Abstract
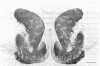
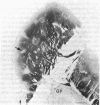
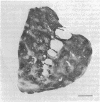
We here report observations on the distribution of acetylcholinesterase (acetylcholine hydrolase, EC 3.1.1.7) in the striatum of the adult human, the rhesus monkey, and the cat. By the histochemical staining methods of Geneser-Jensen and Blackstad and of Karnovsky and Roots, compartments of low cholinesterase activity were identified in parts of the striatum in all three species. In frontal sections, these enzyme-poor zones appeared as a variable number of weakly stained approximately 0.5-mm-wide zones embedded in a darkly stained background. The zones varied in cross-sectional shape from round to elongated and were sometimes branched. They were most prominent in the head of the caudate nucleus. Three-dimensional reconstructions of serial sections through the caudate nucleus in the human and cat suggest that over distances of at least several millimeters, the zones of low enzyme activity form nearly continuous labyrinths.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Butcher L. L., Hodge G. K. Postnatal development of acetylcholinesterase in the caudate-putamen nucleus and substantia nigra of rats. Brain Res. 1976 Apr 23;106(2):223–240. doi: 10.1016/0006-8993(76)91022-2. [DOI] [PubMed] [Google Scholar]
- Geneser-Jensen F. A., Blackstad T. W. Distribution of acetyl cholinesterase in the hippocampal region of the guinea pig. I. Entorhinal area, parasubiculum, and presubiculum. Z Zellforsch Mikrosk Anat. 1971;114(4):460–481. doi: 10.1007/BF00325634. [DOI] [PubMed] [Google Scholar]
- Goldman P. S., Nauta W. J. An intricately patterned prefronto-caudate projection in the rhesus monkey. J Comp Neurol. 1977 Feb 1;72(3):369–386. doi: 10.1002/cne.901710305. [DOI] [PubMed] [Google Scholar]
- HEBB C. O., SILVER A. Choline acetylase in the central nervous system of man and some other mammals. J Physiol. 1956 Dec 28;134(3):718–728. doi: 10.1113/jphysiol.1956.sp005678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobowitz D. M., Palkovits M. Topographic atlas of catecholamine and acetylcholinesterase-containing neurons in the rat brain. I. Forebrain (telencephalon, diencephalon). J Comp Neurol. 1974 Sep 1;157(1):13–28. doi: 10.1002/cne.901570103. [DOI] [PubMed] [Google Scholar]
- KARNOVSKY M. J., ROOTS L. A "DIRECT-COLORING" THIOCHOLINE METHOD FOR CHOLINESTERASES. J Histochem Cytochem. 1964 Mar;12:219–221. doi: 10.1177/12.3.219. [DOI] [PubMed] [Google Scholar]
- Kalil K. Patch-like termination of thalamic fibers in the putamen of the rhesus monkey: an autoradiographic study. Brain Res. 1978 Jan 27;140(2):333–339. doi: 10.1016/0006-8993(78)90464-x. [DOI] [PubMed] [Google Scholar]
- Künzle H. Bilateral projections from precentral motor cortex to the putamen and other parts of the basal ganglia. An autoradiographic study in Macaca fascicularis. Brain Res. 1975 May 2;88(2):195–209. doi: 10.1016/0006-8993(75)90384-4. [DOI] [PubMed] [Google Scholar]
- Mensah P. L. The internal organization of the mouse caudate nucleus: evidence for cell clustering and regional variation. Brain Res. 1977 Nov 25;137(1):53–66. doi: 10.1016/0006-8993(77)91012-5. [DOI] [PubMed] [Google Scholar]
- Olson L., Seiger A., Fuxe K. Heterogeneity of striatal and limbic dopamine innervation: highly fluorescent islands in developing and adult rats. Brain Res. 1972 Sep 15;44(1):283–288. doi: 10.1016/0006-8993(72)90385-x. [DOI] [PubMed] [Google Scholar]
- Pert C. B., Kuhar M. J., Snyder S. H. Opiate receptor: autoradiographic localization in rat brain. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3729–3733. doi: 10.1073/pnas.73.10.3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royce G. J. Autoradiographic evidence for a discontinuous projection to the caudate nucleus from the centromedian nucleus in the cat. Brain Res. 1978 May 5;146(1):145–150. doi: 10.1016/0006-8993(78)90224-x. [DOI] [PubMed] [Google Scholar]
- Tennyson V. M., Barrett R. E., Cohen G., Côté L., Heikkila R., Mytilineou C. The developing neostriatum of the rabbit: correlation of fluorescence histochemistry, electron microscopy, endogenous dopamine levels, and ( 3 H)dopamine uptake. Brain Res. 1972 Nov 13;46:251–285. doi: 10.1016/0006-8993(72)90019-4. [DOI] [PubMed] [Google Scholar]
- Yamamura H. I., Snyder S. H. Muscarinic cholinergic binding in rat brain. Proc Natl Acad Sci U S A. 1974 May;71(5):1725–1729. doi: 10.1073/pnas.71.5.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]