Abstract
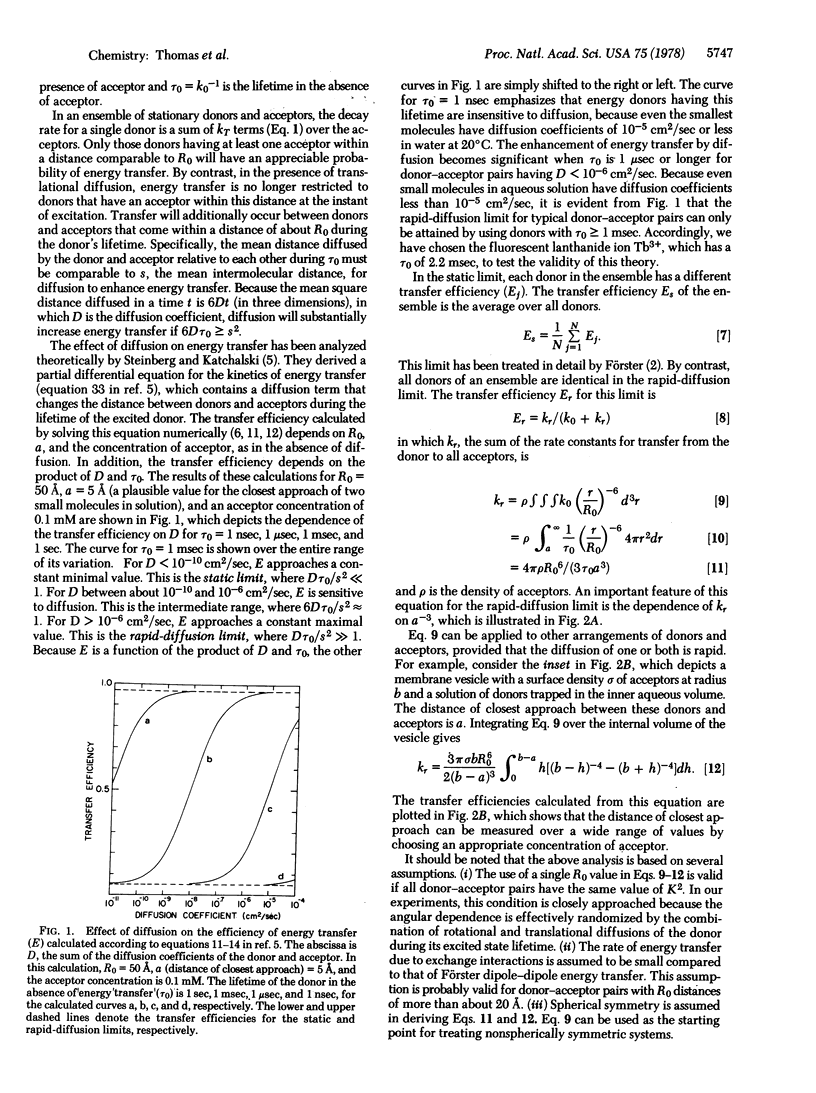
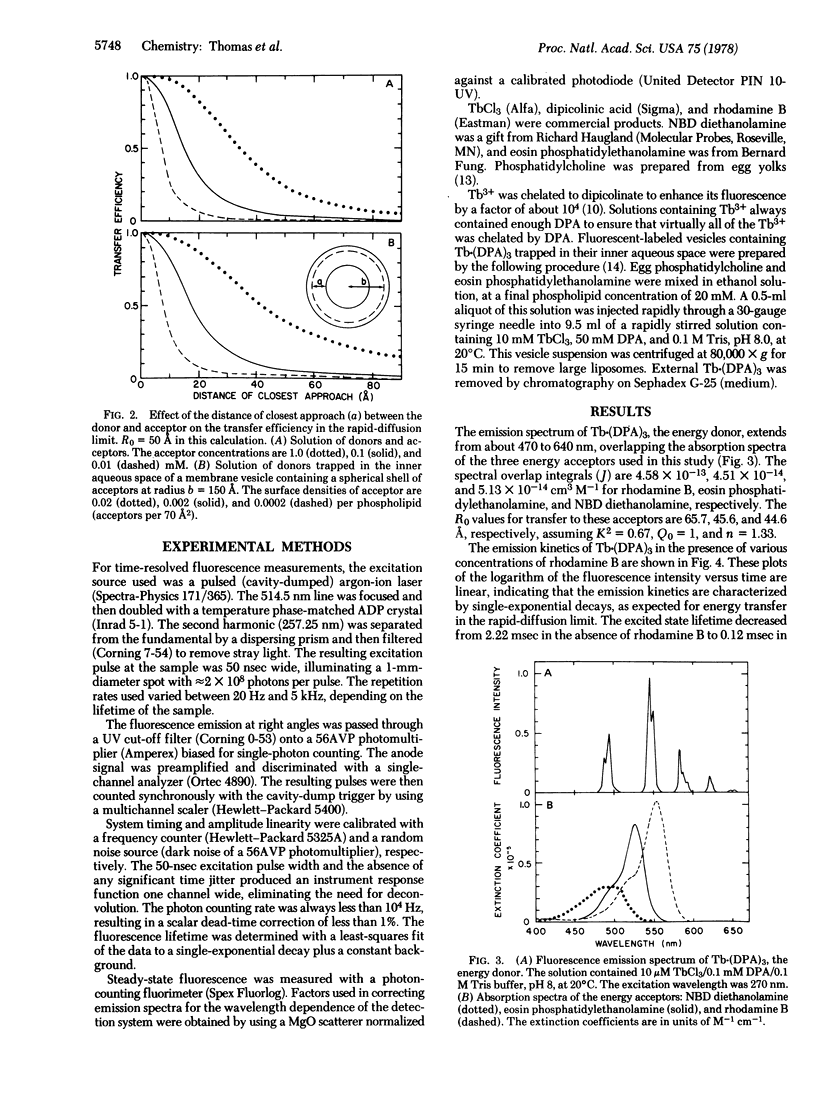
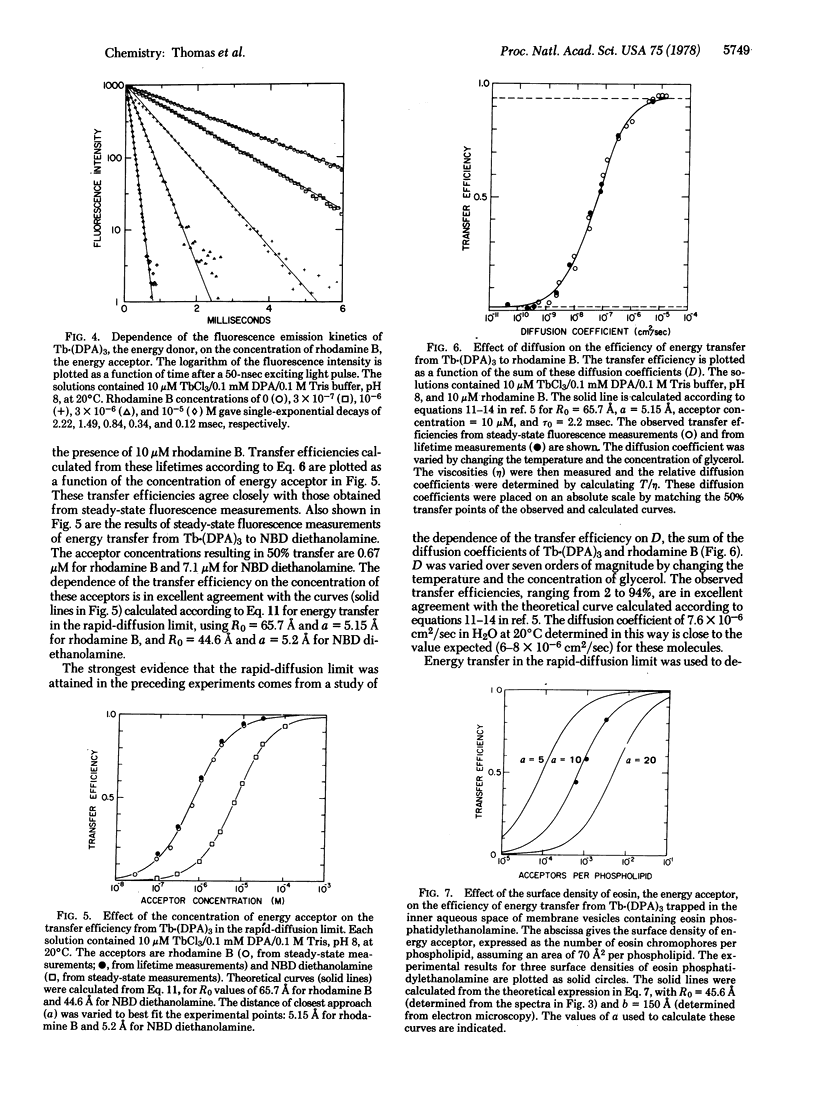
Energy transfer is enhanced by translational diffusion of the donor and acceptor [Steinberg, I. Z. & Katchalski, E. (1968) J. Chem. Phys. 48, 2404-2410]. The effect of diffusion on energy transfer depends on Dτ0/s2, in which D is the sum of the diffusion coefficients of the donor and acceptor, τ0 is the lifetime of the donor in the absence of transfer, and s is the mean distance between donors and acceptors. In most previous studies, Dτ0/s2 ≪ 1, corresponding to the static limit. We report here steady-state and kinetic fluorescence experiments showing that Dτ0/s2 ≫ 1, the rapid-diffusion limit, can be attained by using Tb3+ chelated to dipicolinate as a long-lived energy donor (τ0 = 2.2 msec). The concentration of rhodamine B, the energy acceptor, resulting in 50% transfer was 0.67 μM, which is three orders of magnitude less than the concentration giving 50% transfer in the static limit. The dependence of the transfer efficiency on diffusion coefficients varying from 5 × 10-11 to 1.5 × 10-4 cm2/sec, spanning the range from the static limit to the rapid-diffusion limit, is in excellent agreement with theory. It is evident that energy donors with millisecond or longer excited state lifetimes can be used to probe translational motions in membranes and other assemblies. Energy transfer in the rapid diffusion limit is sensitive to the distance of closest approach (a) of the donor and acceptor. For a Tb·(DPA)3 chelate trapped inside the aqueous space of a membrane vesicle containing eosin phosphatidylethanolamine, a = 10 Å. The transverse location of chromophores in model membranes and biological membranes can be determined by this technique.
Keywords: Förster transfer, spectroscopic rulers, terbium, membrane vesicles
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barela T. D., Sherry A. D. A simple, one-step fluorometric method for determination of nanomolar concentrations of terbium. Anal Biochem. 1976 Apr;71(2):351–357. doi: 10.1016/s0003-2697(76)80004-8. [DOI] [PubMed] [Google Scholar]
- Batzri S., Korn E. D. Single bilayer liposomes prepared without sonication. Biochim Biophys Acta. 1973 Apr 16;298(4):1015–1019. doi: 10.1016/0005-2736(73)90408-2. [DOI] [PubMed] [Google Scholar]
- Horrocks W. D., Jr, Schmidt G. F., Sudnick D. R., Kittrell C., Bernheim R. A. Laser-induced lanthanide ion laminescence lifetime measurements by direct excitation of metal ion levels. A new class of structural probe for calcium-binding proteins and nucleic acids. J Am Chem Soc. 1977 Mar 30;99(7):2378–2380. doi: 10.1021/ja00449a079. [DOI] [PubMed] [Google Scholar]
- Ka Luk C. Study of the nature of the metal-binding sites and estimate of the distance between the metal-binding sites in transferrin using trivalent lanthanide ions as fluorescent probes. Biochemistry. 1971 Jul 20;10(15):2838–2843. doi: 10.1021/bi00791a006. [DOI] [PubMed] [Google Scholar]
- SINGLETON W. S., GRAY M. S., BROWN M. L., WHITE J. L. CHROMATOGRAPHICALLY HOMOGENEOUS LECITHIN FROM EGG PHOSPHOLIPIDS. J Am Oil Chem Soc. 1965 Jan;42:53–56. doi: 10.1007/BF02558256. [DOI] [PubMed] [Google Scholar]
- Saviotti M. L., Galley W. C. Room temperature phosphorescence and the dynamic aspects of protein structure. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4154–4158. doi: 10.1073/pnas.71.10.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stryer L. Fluorescence energy transfer as a spectroscopic ruler. Annu Rev Biochem. 1978;47:819–846. doi: 10.1146/annurev.bi.47.070178.004131. [DOI] [PubMed] [Google Scholar]
- Stryer L., Haugland R. P. Energy transfer: a spectroscopic ruler. Proc Natl Acad Sci U S A. 1967 Aug;58(2):719–726. doi: 10.1073/pnas.58.2.719. [DOI] [PMC free article] [PubMed] [Google Scholar]


