Abstract
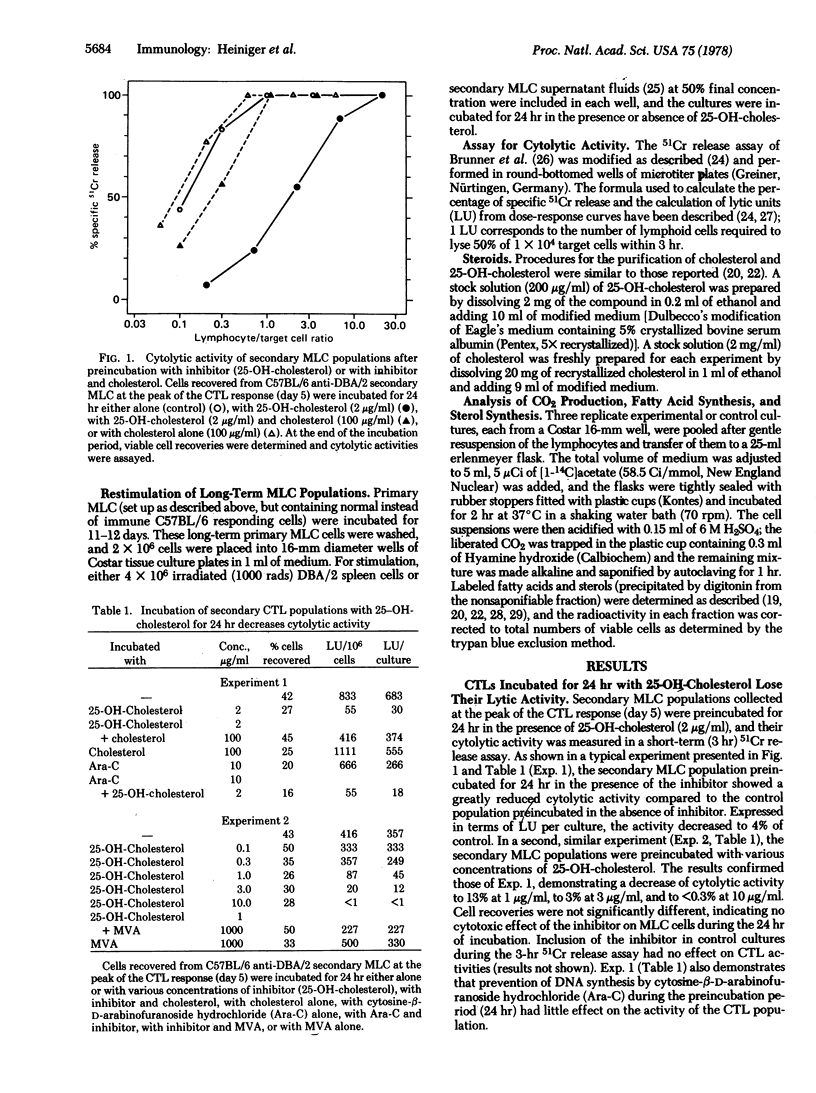
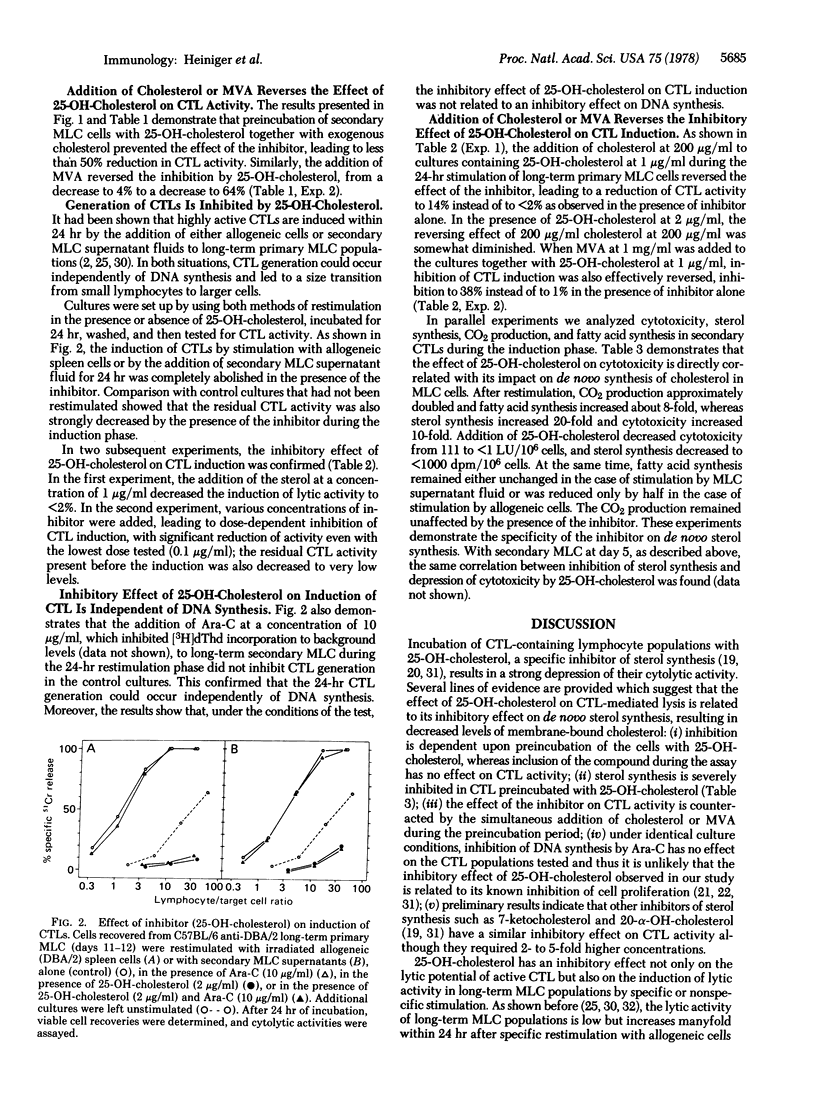
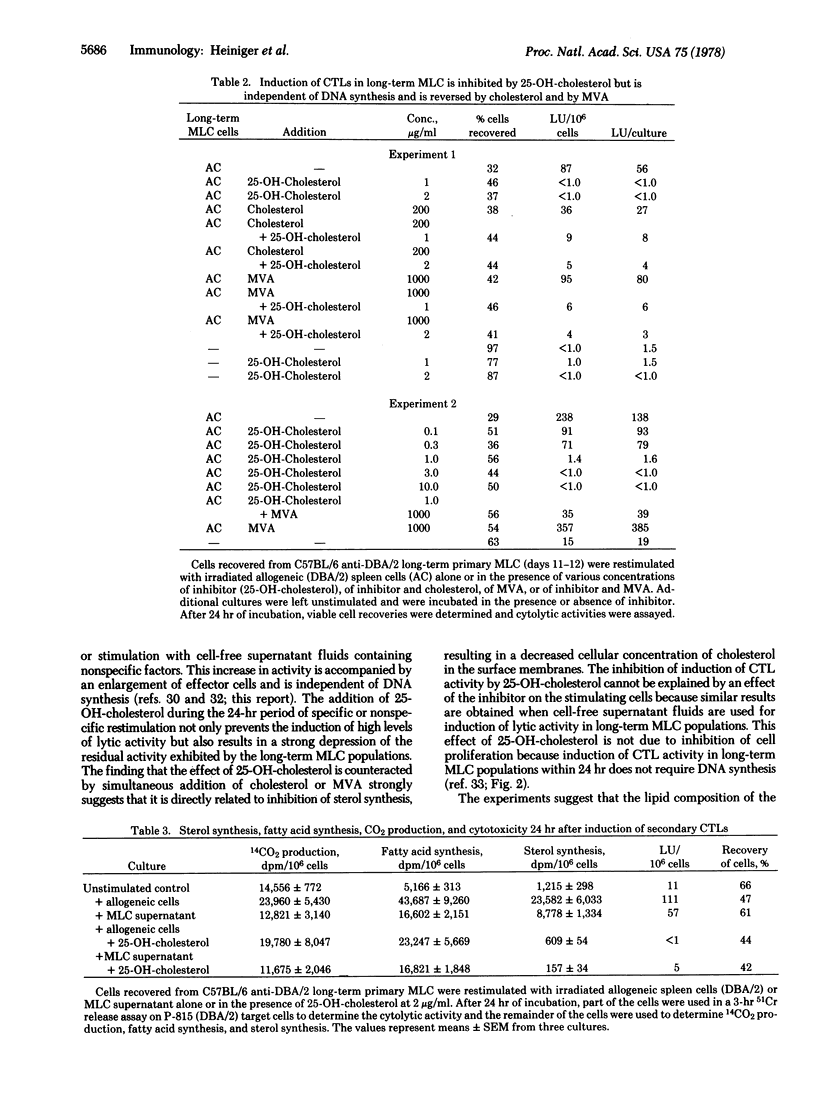
Preincubation of cytolytic T lymphocytes (CTLs) generated in secondary C57BL/6 anti-DBA/2 mixed leukocyte cultures with an inhibitor of cellular cholesterol synthesis (25-OH-cholesterol) for 24 hr strongly depressed the cytolytic activity as determined in a 3-hr 51Cr assay. The effect of the inhibitor was reversed by the simultaneous addition of cholesterol or of mevalonic acid during the preincubation period (mevalonate is the product of the regulatory enzyme in the sterol synthesis pathway, 3-hydroxy-3-methylglutaryl-CoA reductase (NADP) [mevalonate:NADP+ oxidoreductase (CoA-acylating), EC 1.1.1.34]). Because, under the same culture conditions, inhibition of DNA synthesis had no effect on CTL activity, the experiments suggest that the effect of 25-OH-cholesterol is related to its inhibitory effect on sterol synthesis, resulting in decreased levels of membrane-bound cholesterol, rather than to inhibition of cellular proliferation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berke G., Tzur R., Inbar M. Changes in fluorescence polarization of a membrane probe during lymphocyte-target cell interaction. J Immunol. 1978 Apr;120(4):1378–1384. [PubMed] [Google Scholar]
- Brunner K. T., Mauel J., Cerottini J. C., Chapuis B. Quantitative assay of the lytic action of immune lymphoid cells on 51-Cr-labelled allogeneic target cells in vitro; inhibition by isoantibody and by drugs. Immunology. 1968 Feb;14(2):181–196. [PMC free article] [PubMed] [Google Scholar]
- Cerottini J. C., Brunner K. T. Cell-mediated cytotoxicity, allograft rejection, and tumor immunity. Adv Immunol. 1974;18:67–132. doi: 10.1016/s0065-2776(08)60308-9. [DOI] [PubMed] [Google Scholar]
- Cerottini J. C., Engers H. D., Macdonald H. R., Brunner T. Generation of cytotoxic T lymphocytes in vitro. I. Response of normal and immune mouse spleen cells in mixed leukocyte cultures. J Exp Med. 1974 Sep 1;140(3):703–717. doi: 10.1084/jem.140.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman D. Phase transitions and fluidity characteristics of lipids and cell membranes. Q Rev Biophys. 1975 May;8(2):185–235. doi: 10.1017/s0033583500001797. [DOI] [PubMed] [Google Scholar]
- Chen H. W., Heiniger H. J., Kandutsch A. A. Alteration of 86Rb+ influx and efflux following depletion of membrane sterol in L-cells. J Biol Chem. 1978 May 10;253(9):3180–3185. [PubMed] [Google Scholar]
- Chen H. W., Heiniger H. J., Kandutsch A. A. Relationship between sterol synthesis and DNA synthesis in phytohemagglutinin-stimulated mouse lymphocytes. Proc Natl Acad Sci U S A. 1975 May;72(5):1950–1954. doi: 10.1073/pnas.72.5.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H. W., Heiniger H. J. Stimulation of sterol synthesis in peripheral leukocytes of leukemic mice. Cancer Res. 1974 Jun;34(6):1304–1307. [PubMed] [Google Scholar]
- Chen H. W., Kandutsch A. A., Waymouth C. Inhibition of cell growth by oxygenated derivatives of cholesterol. Nature. 1974 Oct 4;251(5474):419–421. doi: 10.1038/251419a0. [DOI] [PubMed] [Google Scholar]
- Demel R. A., De Kruyff B. The function of sterols in membranes. Biochim Biophys Acta. 1976 Oct 26;457(2):109–132. doi: 10.1016/0304-4157(76)90008-3. [DOI] [PubMed] [Google Scholar]
- Edelman G. M. Surface modulation in cell recognition and cell growth. Science. 1976 Apr 16;192(4236):218–226. doi: 10.1126/science.769162. [DOI] [PubMed] [Google Scholar]
- Edelman G. M., Yahara I., Wang J. L. Receptor mobility and receptor-cytoplasmic interactions in lymphocytes. Proc Natl Acad Sci U S A. 1973 May;70(5):1442–1446. doi: 10.1073/pnas.70.5.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiniger H. J., Kandutsch A. A., Chen H. W. Depletion of L-cell sterol depresses endocytosis. Nature. 1976 Oct 7;263(5577):515–517. doi: 10.1038/263515a0. [DOI] [PubMed] [Google Scholar]
- Henney C. S. T-Cell-mediated cytolysis: an overview of some current issues. Contemp Top Immunobiol. 1977;7:245–272. doi: 10.1007/978-1-4684-3054-7_7. [DOI] [PubMed] [Google Scholar]
- Kandutsch A. A., Chen H. W. Consequences of blocked sterol synthesis in cultured cells. DNA synthesis and membrane composition. J Biol Chem. 1977 Jan 25;252(2):409–415. [PubMed] [Google Scholar]
- Kandutsch A. A., Chen H. W., Heiniger H. J. Biological activity of some oxygenated sterols. Science. 1978 Aug 11;201(4355):498–501. doi: 10.1126/science.663671. [DOI] [PubMed] [Google Scholar]
- Kandutsch A. A., Chen H. W. Regulation of sterol synthesis in cultured cells by oxygenated derivatives of cholesterol. J Cell Physiol. 1975 Apr;85(2 Pt 2 Suppl 1):415–424. doi: 10.1002/jcp.1040850408. [DOI] [PubMed] [Google Scholar]
- Ladbrooke B. D., Williams R. M., Chapman D. Studies on lecithin-cholesterol-water interactions by differential scanning calorimetry and X-ray diffraction. Biochim Biophys Acta. 1968 Apr 29;150(3):333–340. doi: 10.1016/0005-2736(68)90132-6. [DOI] [PubMed] [Google Scholar]
- MacDonald H. R., Sordat B., Cerottini J. C., Brunner K. T. Generation of cytotoxic T lymphocytes in vitro. IV. Functional activation of memory cells in the absence of DNA synthesis. J Exp Med. 1975 Sep 1;142(3):622–636. doi: 10.1084/jem.142.3.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald H. R., Engers H. D., Cerottini J. C., Brunner K. T. Generation of cytotoxic T lymphocytes in vitro. II. Effect of repeated exposure to alloantigens on the cytotoxic activity of long-term mixed leukocyte cultures. J Exp Med. 1974 Sep 1;140(3):718–730. doi: 10.1084/jem.140.3.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson G. L. Transmembrane control of the receptors on normal and tumor cells. I. Cytoplasmic influence over surface components. Biochim Biophys Acta. 1976 Apr 13;457(1):57–108. doi: 10.1016/0304-4157(76)90014-9. [DOI] [PubMed] [Google Scholar]
- Ryser J. E., Cerottini J. C., Brunner K. T. Generation of cytolytic T lymphocytes in vitro. IX. induction of secondary CTL responses in primary long-term MLC by supernatants from secondary MLC. J Immunol. 1978 Feb;120(2):370–377. [PubMed] [Google Scholar]
- Schlager S. I., Ohanian S. H., Borsos T. Identification of lipids synthesized and released by tumor cells under attack by antibody and complement. J Immunol. 1978 May;120(5):1644–1650. [PubMed] [Google Scholar]
- Schlager S. I., Ohanian S. H., Borsos T. Stimulation of the synthesis and release of lipids in tumor cells under attack by antibody and C. J Immunol. 1978 Mar;120(3):895–901. [PubMed] [Google Scholar]
- Snell G. D. T cells, T cells recognition structures, and the major histocompatibility complex. Immunol Rev. 1978;38:3–69. doi: 10.1111/j.1600-065x.1978.tb00384.x. [DOI] [PubMed] [Google Scholar]


