 10.1073/pnas.50.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale A. H., Witte O. N., Baltimore D., Eisen H. N. Vesicular stomatitis virus glycoprotein is necessary for H-2-restricted lysis of infected cells by cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 1978 Feb;75(2):970–974. doi: 10.1073/pnas.75.2.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht T. T., Summers D. F. Interactions of vesicular stomatitis virus with murine cell surface antigens. J Virol. 1976 Sep;19(3):833–845. doi: 10.1128/jvi.19.3.833-845.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A., Morein B., Fries E., Simons K., Robinson P., Schirrmacher V., Terhorst C., Strominger J. L. Human (HLA-A and HLA-B) and murine (H-2K and H-2D) histocompatibility antigens are cell surface receptors for Semliki Forest virus. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3846–3850. doi: 10.1073/pnas.75.8.3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning R., Schrader J. W., Edelman G. M. Antiviral antibodies inhibit the lysis of tumour cells by anti-H--2 sera. Nature. 1976 Oct 21;263(5579):689–691. doi: 10.1038/263689a0. [DOI] [PubMed] [Google Scholar]
- Johansson K., Persson H., Lewis A. M., Pettersson U., Tibbetts C., Philipson L. Viral DNA sequences and gene products in hamster cells transformed by adenovirus type 2. J Virol. 1978 Sep;27(3):628–639. doi: 10.1128/jvi.27.3.628-639.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Koszinowski U., Ertl H. Lysis mediated by T cells and restricted by H-2 antigen of target cells infected with vaccinia virus. Nature. 1975 Jun 12;255(5509):552–554. doi: 10.1038/255552a0. [DOI] [PubMed] [Google Scholar]
- Koszinowski U., Thomssen R. Target cell-dependent T cell-mediated lysis of vaccinia virus-infected cells. Eur J Immunol. 1975 Apr;5(4):245–251. doi: 10.1002/eji.1830050405. [DOI] [PubMed] [Google Scholar]
- Kvist S., Ostberg L., Peterson P. A. Reactions and crossreactions of a rabbit anti-H2 antigen serum. Scand J Immunol. 1978 Apr;7(4):265–276. doi: 10.1111/j.1365-3083.1978.tb00454.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lewis J. B., Atkins J. F., Baum P. R., Solem R., Gesteland R. F., Anderson C. W. Location and identification of the genes for adenovirus type 2 early polypeptides. Cell. 1976 Jan;7(1):141–151. doi: 10.1016/0092-8674(76)90264-6. [DOI] [PubMed] [Google Scholar]
- Ostberg L., Rask L., Nilsson K., Peterson P. A. Independent expression of the two HL-A antigen polypeptide chains. Eur J Immunol. 1976 Jul;5(7):462–468. doi: 10.1002/eji.1830050707. [DOI] [PubMed] [Google Scholar]
- Ostberg L., Sege K., Rask L., Peterson P. A. Isolation of radiolabelled H-2 antigens [proceedings]. Folia Biol (Praha) 1976;22(6):372–373. [PubMed] [Google Scholar]
- POPE J. H., ROWE W. P. IMMUNOFLUORESCENT STUDIES OF ADENOVIRUS 12 TUMORS AND OF CELLS TRANSFORMED OR INFECTED BY ADENOVIRUSES. J Exp Med. 1964 Oct 1;120:577–588. doi: 10.1084/jem.120.4.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson P. A., Rask L., Lindblom J. B. Highly purified papain-solubilized HL-A antigens contain beta2-microglobulin. Proc Natl Acad Sci U S A. 1974 Jan;71(1):35–39. doi: 10.1073/pnas.71.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson P. A., Rask L., Sege K., Klareskog L., Anundi H., Ostberg L. Evolutionary relationship between immunoglobulins and transplantation antigens. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1612–1616. doi: 10.1073/pnas.72.4.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipson L., Pettersson U., Lindberg U. Molecular biology of adenoviruses. Virol Monogr. 1975;14:1–115. doi: 10.1007/978-3-7091-8391-5_1. [DOI] [PubMed] [Google Scholar]
- SANDERSON A. R. APPLICATIONS OF ISO-IMMUNE CYTOLYSIS USING RADIOLABELLED TARGET CELLS. Nature. 1964 Oct 17;204:250–253. doi: 10.1038/204250a0. [DOI] [PubMed] [Google Scholar]
- Schrader J. W., Cunningham B. A., Edelman G. M. Functional interactions of viral and histocompatibility antigens at tumor cell surfaces. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5066–5070. doi: 10.1073/pnas.72.12.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader J. W., Edelman G. M. Participation of the H-2 antigens of tumor cells in their lysis by syngeneic T cells. J Exp Med. 1976 Mar 1;143(3):601–614. doi: 10.1084/jem.143.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearer G. M., Rehn T. G., Schmitt-Verhulst A. M. Role of the murine major histocompatibility complex in the specificity of in vitro T-cell-mediated lympholysis against chemically-modified autologous lymphocytes. Transplant Rev. 1976;29:222–246. doi: 10.1111/j.1600-065x.1976.tb00203.x. [DOI] [PubMed] [Google Scholar]
- Sjögren H. O., Minowada J., Ankerst J. Specific transplantation antigens of mouse sarcomas induced by adenovirus type 12. J Exp Med. 1967 Apr 1;125(4):689–701. doi: 10.1084/jem.125.4.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terhorst C., Robb R., Jones C., Strominger J. L. Further structural studies of the heavy chain of HLA antigens and its similarity to immunoglobulins. Proc Natl Acad Sci U S A. 1977 Sep;74(9):4002–4006. doi: 10.1073/pnas.74.9.4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitetta E. S., Baur S., Uhr J. W. Cell surface immunoglobulin. II. Isolation and characterization of immunoglobulin from mouse splenic lymphocytes. J Exp Med. 1971 Jul 1;134(1):242–264. doi: 10.1084/jem.134.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIGZELL H. QUANTITATIVE TITRATIONS OF MOUSE H-2 ANTIBODIES USING CR-51-LABELLED TARGET CELLS. Transplantation. 1965 May;3:423–431. doi: 10.1097/00007890-196505000-00011. [DOI] [PubMed] [Google Scholar]
- Walter G., Maizel J. V., Jr The polypeptides of adenovirus. IV. Detection of early and late virus-induced polypeptides and their distribution in subcellular fractions. Virology. 1974 Feb;57(2):402–408. doi: 10.1016/0042-6822(74)90180-9. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Callahan G. N., Althage A., Cooper S., Klein P. A., Klein J. On the thymus in the differentiation of "H-2 self-recognition" by T cells: evidence for dual recognition? J Exp Med. 1978 Mar 1;147(3):882–896. doi: 10.1084/jem.147.3.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. The concept that surveillance of self is mediated via the same set of genes that determines recognition of allogenic cells. Cold Spring Harb Symp Quant Biol. 1977;41(Pt 2):505–510. doi: 10.1101/sqb.1977.041.01.058. [DOI] [PubMed] [Google Scholar]


