Abstract
Progranulin (PGRN) is a highly unusual molecule with both neuronal and microglial expression with two seemingly unrelated functions, i.e., as a neuronal growth factor and a modulator of neuroinflammation. Haploinsufficiency due to loss of function mutations lead to a fatal presenile dementing illness (frontotemporal lobar degeneration), indicating that adequate expression of PGRN is essential for successful aging. PGRN might be a particularly relevant factor in the pathogenesis of HIV encephalitis (HIVE) and HIV-associated neurocognitive disorders (HAND). We present emerging data and a review of the literature which show that cells of myeloid lineage such as macrophages and microglia are the primary sources of PGRN and that PGRN expression contributes to pathogenesis of CNS diseases. We also present evidence that PGRN is a macrophage antiviral cytokine. For example, PGRN mRNA and protein expression are significantly upregulated in brain specimens with HIVE, and in HIV-infected microglia in vitro. Paradoxically, our preliminary CHARTER data analyses indicate that lower PGRN levels in CSF trended towards an association with HAND, particularly in those without detectable virus. Based upon these findings, we introduce the hypothesis that PGRN plays dual roles in modulating antiviral immunity and neuronal dysfunction in the context of HIV infection. In the presence of active viral replication, PGRN expression is increased functioning as an anti-viral factor as well as a neuroprotectant. In the absence of active HIV replication, ongoing inflammation or other stressors suppress PGRN production from macrophages/microglia contributing to neurocognitive dysfunction. We propose CSF PGRN as a candidate surrogate marker for HAND.
Keywords: microglia, cytokines, human, growth factor, innate immunity, HIV encephalitis (HIVE), HIV-associated neurocognitive disorder (HAND)
INTRODUCTION
Progranulin (PGRN) is a multifunctional growth factor widely expressed in various tissues with highest levels in epithelial and myeloid cells (Bateman and Bennett, 2009). PGRN is involved in cell proliferation, wound healing and modulation of inflammation (Ahmed et al., 2007). Haploinsufficiency resulting from PGRN gene (GRN) mutations causes frontotemporal lobar dementia (FTLD), a fatal progressive neurodegenerative disorder affecting young (<60 yrs) individuals (Baker et al., 2006; Cruts et al., 2006). This suggests that adequate expression of PGRN may play a critical role in successful aging of the central nervous system (CNS). However, the mechanisms by which reduced levels of PGRN lead to dementia are poorly understood. In the CNS, neurons express PGRN constitutively, while increased microglial PGRN immunoreactivity is observed in several neurodegenerative or neuroinflammatory diseases such as Alzheimer disease (AD), FTLD and multiple sclerosis (MS) (Sleegers et al., 2009). There is also emerging evidence that PGRN is important in antimicrobial innate immunity. However, whether PGRN expression is modulated in HIV-infected individuals and whether PGRN plays a role in the antiviral immunity or in neurocognitive impairment (NCI) termed HIV-associated neurocognitive disorders (HAND) are unknown.
Despite effective and widely available combination antiretroviral therapy (cART), HIV-associated NCI still has a prevalence of about 50% of HIV infected individuals (McArthur et al., 2010; Kamat et al., 2012). HAND ranges in severity from very mild (asymptomatic neurocognitive impairment) to disabling (HIV-associated dementia, or HAD). HAD is strongly associated with active HIV replication in the CNS and a neuropathologic response known as HIV encephalitis (HIVE). In the cART era the prevalence of HAD/HIVE has decreased, and the prevalence of the milder forms has increased. Moreover, cognitive deterioration can occur despite the viral suppression and immune recovery induced by cART. These changes could result from inadequate penetration of cART and residual infection in the brain, chronic CNS inflammatory and oxidative stress responses, or neurotoxic effects of long-term cART treatment (Heaton et al., 2010; Perry et al., 2005; Kaul, 2009). The pathogenesis of HAND is complex and not clearly understood. While much has been investigated on the role of neurotoxic viral and host factors released from HIV-infected macrophages, more understanding of key molecules and cellular pathways that modulate HIV-macrophage (including microglia) interactions is needed.
Interestingly, a recent gene array study by the National NeuroAIDS Tissue Consortium (NNTC) suggests that the pathogenesis of HIV-associated NCI is heterogeneous (Gelman et al., 2012a). Specifically, the study indicates that the pathophysiology of HIV-associated NCI may differ depending on the presence or absence of HIVE. The consortium has proposed new designations, Type I and Type II NCI, to refer to NCI with and without HIVE, respectively. These results are important since they indicate that there are “molecular subtypes” of HAND that might respond differently to the same treatment. The recent NNTC clinico-neurovirological correlation study (Gelman et al., 2012a; Gelman et al., 2012b) has provided added evidence for two types of NCI in patients with HAND.
In this manuscript, we review the literature regarding the known expression of PGRN in normal and diseased CNS and systemic organs, as well as PGRN determinations in plasma and the cerebrospinal fluids (CSF) of individuals with several neurological diseases. The role of PGRN as a neuronal growth factor and an innate immune modulator are discussed based on evidence from patients as well as animal and cell models. We also present data which suggest that PGRN may play a role in the pathogenesis of HIVE and HAND, could potentially serve as a surrogate biomarker and also might prove to be an efficacious therapeutic target.
PART 1: PGRN EXPRESSION
PGRN expression in the periphery
Progranulin was initially isolated and characterized in inflammatory leukocytes collected from peritonitis patients and bone marrow (Bhandari et al., 1992). In rodents, Bateman and colleagues have shown by in situ hybridization that Grn mRNA is most abundant in the spleen and several tissues of endocrine origin with no expression in skeletal, cardiac muscles or liver (Daniel et al., 2000). Grn mRNA was detected in a restricted set of epithelial cells in mouse tissues. Skin, GI tract and glandular epithelium expressed high levels and bronchial epithelium expressed lower levels.. Grn in the spleen was expressed predominantly in lymphoid cells. When the expression of PGRN mRNA was measured in different human cell lines, myelogenous leukemic cell lines (HL-60, U937) and epithelial cell lines were found to express high levels (Ong et al., 2006). GRN is the 17th and 30th most abundant transcript in human macrophage and monocyte-derived dendritic cells, respectively (Chantry et al., 1998).
PGRN expression in CNS diseases
In the CNS, Grn was identified as a microglial gene uniquely modulated in mice with Creutzfeldt-Jakob disease (CJD) (Baker and Manuelidis, 2003). When the gene expression profile of CJD microglia was compared with that of microglia isolated from uninfected mouse brain tissue, Grn mRNA expression was higher with CJD. In vivo studies of developing and mature mice also reported Grn mRNA expression in brain microglial cells, and in neurons. In a most recent study, Grn gene (heterozygote knock-in) and protein expression (by immunohistochemistry) were examined and showed that neuronal PGRN expression increased with the cells’ maturity, while microglial cell expression varied with the state of immune activation (Petkau et al., 2010). Predominantly neuronal and microglial GRN expression also has been reported in autopsy studies (Ahmed et al., 2007). While neuronal PGRN immunoreactivity did not appear to be highly variable in diseased human brain tissue, its expression was increased in the activated microglial cells of several CNS diseases including AD, FTLD, amyotrophic lateral sclerosis (ALS) and MS (Sleegers et al., 2009; Vercellino et al., 2011).
Modulation of GRN expression at the gene level has also been well-documented. A detailed analysis of GRN mRNA in FTLD brains (with loss of function PGRN mutations and haploinsufficiency) showed an overall increase in several brain areas, indicating that PGRN transcription from the normal allele can be upregulated (Chen-Plotkin et al., 2010). These findings also suggest that PGRN expression is differentially regulated in neurons and microglia. Increased GRN mRNA expression also was found in the spinal cord of ALS patients (Malaspina et al., 2001). PGRN dysregulation also occurs in the blood of patients with CNS diseases (see below), but the cellular origins and mechanisms have not been fully elucidated.
Genetic mechanisms of PGRN regulation
Loss-of-function mutations in GRN cause FTLD, a progressive neurodegenerative disease affecting 10% of early-onset dementia patients. The majority of GRN mutations consist of nonsense mutations, small insertion/deletion mutations that result in a shift in the reading frame or splice-site mutations (Sleegers et al., 2009). In addition, several GRN single nucleotide polymorphisms (SNPs) have been proposed to modulate PGRN levels. Rademakers et al. demonstrated that a common genetic variant (rs5848) in the 3′-untranslated region (UTR) of GRN in a binding-site for miR-659 is a major susceptibility factor for ubiquitin-positive FTLD (FTLD-U) (Rademakers et al., 2008). In a subgroup of non-GRN mutation carriers (n = 339), they found that carriers for the TT-allele of rs5848 have a 3.2-fold increased risk for FTLD-U compared with CC-allele carriers; rs5848 also could influence serum PGRN level in AD, FTLD, and other dementias (see below). A significant association between disease onset and another GRN SNP (rs9897526) located 21 bp downstream of the intron 2 splice donor site was observed in ALS in Belgian population (n=230) (Sleegers et al., 2008) Additionally, two recent studies identified polymorphisms in binding sites for miR-29b and miR-107 in the 3′-UTR as potential regulators of GRN expression (Wang et al., 2010). GRN mutation carriers show a high variability in disease onset and pathologic presentation, even with identical mutations, suggesting that environmental influences or additional genetic factors modify the disease manifestation. Genome wide approaches have identified rs646776 near the sortilin gene as a potential determinant of PGRN levels in human plasma. Each copy of the minor C allele was associated with a lower plasma PGRN level (Carrasquillo et al., 2010). Another gene implicated in PGRN regulation is TMEM106B. TMEM106B is regulated by the microRNA-132/212 cluster and affects the PGRN pathway. The TMEM106B SNP rs1990622 may also influence risk for FTLD by modulating the rate that PGRN is secreted (Van, V et al., 2010; Cruchaga et al., 2011; Chen-Plotkin et al., 2012).
Given the high prevalence of the T allele of rs5848 and its potentially negative impact on PGRN protein expression, the influence of GRN gene variability on susceptibility to AD and PGRN expression were evaluated. This study involved Italian (n=377) and American (n=355) patients, with the AD diagnosis confirmed by autopsy in 68 Americans (Fenoglio et al., 2009). This study found that GRN mRNA expression in the parietal lobe of AD cases was 0.76-fold lower compared to controls, and the decedents with the rs5848 TT haplotype had the lowest GRN expression levels. Although no significant differences in GRN expression were found in blood mononuclear cells (PBMC) and cerebrospinal fluid (CSF) cells, stratifying subjects according to rs5848 T and C allelic frequencies showed a 0.57-fold decrease in PBMC GRN mRNA levels in T versus C haplotypes. These data argue against a direct role of GRN as a susceptibility factor for sporadic AD, but they support a possible role as a disease-modifying gene that can influence neuronal survival. As well, the T allele of the rs5848 SNP of GRN is a possible underlying predisposing condition for neurocognitive decline in non-FTLD dementias.
PGRN as a biomarker/surrogate marker (Table 1: serum or plasma levels)
Table 1.
Summary of literature of the plasma or serum determination of PGRN levels in CNS diseases
| conditions | number of cases | PGRN concentration (ng/ml) | statistics | method | source | reference | |
|---|---|---|---|---|---|---|---|
| 1 | control | 40 | Median (range) 109 (79–185) | ELISA (adipogene) | serum | (Steinacker et al., 2011) | |
| ALS | 68 | 105(52–269) | ns | ||||
| PD | 20 | 110(65–196) | ns | ||||
| 2 | control | 56 | Mean ±SEM 231±24 | ELISA (R&D) | plasma | (Philips et al., 2010) | |
| ALS | 91 | 261±25 | ns | ||||
| 3 | Healthy control | 75 | Mean ±SD 172.2±62.7 | ELISA (adipogene) | plasma | (Ghidoni et al., 2008) | |
| Unaffected, no PGRN mutation (relatives of FTLD) | 51 | 167.1±51.8 | ns | ||||
| Unaffected, mutation | 22 | 42.5±11.7 | * | ||||
| FTLD, no mutation | 63 | 197.9±67.6 | ns | ||||
| FTLD, mutation | 8 | 61.6±25.9 | ** | ||||
| 5 | Healthy non-carrier (relatives of FTLD) | 9 | Median (range) 135.3(110.9–144.4) | ELISA (R&D) | serum | (Sleegers et al., 2009) | |
| Community controls | 22 | 226.5 (101–387) | |||||
| FTLD, Mutation carrier | 14 | 62.4 (48.7–81.0) | *** | ||||
| 6 | Control (Italian) | 29 | Mean ±SEM 180.81±18.39 | ELISA (adipogene) | plasma | (Galimberti et al., 2012) | |
| Bipolar disorder (Italian) | 61 | 116.14±5.80 | *** | ||||
| Bipolar disorder(German) | 26 | 89.69±3.97 | *** | ||||
| 7 | control | 40 | median (* IQR) 87.00 (81) | ELISA (R&D) | plasma | (Al-Ayadhi and Mostafa, 2011) | |
| autism | 40 | 77.50 (19) | *** | ||||
| 8 | control | 70 | Mean ±SD 228±50 | ELISA (adipogene) | plasma | (Finch et al., 2009) | |
| FTLD without mutation | 191 | 220±47 | ns | ||||
| GRN mutation carrier | 8 | 68±16 | *** | ||||
| AD with mutation | 1 | 74 | |||||
| AD without mutation | 71 | 238±53 | ns | ||||
| 9 | Minor cognitive impairment | 56 | Mean ±SD 228.51±10.63 | ELISA (adipogene) | plasma | (Carecchio et al., 2009) | |
| AD | 75 | 221.25±10.05 | ns | ||||
| FTLD | 45 | 216.00±12.26 | ns | ||||
| 10 | control | 36 | Mean, range 195 (180–211) | ELISA (adipogene) | serum | (Hsiung et al., 2011) | |
| Amnestic mild cognitive impairment (MCI) | 13 | 228 (196–261) | ns | ||||
| AD | 57 | 202 (188–217) | ns | ||||
| FTD-ALS | 12 | 187 (167–204) | ns | ||||
| Other dementia | 18 | 223 (197–250) | ns | ||||
| GRN mutation carriers | 6 | 50.5 (43.8–57.2) | *** | ||||
| 11 | Control (similar rs5848 genotype frequency) | 114 | Mean ±SEM 125.6±36.1 | ELISA (adipogene) | plasma | (Kamalainen et al., 2013) | |
| AD | 258 | 138.1±36.1 | ** |
IQR = interquartile range (Q3 – Q1) (* p <0.05, ** p <0.01, *** p <0.001, ns = not significant)
PGRN levels have been determined in a diverse category of human diseases. PGRN expression by epithelial cells and its mitogenic effect have suggested a potential role in cancer. These studies reported increased plasma PGRN levels in various cancer patients including those with glioblastoma multiforme (Tkaczuk et al., 2011; Koo et al., 2012; Wang et al., 2012). Moreover, PGRN is highly expressed in adipose tissues including in macrophages, and PGRN dysregulation has been proposed as a mechanism in metabolic disorders such as type II diabetes (Youn et al., 2009; Tonjes et al., 2010). Increased PGRN levels in the plasma of patients with diabetes have been correlated with visceral fat accumulation and dyslipidemia. Elevated serum PGRN levels have also been reported in autoimmune disorders such as systemic lupus erythematosus and certain infectious conditions (Tanaka et al., 2012). Serum PGRN levels are also markedly elevated in mothers during gestation and show strong positive correlation with estrogen and progesterone (Todoric et al., 2012).
Given that PGRN is a crucial neurotrophic factor and a modulator of neuroinflammation, plasma/serum PGRN levels have been examined in patients with several CNS diseases. CSF and plasma of FTLD subjects carrying PGRN mutations show severely reduced PGRN protein levels (up to 3.93-fold) regardless of clinical affliction (Ghidoni et al., 2008; Sleegers et al., 2009). As such, low plasma PGRN (74.4 ng/ml cutoff) can serve as a biological predictor of incident FTLD. Findings in other CNS diseases are more varied. Blood PGRN levels have been determined in AD, ALS, MS and other neurological diseases (see Table 1 for summary). In AD blood cell PGRN mRNA levels were reported to be increased (Coppola et al., 2008), but a subsequent study reported that plasma PGRN protein levels in AD were not abnormal (Carecchio et al., 2009). The latter study also found no significant change in plasma PGRN levels in (non-PGRN mutation) FTLD or in minor cognitive impairment. Similarly, plasma PGRN levels were not significantly different in ALS patients without GRN mutations (Philips et al., 2010). Conflicting results were reported in another study, in which plasma PGRN levels were reported to be significantly increased in AD and with increasing age (Kamalainen et al., 2013). Thus, larger studies are probably needed to fully elucidate the potential relationship to AD. There is also evidence that plasma PGRN levels are low in patients with certain psychiatric disorders. For example, plasma PGRN protein levels were reportedly decreased in children with autism (Al-Ayadhi and Mostafa, 2011). Plasma PGRN levels also were reported to be low in people with bipolar disorder (Galimberti et al., 2012). Again, while larger studies are necessary to confirm these findings, the available preliminary reports suggest that PGRN expression could play a role in the pathogenesis of abnormal brain function observed in certain neuropsychiatric conditions.
CSF PGRN levels in CNS diseases (Table 2)
Table 2.
Summary of literature of the CSF determination of PGRN levels in CNS diseases
| conditions | case number | PGRN concentration (ng/ml) | statistics | method | Ref | ||
|---|---|---|---|---|---|---|---|
| 1 | Control | 40 | Median (range) 4.3 (2.3–7.0) | ELISA (adipogene) | (Steinacker et al., 2011) | ||
| ALS | 68 | 3.9 (2.2–8.2) | ns | ||||
| PD | 20 | 4.1(2.6–4.5) | ns | ||||
| 2 | MS | total | 40 | Mean ±SD 21.32±4.2 | ELISA (adipogene) | (Vercellino et al., 2011) | |
| * RRMS during remission | 20 | 14.56±4.16 | |||||
| RRMS during relapse | 10 | 31.26±5.62 | ** | ||||
| * SPMS | 10 | 24.92±3.03 | ** | ||||
| Non-inflammatory control, with neurological diseases | 15 | 17.15±4.92 | ns | ||||
| Viral encephalitis; Inflammatory control | 5 | 76.38±85.08 | ** | ||||
| 3 | MS | 55 | Mean ±SEM 5.99±0.25 | ELISA (adipogene) | (De et al., 2010) | ||
| Non-inflammatory Neurologic diseases | 35 | 5.95±0.35 | ns | ||||
| Viral meningitis, optic neuritis, SLE (Other inflammatory neurologic diseases) | 7 | 8.20±1.69 | ns | ||||
| Control | 8 | 6.48±0.65 | ns | ||||
| 4 | control | 31 | Mean ±SEM 6.4±0.6 | ELISA (R&D ) | (Philips et al., 2010) | ||
| ALS | 29 | 7.0±0.5 | ns | ||||
| FTLD no PGRN mutation | 24 | 6.9±0.6 | ns | ||||
| FTLD, PGRN mutation | 3 | 2.2±1.0 | * | ||||
| FTLD+ALS | 4 | 5.5±0.7 | ns | ||||
| AD | 34 | 6.0±0.5 | ns | ||||
| 5 | Healthy control | 5 | Mean ±SD 8.2±2.7 | ELISA (adipogene) | (Ghidoni et al., 2008) | ||
| FTLD, no PGRN mutation | 17 | 8.7±2.1 | ns | ||||
| FTLD, PGRN mutation | 5 | 3.7±0.6 | * | ||||
RRMS = relapsing remitting MS, SPMS = secondary progressive MS (* p <0.05, ** p <0.01, ns = not significant)
Although PGRN levels in blood might be a practical disease biomarker due to its easy clinical accessibility, CSF levels of PGRN may be of greater biological relevance to CNS diseases. The normal CSF concentration of PGRN is low (~ 1/50 of the blood level), which could suggest that the CNS is much more vulnerable to PGRN deficiency. For example, the average plasma PGRN levels in HIV-infected subjects of the CHARTER cohort (Heaton et al., 2010) that we determined were 129.7 ± 8.3 ng/ml, versus the CSF concentration of 3.08 ± 0.19 (Mean ± SEM) (also see Tables 1 and 2 for other examples). In multiple sclerosis (MS) patients, CSF PGRN levels were found to be increased in the relapsing-remitting (RR) type during clinical relapses, and also in the secondary progressive (SP) type, indicating that disease activity is the primary determinant of PGRN elevation in MS (Vercellino et al., 2011). Interestingly, the comparison subjects with viral encephalitis had significantly higher CSF PGRN compared to MS or non-inflammatory control groups. Another study of PGRN in MS patients found similar trends which did not reach statistical significance (De et al., 2010). The same study found no evidence that CSF PGRN levels vary according to gender or age. Other studies found no significant change of CSF PGRN levels in FTLD, ALS and AD (Philips et al., 2010), while another study reported a tendency of CSF PGRN to be reduced with ongoing ALS and disease duration (Steinacker et al., 2011).
Cells of monocyte-lineage are the strongest expressers of PGRN
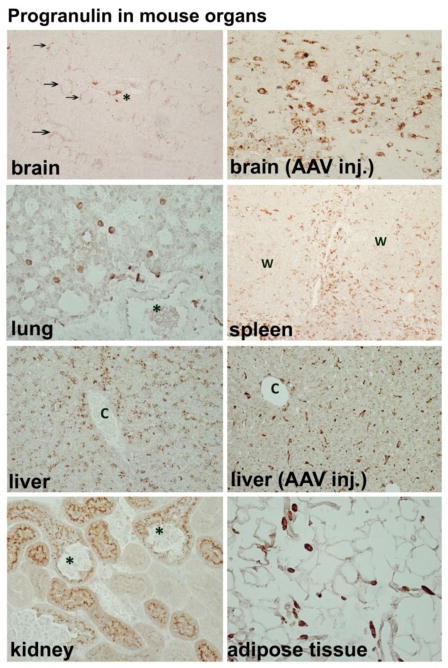
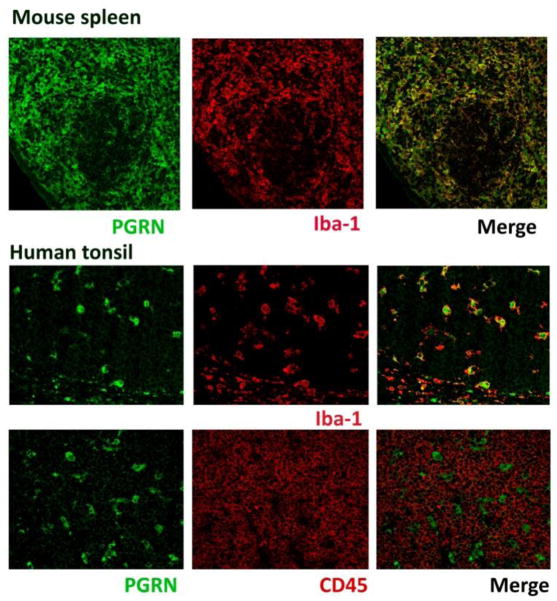
Since the cellular origins and the molecular mechanisms underlying PGRN dysregulation in these patient populations are unknown, we performed an immunohistochemical study to determine the origin of PGRN based on protein expression in select mouse and human organs in vivo. Our results show that PGRN expression in vivo is limited to certain epithelial and myeloid cells, and that tissue macrophages and dendritic cells exhibit the most PGRN expression in vivo. Serial single labeling or double labeling with macrophage and lymphoid cell markers demonstrated that lymphocytes are not a major PGRN-expressing cell type (Figs. 1 and 2). PGRN expression was low in lung and heart tissue, and staining was limited to rare isolated macrophages. In the kidney select proximal tubular epithelial cells were PGRN+ (Fig. 1 and other data not shown). Macrophages associated with visceral adipose tissue were strongly positive throughout the body. In the liver hepatocytes were positive, and hepatic macrophages (Kupffer cells) became positive when they are activated, such as with post viral vector (AAV9) injection. In the spleen large populations of strong PGRN-positive cells were present in the red pulp and in the subcapsular sinuses, and were fewer in the white pulp. The thymus similarly demonstrated large numbers of strong PGRN+ cells (not shown). Human tonsils also contained many strongly PGRN immunoreactive cells (see Figure 2). In mouse spleen and human tonsils, the distribution and morphology of PGRN+ cells, as well as Iba-1 and CD45 double labeling confirmed their identity as macrophages and dendritic cells, rather than lymphocytes (Figure 2). Tonsillar surface epithelial cells were only weakly PGRN+ (not shown). These results show that the amount of tissue PGRN expression is determined in part by the number of resident macrophages/dendritic cells and their activation status. Lymphoid organs such as spleen, thymus and tonsil are among the highest PGRN-expressing organs, with brain and liver showing intermediate levels, and heart and lung showing the lowest levels of PGRN. There were also qualitative differences between epithelial and myeloid PGRN immunoreactivity. For example, in the CNS microglia/macrophage PGRN staining was sharp and strong while neuronal PGRN was weak and punctate (Figure 1). In the cerebellum, Purkinje cells had no immunoreactivity, while cells in close contact with Purkinje cells including some microglia were positive (not shown). Choroid plexus epithelium and ependymal cells were negative (not shown). Together, these results illustrate that cells of myeloid lineage including macrophages, dendritic cells and microglia constitute a major cell type that expresses PGRN in vivo. Our data also suggest that lymphocytes are not a large source of PGRN. These results have implications for the understanding of the biomarker data (see below).
Figure 1. PGRN expression in the mouse.
PGRN expression is highly organ- and cell type-specific. (A) In the brain, neurons show fine punctate cytoplasmic staining (arrows) while perivascular macrophages (asterisk) and microglia (not shown) also show immunoreactivity (X 200). (B) In injured brain, such as following intracerebral AAV vector injection as shown here, ameboid microglia show strong PGRN immunoreactivity (X 400). (C) In other organs such as lung, PGRN immunoreactivity is minimal, limited to scattered macrophages (X 400). Asterisk indicates a bronchial lumen with little PGRN expression in the epithelium. (D) Lymphoid organs such as spleen had numerous PGRN+ cells that are consistent with macrophages and dendritic cells (X 100). (W = white pulp) (E) Normal liver shows punctate granular staining of the hepatocytes (X 200). (F) However, strong PGRN immunoreactivity emerges in liver macrophages (Kupffer cells) following systemic AAV injection (X 100). (C = central vein) (G) In the kidney, PGRN immunoreactivity is limited to select proximal tubular epithelial cells, accentuated in their luminal border (X 400). Asterisks mark PGRN-negative glomeruli. (F) Macrophages associated with visceral adipose tissue throughout the body show strongly PGRN positivity (X 400). Methods: Paraffin-embedded sections of mouse organs were subjected to PGRN immunohistochemistry using antigen retrieval, goat anti-PGRN IgG (R&D Systems, 1:80 dilution) and the micropolymer-based secondary antibody (ImmPress) methods, as previously described (Tarassishin et al., 2013).
Figure 2. PGRN expression in mouse spleen and human tonsil.
Double label immunofluorescence studies were performed for PGRN (green) and macrophage marker (Iba-1: red) or with lymphocyte marker (CD45: red). In mouse spleen, PGRN immunoreactivity (predominantly in red pulp) co-localizes with Iba-1. In human tonsil, PGRN immunoreactivity co-localizes with Iba-1 but little with CD45 (lymphocyte common antigen). PGRN is intracellular localized in endosomal reticulum, Golgi or lysosome (Hu et al., 2010). Iba-1 is localized predominantly on the cell membrane. Methods: paraffin-embedded sections of mouse spleen or human tonsillectomy specimens were subjected to antigen retrieval, incubation with primary antibodies (goat anti-PGRN from R&D Systems at 1:80, rabbit anti-Iba-1 from WACO at 1:500 or mouse anti-human CD45 from DAKO at 1:50) followed by Alexa 488 chicken anti-goat, Alexa 555 donkey anti-rabbit or Alexa 555 anti mouse IgG at a 1:1000 dilution.
PGRN expression is upregulated in HIV encephalitis (HIVE)
Despite the strong emerging evidence pointing to the important role of PGRN in antimicrobial immune function, studies that directly examine the expression/role of PGRN in infectious diseases are rare. We performed analysis of PGRN expression in human brain specimens with HIV encephalitis (HIVE) and normal controls. Paraffin-embedded white matter sections of HIVE and control brains were obtained from the NNTC cohort; PGRN expression was determined by immunohistochemistry as described for Figures 1 and 2. Double immunofluorescence for PGRN and the macrophage/microglial marker CD68, or HIV gag p24, were performed as previously described (Suh et al., 2010; Cosenza-Nashat et al., 2006). HIVE lesions showed markedly increased PGRN immunoreactivity in macrophages and microglia, as determined by morphology (Figure 3) as well as CD68 double labeling (Figure 4). PGRN was prominently expressed in multinucleated giant cells, a morphologic hallmark of macrophages that are productively infected with HIV (Budka, 1986). Double labeling showed frequent co-localization of PGRN with HIV p24 antigen (Figure 4). These histological results were compared to brain gene array data obtained from subjects in the NNTC cohort (Figure 5A). GRN signal intensity was significantly higher in white matter and basal ganglia, but not in neocortex, of HIVE cases compared to controls. Control groups included brains from uninfected individuals (CON), brains from HIV-seropositive individuals without neurocognitive impairment or HIVE (HIV), or brains from HIV+ individuals with neurocognitive impairment but without HIVE (DEM). The increased PGRN mRNA expression in the basal ganglia of HIVE cases was confirmed by quantitative real-time PCR (Figure 5B). The absence of PGRN increase in the cortex of HIVE likely represents the known paucity HIVE pathologies in the cerebral cortex of affected individuals (Kure et al., 1990). Together, these results indicate that HIV replication in microglia and macrophages produces a marked increase in GRN gene transcription and translation.
Figure 3. PGRN expression in HIV encephalitis and control brains.
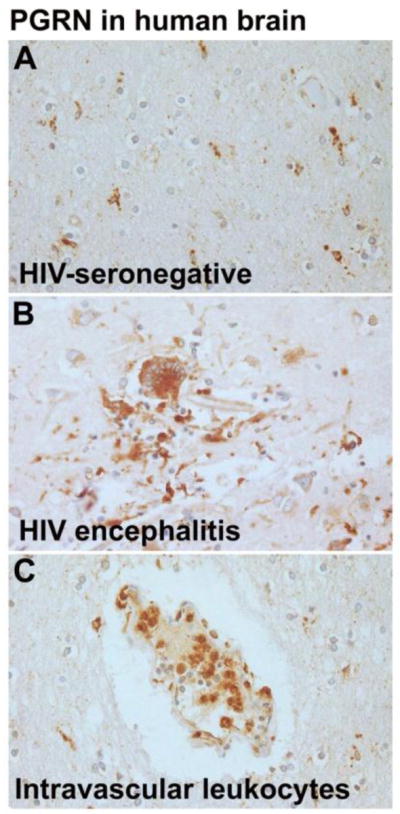
Formalin-fixed, paraffin-embedded postmortem human brain sections from HIVE or control brains from NNTC were subjected to PGRN immunohistochemistry as described in Figure 1 legend. (A) A white matter section from a HIV-negative brain shows numerous ramified microglial cells with fine processes showing PGRN immunoreactivity. (B) A section from HIVE shows a microglial nodule containing multinucleated giant cells with strong PGRN immunoreactivity. This contrasts with weak staining of the neurons and reactive astrocytes in the background. (C) A section from a HIV-negative control brain shows a vessel with PGRN+ intravascular leukocytes. Methods: See Figure 1 legend. All sections were lightly counterstained with hematoxylin to visualize all nuclei.
Figure 4. PGRN expression in HIVE by double-label immunofluorescence.
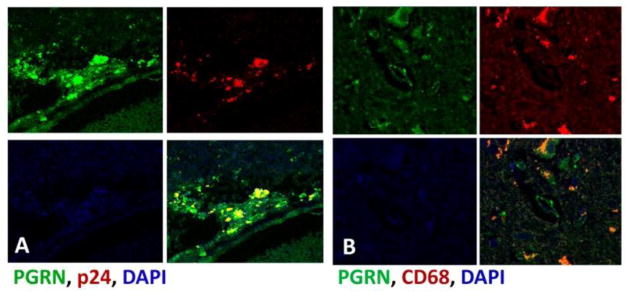
(A) HIVE brain showing co-localization of PGRN (green) with HIV gag antigen p24 (red). (B) HIVE brain showing colocalization of PGRN (green) with the macrophage and microglial marker CD68 (red). Methods: Double immunofluorescence study was performed as described previously (Cosenza-Nashat et al., 2011). Also see Figure 2 legend. All sections were counterstained with DAPI for nuclear stain.
Figure 5. Gene arrays studies demonstrate increase of PGRN mRNA in HIVE.
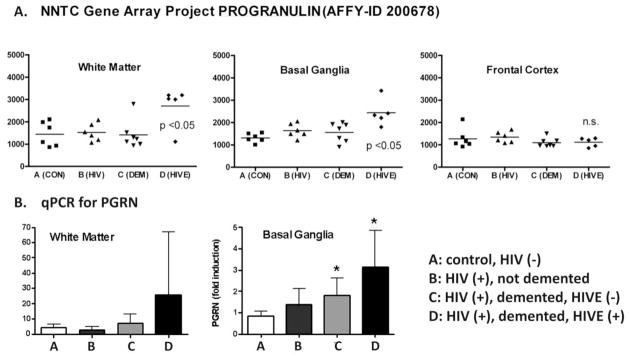
(A) Data from the NNTC brain gene array (Gelman et al., 2012a) demonstrate that GRN expression is significantly elevated in the white matter and basal ganglia but not in frontal cortex of HIVE cases compared to controls. Control groups included A: brains from uninfected individuals (CON), B: brains from HIV-seropositive individuals without HIVE (HIV), and C: brains from HIV+ individuals with neurocognitive impairment but without HIVE (DEM). Mean values from 5–8 brains (horizontal bar) are shown. P<0.05 by ANOVA and Bonferroni post-hoc analysis, n.s. = not significant. (B) Real-time reverse transcription PCR (qPCR) was performed to confirm the gene array data. * p<0.05 vs. control
Regulation of PGRN production in vitro
Despite the importance of macrophage and microglial PGRN, little information is available regarding the mechanism of regulation of expression PGRN in these cells. There is evidence that growth factors (including neural growth factors) are differentially regulated by the “proinflammatory” (Th1/M1) and “immunoregulatory” (Th2/M2) cytokines. For example, IFNγ suppresses and IL-4 induces insulin-like growth factor 1 (IGF1) in murine macrophages and microglia (Arkins et al., 1995; Wynes and Riches, 2003; Butovsky et al., 2006). In cultures of primary human CNS human cells, microglial cells are the primary source of PGRN with production of nanogram/ml concentrations at the “basal” level. Furthermore, the Th1/M1 cytokines (such as IL-1/IFNγ) and Th2/M2 cytokines (IL-4 or IL-13) decrease and increase microglial PGRN production, respectively (Suh et al., 2013). The toll-like receptor 4 (TLR4) ligand LPS potently suppresses production of PGRN, as well as IGF1, from microglia (Suh et al., 2013). Similar LPS-induced PGRN suppression has been reported in murine microglia (Baker and Manuelidis, 2003). These results together suggest the presence of common regulatory elements that control the expression of neuronal growth factors in macrophages and microglia. There is also evidence that the regulation of PGRN expression is cell type-dependent. For example, the Th1/M1 stimuli increase PGRN production from non-macrophage cell types such as astrocytes and fibroblasts (Li et al., 2002; Suh et al., 2012). Although the amount of PGRN produced by these cells is much smaller than macrophage cell types (Suh et al., 2012), the cell-type dependent responses to the same stimuli clearly add to the complexity of biomarker data interpretation. These results may be particularly relevant to the role of inflammation and PGRN in epithelial-lineage cancers. For example, although PGRN expression in reactive astrocytes in vivo is only rarely detected, malignant astrocytoma cells in situ express PGRN, with the degree of expression correlating with the tumor grade (Wang et al., 2012).
The interpretations of macrophage/microglia LPS response in vitro are also complicated given the immunohistochemistry studies of microglia in vivo which report only increases of PGRN immunoreactivity. There are several reasons for this discrepancy. First, LPS may not be a relevant stimulus for most CNS conditions. Second, LPS induces both M1 and M2 cytokine production from macrophages each inducing different (sometimes opposing) responses. Third, cultured microglia are already activated due to the presence of serum in the media and absence of other neural cell types (astrocytes, neurons) that are known to exert inhibitory influence on microglia (Wang et al., 2008; Liu et al., 1994). Therefore, the “basal” status of microglia in vivo and in vitro are not comparable. Nevertheless, the molecular and biochemical mechanisms elucidated in vitro shed light into the nature of the PGRN regulation, which appear to be different from most other immunologically regulated macrophage products.
PART 2: PGRN FUNCTION
Neurodegeneration and immune function of PGRN
To model FTLD caused by genetic PGRN deficiency and to study the role of Grn in neurodegeneration and innate immune functions, several gene knockout (Grn−/−) mice have been generated and characterized. While these studies support the general idea that PGRN contributes to normal aging, the CNS abnormalities in these mice are very subtle even though they are homozygous knockouts (Ahmed et al., 2010). The changes in these mice consist of increased neuronal lipofuscin accumulation and gliosis. Importantly, Grn−/− mice show exaggerated inflammation and cytokine production and impaired host defense, when challenged with pathogens or pathogenic components. In vitro, LPS-challenged Grn−/− macrophages reportedly produce higher amounts TNFα and IL-6 but less IL-10. Grn−/− mice also show defective clearance of Listeria monocytogenes from the brain (Yin et al., 2010). The cytokine-suppressive effect of (murine) microglial Grn has also been demonstrated in mice with CD11b-specific deletion of Grn (Martens et al., 2012). When challenged with 1-methyl-4-(2′-methylphenyl)-1, 2, 3, 6-tetrahydrophine (MPTP, a model of Parkinson’s disease), these mice showed exaggerated neuronal loss and over-expression microglial cytokines in vivo and in vitro. In a traumatic brain injury model, Grn-deficiency also led to an exacerbated inflammatory response and activation of microglia (Tanaka et al., 2013). These data together suggest that microglial Grn plays a crucial role in downmodulating cytokine production and neuroinflammation and ameliorating neuronal death through downmodulation of inflammation.
In contrast to these studies, others have reported cytokine-enhancing role of PGRN. For example, Grn was reported to be a necessary cofactor for TNFα and IL-6 production in mouse macrophages challenged with the TLR9 ligand (Park et al., 2011). Furthermore, Okura et al., reported that exogenous PGRN augmented and anti-PGRN antibody reduced the expression of TNFα and IL-1β from human macrophages (Okura et al., 2010). Our own study in primary human fetal microglia showed that GRN was involved in the induction of cytokines, especially TNFα. Using siRNA-mediated PGRN knockdown, and LPS and poly IC as stimuli, there was a~ 50% reduction in LPS- or poly IC-induced TNFα protein production in PGRN-knocked down microglia (Suh et al., 2012). Other cytokines and chemokines examined were less affected by PGRN knockdown. In these studies we did not observe any reduction in cytokine production by PGRN. Our results appear to contradict other data suggesting that PGRN can suppress cytokine production (Yin et al., 2010; Tang et al., 2011), yet they have important implications for a species-specific and stimulus-specific role of PGRN in the regulation of inflammation and host defense (see below).
Other aspects of macrophage biology have been shown to be affected by PGRN including phagocytosis, migration, and TNFα signaling (Eriksen and Mackenzie, 2008; Tang et al., 2011). Tang et al. showed that PGRN functions as a TNFα receptor antagonist in macrophages and proposed that PGRN and its engineered mutant are potentially efficacious in treating rheumatoid arthritis. The latter study reinforces the idea that PGRN plays a unique role in the regulation of TNFα expression/function. However, in human fetal microglia, we saw no evidence that PGRN acts as a TNFα antagonist.
Regulation of PGRN protein processing and its functional implications
The production of PGRN degradation products, granulins, and its biological consequences has been studied in detail in neutrophils and epithelial cells. These studies report that PGRN and granulins have opposing activities (Zhu et al., 2002). Information regarding the mechanism of granulin production from macrophages and microglia and the activity of granulin in macrophage biology has not been available. We have recently identified macrophage elastase (MMP-12) as a PGRN proteolytic enzyme and SLPI as an inhibitor of proteolysis in human microglia (and in a cell-free system) establishing MMP-12 and SLPI as potentially important modulators of PGRN biology in microglia (Suh et al., 2012). Previously, PGRN has been shown to be cleaved by MMP-14, MMP-9, and ADAMTS7, in addition to neutrophil elastase, making it likely that multiple enzymes are involved in producing granulins in the CNS (De and Van, 2011). Curiously, microglial MMP-12 activation and PGRN cleavage occurred intracellularly but not extracellularly. MMP-12 activity is regulated by several factors including Ca2+ concentration and pH (Gossas and Danielson, 2006), thus it is likely that extracellular PGRN proteolysis will occur under the conditions that sharply increase the Ca2+ levels such as during inflammation and cell death. These results also support the scenario that under inflammatory CNS conditions, PGRN production is suppressed while granulin production is increased, possibly switching the role of microglia from a neurotrophic one to an inflammatory one.
However, there are several pieces of information that contradict the idea that PGRN and granulins have opposing roles in macrophage inflammation. First, our study of human fetal microglia shows that PGRN conversion to granulins is minimal even under the strongest inflammatory conditions such as stimulation with LPS. To our knowledge, no data exists that demonstrate the amount of PGRN degradation to granulin in murine macrophages or neutrophils. Second, as mentioned earlier, PGRN increased the production of cytokines and chemokine including TNFα in human fetal microglia, which is opposite to the findings in murine macrophages.
Unexpectedly, we found no evidence that human fetal microglia produce SLPI (while inflammatory astrocytes produced large amounts of SLPI in vitro). In rodents, macrophages have been shown to be a significant source of SLPI (Jin et al., 1997). Few reports exist that demonstrate SLPI production by human macrophages (Taggart et al., 2005) and possibly in human microglia and macrophages SLPI expression is silenced in a fashion that is akin to iNOS (Denis, 1994; Schneemann et al., 1993; Albina, 1995). SLPI’s other known functions include inhibition of HIV infection and suppression of macrophage activation (Jin et al., 1997), in addition to anti-proteolytic activity. For example, extracellular SLPI has been shown to be taken up by macrophages and inhibit NF-κB activation and cytokine production following nuclear translocation (Jin et al., 1997). The low to absent SLPI expression by human microglia (and macrophages) might thus render these cells more prone to proinflammatory activation. These findings are further indications of important species-dependent regulatory mechanisms in PGRN biology.
PGRN as a neuronal growth factor
There is ample evidence for the essential role of PGRN in neuronal survival and function, the most important one being the clinical data that PGRN haploinsufficiency causes FTLD. Recombinant progranulin in primary neuronal cultures also show neutrophic effects (Van et al., 2008). The receptor for PGRN on neurons has been found to be sortilin which captures and internalizes extracellular PGRN for endosomal processing (Hu et al., 2010). Studies of mice with Grn deletion demonstrate that in mice, PGRN does not play an essential neurotrophic role as in humans. In addition, unlike other growth factor gene knockouts such as Igf1, which results in developmental anomaly (small brains, etc), PGRN deficiency (in both mice and humans) manifests only at later stages of life. These findings indicate that PGRN’s role in neuronal function is fundamentally different from those of neurotrophins and IGFs.
Based on the assumption that PGRN might induce neurotrophic gene expression in CNS cells, we performed preliminary gene expression analysis in human astrocyte cultures exposed to recombinant PGRN. PGRN induced the expression of mRNAs associated with neuroprotection and defense against oxidative injury including interleukin-11, leukemia inhibitory factor, thioredoxin and metallothionein 2A (data not shown). These results suggest a scenario in which microglial and neuronal GRN is secreted to adjacent cells such as astrocytes, which then induce neuroprotective and antioxidant factors (see Figure 6 below for hypothesis). We hypothesize that constitutive expression of PGRN in neurons and microglia helps to maintain normal CNS trophic environment through multiple cell-cell interactions. Conversely, chronic depletion of PGRN caused by gene mutations, polymorphisms, chronic proinflammatory states, and other as yet unidentified mechanisms can lead to neurodegeneration by suppressing neurotrophic and antioxidant mechanisms in the brain.
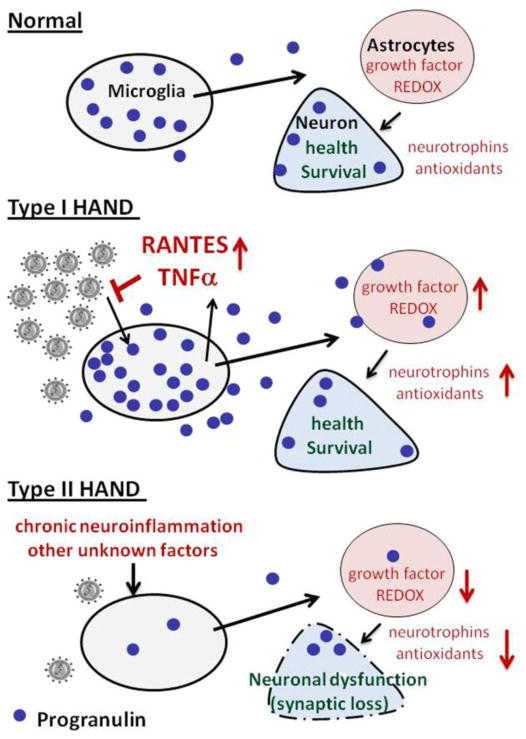
Figure 6. Hypothesis.
Based on all available data, we hypothesize that PGRN plays a dual role in the pathogenesis of HIV-associated neurocognitive disorders. (A) In normal brain, constitutive PGRN expression maintains normal neuronal survival in part through promoting neurotrophic and antioxidant gene expression in a paracrine and cell-cell interactions involving microglia, astrocytes and neurons. (B) In the CNS of individuals with active HIV replication (such as in HIVE), macrophage and microglial PGRN expression is upregulated which is reflected in the elevated levels of PGRN. PGRN expressed in the HIV-infected cells mainly functions as an innate antiviral factor, as well as to protect neurons from damaging viral and host products, via mechanisms described above. We speculate that unlike in murine cells, PGRN’s role in human microglia is to promote production of cytokines and chemokines that function as antimicrobial factors during acute infection. (C) In most HIV-associated neurocognitive disorders that are not associated with active viral replication (Type II HAND), PGRN production from macrophages and microglia is reduced by proinflammatory mediators and other unknown stressor contributing to neuronal dysfunction. We propose CSF PGRN as a surrogate disease marker for HAND not associated with active viral replication.
PGRN in innate immunity against pathogens
The observation that PGRN is primarily a product of cells of the innate immune system (neutrophils, monocytes and macrophages) suggests its role in antimicrobial activity. Its known function in modulating cytokine production (though the details vary depending of the report) also suggests such a role. Its antimicrobial role has been alluded to in an earlier study which showed delayed clearance of bacteria from the brains of Grn−/− mice (Yin et al., 2010). PGRN mRNA is highly expressed in the CNS with virulent Sindbis virus infection (Johnston et al., 2001). Limited clinical studies also suggest its possible role in infectious diseases. For example, PGRN expression was induced in gastric epithelial cells infected with Helicobacter pylori (Wex et al., 2011). The induction in vitro required direct contact between epithelial cells and live bacteria. H-pylori-infected subjects also had increased serum PGRN levels. These results suggest that increased PGRN expression is a natural response of gastric epithelial cells to infection.
As shown earlier, our analysis of HIVE also demonstrates co-expression of PGRN and the HIV p24, suggesting PGRN expression is part of the microglial response to HIV infection. Furthermore, gene array data show that PGRN mRNA expression is significantly upregulated in HIVE. The strongest link between HIV infection and PGRN production comes from our CHARTER sample analysis which shows highly significant (p <0.001) correlation between PGRN levels and the viral loads in both plasma and the CSF (manuscript in preparation). In vitro, PGRN is increased by HIV infection of microglia and this requires active viral replication, as the reverse transcriptase inhibitor, AZT, abolishes this effect (not shown). Our preliminary data employing PGRN siRNA suggest that the increased PGRN expression may act as a protective response to restrict HIV infection in the microglia. Taken together, these results strongly suggest that PGRN plays a role in the innate immune response to HIV infection. Potential mechanisms include PGRN-mediated production of antiviral cytokines and chemokines such as TNFα, RANTES, MIP-1α and MIP-1β (Si et al., 2002; Moore and Dragic, 1999). Another potential mechanism has been suggested by studies which demonstrated that PGRN directly binds to HIV-1 and HIV-2 Tat proteins and suppresses Tat-responsive promoters (Trinh et al., 1999; Shoham et al., 2003; Hoque et al., 2010).
Other studies have found that PGRN affects additional aspects of macrophage and microglial biology such as proliferation, recruitment, differentiation, activation and endocytosis/phagocytosis, all of which may be relevant to HIV-macrophage interactions. Pickford et al. demonstrated that PGRN acted as a chemoattractant for microglia in vitro, and its over-expression in vivo increased the number of activated microglia in the brain (Pickford et al., 2011). PGRN also enhanced microglial endocytosis of β-amyloid. Studies of the role of PGRN in phagocytosis using Grn −/− macrophages reported that PGRN inhibits phagocytosis of apoptotic cells (Kao et al., 2011). Another study found no differences in the phagocytic capacity between the wildtype and Grn −/− macrophages(Yin et al., 2010). PGRN is also implicated in macrophage and dendritic cell antigen presentation (Jian et al., 2013).
PGRN also plays a prominent role in endosomal lysosomal protein transport/processing. Importantly, a recent study in humans reports adult-onset lysosomal storage disease caused by homozygous PGRN deficiency (Cenik et al., 2012). PGRN also shows high affinity binding to the lysosomal transport protein sortilin, a purported neuronal receptor for PGRN (Hu et al., 2010). Furthermore, impaired autophagy-lysosomal pathway resulting in increased accumulation of autophagy-related receptor p62 and lysosomal proteases were reported in aged Grn −/− mice (Wils et al., 2012). Overexpression of TMEM106B, a protein localized in the late endosome/lysosome, induces impairment of endosomal and lysosomal degradation and elevated levels of PGRN in neuronal cells (Brady et al., 2013). As the endosomal-lysosomal pathway plays a major role in HIV biogenesis in macrophages and microglia, these results also implicate endosomal mechanisms for the PGRN activity observed in microglia. Thus, PGRN may modulate anti-HIV immunity through several mechanisms.
PGRN as a surrogate marker for HIV-associated NCI
A number of studies have attempted to identify biomarkers to monitor HIV immunopathogenesis in patients on cART, but thus far no candidate biomarker has proved to be useful clinically. Based on our novel preliminary data that microglial PGRN is induced by HIV infection and is involved in anti-HIV innate immune responses, but suppressed by proinflammatory stimuli and in the CSF of a subset of individuals with HAND, we hypothesize that PGRN plays a dual role with respect to the antiviral immunity and neuronal dysfunction (Figure 6).
In normal brain, constitutive PGRN expression maintains neuronal survival through promoting neurotrophic and antioxidant gene expression in a paracrine and cell-cell interactions involving microglia, astrocytes and neurons. In HIVE or in patients with active systemic viral replication, PGRN is upregulated and it plays a role in antiviral immunity. In patients with undetectable virus, progranulin from macrophages and microglia might be depleted due to several factors including ongoing chronic neuroinflammation. We propose Type I and Type II HAND designations for neurocognitively impaired individuals with and without detectable HIV, respectively. Our preliminary analyses of CHARTER samples demonstrate that the CSF PGRN levels tend to be lower in individuals with neurocognitive impairment, even after adjusting for CSF HIV RNA, age and CSF TNFα by multivariable analyses (manuscript in preparation). Furthermore, this trend becomes more accentuated in individuals with no detectable CSF viral load. We propose that low CSF progranulin levels in these patients contribute to neuronal dysfunction. We further propose that CSF PGRN levels may serve as a surrogate biomarker for type II HAND, with decreasing levels indicating poorer prognosis or a suboptimal response to therapy. If the CSF data are confirmed in a larger study, PGRN replacement therapy might be considered.
Concluding remarks and future studies
The available studies shed light into the PGRN biology in microglia and macrophages but also open up several important questions, such as the molecular mechanisms underlying microglial PGRN gene repression, and the signals that upregulate microglial PGRN expression in vivo. It is also unclear whether neuronal PGRN is under similar regulation. The fundamentally different role of microglial (macrophage) PGRN in inflammation (as opposed to PGRN’s generally implicated neurotrophic role) might suggest that neuron-autonomous PGRN might be important in the maintenance of normal neuronal physiology. The results also suggest that complete absence of PGRN in Grn−/− mice may not accurately reflect the relationship between neuronal and microglial PGRN (such as that occurs in FTLD), due to abolition of the reactive PGRN arm, such as that exists in myeloid cells. Future studies addressing these issues could aid the understanding of the mechanism by which PGRN deficiency predisposes to neurodegeneration. With regard to PGRN and antiviral immunity and neurocognitive decline, whether PGRN expression affects the risk of developing other neurodegenerative diseases such as HIV-associated neurocognitive impairment remains to be determined. There is also a need to determine whether the common GRN gene variants such as rs5848 predispose to neurocognitive impairments in HIV-infected individuals.
Acknowledgments
The authors thank the National NeuroAIDS Tissue Consortium (NNTC) for providing brain tissue and brain gene array data. We thank the CHARTER Group for providing clinical samples of blood plasma and cerebrospinal fluid. We also thank Dr Namjong Choi for excellent immunohistochemistry. We are grateful to Drs Scott Letendre, Yungtai Lo, Susan Morgello, and Yousin Suh for many helpful discussions. This study was supported by the NIH grants RO1MH55477, KO1MH084705 and the Einstein CFAR grant (P30AI051519). The NNTC is supported by the NIH including U01 MH083507, U01 MH083506, U01 MH083501, U01 MH083500, and U01 MH083545. The CHARTER study was supported by N01 MH22005, HHSN271201000036C and HHSN271201000030C from the National Institutes of Health.
ABBREVIATIONS
- AAV
adeno-associated virus
- AD
Alzheimer’s disease
- ALS
amyotrophic lateral sclerosis
- cART
combination antiretroviral therapy
- AZT
azidothymidine
- CHARTER
CNS HIV Anti-Retroviral Therapy Effects Research
- CJD
Creutzfeldt-Jakob disease
- CNS
central nervous system
- CSF
cerebrospinal fluid
- FTLD
frontotemporal lobar degeneration
- FTLD-U
FTLD-ubiquitin-positive
- GRN
human progranulin gene
- Grn
mouse or rat progranulin gene
- HAD
HIV-associated dementia
- HIVE
HIV encephalitis
- IGF1
insulin-like growth factor 1
- LPS
lipopolysaccharide
- M1
macrophage classical activation phenotype
- M2
macrophage alternative activation phenotype
- MMP-12
matrix metalloproteinase-12 (macrophage elastase)
- MS
multiple sclerosis
- NCI
neurocognitive impairment
- NNTC
National NeuroAIDS Consortium
- PGRN
progranulin
- SLPI
secretory leukocyte protease inhibitor
- SNP
single nucleotide polymorphism
- TLR
toll-like receptor
- VSVg-env HIV
vesicular stomatitis virus G envelope HIV
Footnotes
Competing interests: The authors declare no competing interests.
Reference List
- Ahmed Z, Mackenzie IR, Hutton ML, Dickson DW. Progranulin in frontotemporal lobar degeneration and neuroinflammation. J Neuroinflammation. 2007;4:7. doi: 10.1186/1742-2094-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed Z, Sheng H, Xu YF, Lin WL, Innes AE, Gass J, Yu X, Wuertzer CA, Hou H, Chiba S, Yamanouchi K, Leissring M, Petrucelli L, Nishihara M, Hutton ML, McGowan E, Dickson DW, Lewis J. Accelerated lipofuscinosis and ubiquitination in granulin knockout mice suggest a role for progranulin in successful aging. Am J Pathol. 2010;177:311–324. doi: 10.2353/ajpath.2010.090915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Ayadhi LY, Mostafa GA. Low plasma progranulin levels in children with autism. J Neuroinflammation. 2011;8:111. doi: 10.1186/1742-2094-8-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albina JE. On the expression of nitric oxide synthase by human macrophages. Why no NO? J Leukoc Biol. 1995;58:643–649. doi: 10.1002/jlb.58.6.643. [DOI] [PubMed] [Google Scholar]
- Arkins S, Rebeiz N, Brunke-Reese DL, Biragyn A, Kelley KW. Interferon-gamma inhibits macrophage insulin-like growth factor-I synthesis at the transcriptional level. Mol Endocrinol. 1995;9:350–360. doi: 10.1210/mend.9.3.7776981. [DOI] [PubMed] [Google Scholar]
- Baker CA, Manuelidis L. Unique inflammatory RNA profiles of microglia in Creutzfeldt-Jakob disease. Proc Natl Acad Sci U S A. 2003;100:675–679. doi: 10.1073/pnas.0237313100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker M, et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. 2006;442:916–919. doi: 10.1038/nature05016. [DOI] [PubMed] [Google Scholar]
- Bateman A, Bennett HP. The granulin gene family: from cancer to dementia. Bioessays. 2009;31:1245–1254. doi: 10.1002/bies.200900086. [DOI] [PubMed] [Google Scholar]
- Bhandari V, Palfree RG, Bateman A. Isolation and sequence of the granulin precursor cDNA from human bone marrow reveals tandem cysteine-rich granulin domains. Proc Natl Acad Sci U S A. 1992;89:1715–1719. doi: 10.1073/pnas.89.5.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady OA, Zheng Y, Murphy K, Huang M, Hu F. The frontotemporal lobar degeneration risk factor, TMEM106B, regulates lysosomal morphology and function. Hum Mol Genet. 2013;22:685–695. doi: 10.1093/hmg/dds475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budka H. Multinucleated giant cells in brain: a hallmark of the acquired immune deficiency syndrome (AIDS) Acta Neuropathol. 1986;69:253–258. doi: 10.1007/BF00688301. [DOI] [PubMed] [Google Scholar]
- Butovsky O, Landa G, Kunis G, Ziv Y, Avidan H, Greenberg N, Schwartz A, Smirnov I, Pollack A, Jung S, Schwartz M. Induction and blockage of oligodendrogenesis by differently activated microglia in an animal model of multiple sclerosis. J Clin Invest. 2006;116:905–915. doi: 10.1172/JCI26836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carecchio M, Fenoglio C, De RM, Guidi I, Comi C, Cortini F, Venturelli E, Restelli I, Cantoni C, Bresolin N, Monaco F, Scarpini E, Galimberti D. Progranulin plasma levels as potential biomarker for the identification of GRN deletion carriers. A case with atypical onset as clinical amnestic Mild Cognitive Impairment converted to Alzheimer’s disease. J Neurol Sci. 2009;287:291–293. doi: 10.1016/j.jns.2009.07.011. [DOI] [PubMed] [Google Scholar]
- Carrasquillo MM, Nicholson AM, Finch N, Gibbs JR, Baker M, Rutherford NJ, Hunter TA, Jesus-Hernandez M, Bisceglio GD, Mackenzie IR, Singleton A, Cookson MR, Crook JE, Dillman A, Hernandez D, Petersen RC, Graff-Radford NR, Younkin SG, Rademakers R. Genome-wide screen identifies rs646776 near sortilin as a regulator of progranulin levels in human plasma. Am J Hum Genet. 2010;87:890–897. doi: 10.1016/j.ajhg.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenik B, Sephton CF, Kutluk CB, Herz J, Yu G. Progranulin: a proteolytically processed protein at the crossroads of inflammation and neurodegeneration. J Biol Chem. 2012;287:32298–32306. doi: 10.1074/jbc.R112.399170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantry D, DeMaggio AJ, Brammer H, Raport CJ, Wood CL, Schweickart VL, Epp A, Smith A, Stine JT, Walton K, Tjoelker L, Godiska R, Gray PW. Profile of human macrophage transcripts: insights into macrophage biology and identification of novel chemokines. J Leukoc Biol. 1998;64:49–54. doi: 10.1002/jlb.64.1.49. [DOI] [PubMed] [Google Scholar]
- Chen-Plotkin AS, Unger TL, Gallagher MD, Bill E, Kwong LK, Volpicelli-Daley L, Busch JI, Akle S, Grossman M, Van DV, Trojanowski JQ, Lee VM. TMEM106B, the risk gene for frontotemporal dementia, is regulated by the microRNA-132/212 cluster and affects progranulin pathways. J Neurosci. 2012;32:11213–11227. doi: 10.1523/JNEUROSCI.0521-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen-Plotkin AS, Xiao J, Geser F, Martinez-Lage M, Grossman M, Unger T, Wood EM, Van DV, Trojanowski JQ, Lee VM. Brain progranulin expression in GRN-associated frontotemporal lobar degeneration. Acta Neuropathol. 2010;119:111–122. doi: 10.1007/s00401-009-0576-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppola G, Karydas A, Rademakers R, Wang Q, Baker M, Hutton M, Miller BL, Geschwind DH. Gene expression study on peripheral blood identifies progranulin mutations. Ann Neurol. 2008;64:92–96. doi: 10.1002/ana.21397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosenza-Nashat MA, Bauman A, Zhao ML, Morgello S, Suh HS, Lee SC. Cannabinoid receptor expression in HIV encephalitis and HIV-associated neuropathologic comorbidities. Neuropathol Appl Neurobiol. 2011;37:464–483. doi: 10.1111/j.1365-2990.2011.01177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosenza-Nashat MA, Kim MO, Zhao ML, Suh HS, Lee SC. CD45 isoform expression in microglia and inflammatory cells in HIV-1 encephalitis. Brain Pathol. 2006;16:256–265. doi: 10.1111/j.1750-3639.2006.00027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruchaga C, Graff C, Chiang HH, Wang J, Hinrichs AL, Spiegel N, Bertelsen S, Mayo K, Norton JB, Morris JC, Goate A. Association of TMEM106B gene polymorphism with age at onset in granulin mutation carriers and plasma granulin protein levels. Arch Neurol. 2011;68:581–586. doi: 10.1001/archneurol.2010.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruts M, et al. Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Nature. 2006;442:920–924. doi: 10.1038/nature05017. [DOI] [PubMed] [Google Scholar]
- Daniel R, He Z, Carmichael KP, Halper J, Bateman A. Cellular localization of gene expression for progranulin. J Histochem Cytochem. 2000;48:999–1009. doi: 10.1177/002215540004800713. [DOI] [PubMed] [Google Scholar]
- De ML, Van DP. Cellular Effects of Progranulin in Health and Disease. J Mol Neurosci. 2011 doi: 10.1007/s12031-011-9553-z. [DOI] [PubMed] [Google Scholar]
- De RM, Galimberti D, Fenoglio C, Piccio LM, Scalabrini D, Venturelli E, Pietroboni A, Piola M, Naismith RT, Parks BJ, Fumagalli G, Bresolin N, Cross AH, Scarpini E. Cerebrospinal fluid progranulin levels in patients with different multiple sclerosis subtypes. Neurosci Lett. 2010;469:234–236. doi: 10.1016/j.neulet.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis M. Human monocytes/macrophages: NO or no NO? J Leukoc Biol. 1994;55:682–684. doi: 10.1002/jlb.55.5.682. [DOI] [PubMed] [Google Scholar]
- Eriksen JL, Mackenzie IR. Progranulin: normal function and role in neurodegeneration. J Neurochem. 2008;104:287–297. doi: 10.1111/j.1471-4159.2007.04968.x. [DOI] [PubMed] [Google Scholar]
- Fenoglio C, Galimberti D, Cortini F, Kauwe JS, Cruchaga C, Venturelli E, Villa C, Serpente M, Scalabrini D, Mayo K, Piccio LM, Clerici F, Albani D, Mariani C, Forloni G, Bresolin N, Goate AM, Scarpini E. Rs5848 variant influences GRN mRNA levels in brain and peripheral mononuclear cells in patients with Alzheimer’s disease. J Alzheimers Dis. 2009;18:603–612. doi: 10.3233/JAD-2009-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch N, Baker M, Crook R, Swanson K, Kuntz K, Surtees R, Bisceglio G, Rovelet-Lecrux A, Boeve B, Petersen RC, Dickson DW, Younkin SG, Deramecourt V, Crook J, Graff-Radford NR, Rademakers R. Plasma progranulin levels predict progranulin mutation status in frontotemporal dementia patients and asymptomatic family members. Brain. 2009;132:583–591. doi: 10.1093/brain/awn352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galimberti D, et al. Progranulin gene variability and plasma levels in bipolar disorder and schizophrenia. PLoS ONE. 2012;7:e32164. doi: 10.1371/journal.pone.0032164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman BB, Chen T, Lisinicchia JG, Soukup VM, Carmical JR, Starkey JM, Masliah E, Commins DL, Brandt D, Grant I, Singer EJ, Levine AJ, Miller J, Winkler JM, Fox HS, Luxon BA, Morgello S. The National NeuroAIDS Tissue Consortium brain gene array: two types of HIV-associated neurocognitive impairment. PLoS ONE. 2012a;7:e46178. doi: 10.1371/journal.pone.0046178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman BB, Lisinicchia JG, Morgello S, Masliah E, Commins D, Achim CL, Fox HS, Kolson DL, Grant I, Singer E, Yiannoutsos CT, Sherman S, Gensler G, Moore DJ, Chen T, Soukup VM. Neurovirological correlation with HIV-associated neurocognitive disorders and encephalitis in a HAART-era cohort. J Acquir Immune Defic Syndr. 2012b;62:487–495. doi: 10.1097/QAI.0b013e31827f1bdb. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghidoni R, Benussi L, Glionna M, Franzoni M, Binetti G. Low plasma progranulin levels predict progranulin mutations in frontotemporal lobar degeneration. Neurology. 2008;71:1235–1239. doi: 10.1212/01.wnl.0000325058.10218.fc. [DOI] [PubMed] [Google Scholar]
- Gossas T, Danielson UH. Characterization of Ca2+ interactions with matrix metallopeptidase-12: implications for matrix metallopeptidase regulation. Biochem J. 2006;398:393–398. doi: 10.1042/BJ20051933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75:2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoque M, Mathews MB, Pe’ery T. Progranulin (granulin/epithelin precursor) and its constituent granulin repeats repress transcription from cellular promoters. J Cell Physiol. 2010;223:224–233. doi: 10.1002/jcp.22031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiung GY, Fok A, Feldman HH, Rademakers R, Mackenzie IR. rs5848 polymorphism and serum progranulin level. J Neurol Sci. 2011;300:28–32. doi: 10.1016/j.jns.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu F, Padukkavidana T, Vaegter CB, Brady OA, Zheng Y, Mackenzie IR, Feldman HH, Nykjaer A, Strittmatter SM. Sortilin-mediated endocytosis determines levels of the frontotemporal dementia protein, progranulin. Neuron. 2010;68:654–667. doi: 10.1016/j.neuron.2010.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian J, Konopka J, Liu C. Insights into the role of progranulin in immunity, infection, and inflammation. J Leukoc Biol. 2013;93:199–208. doi: 10.1189/jlb.0812429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin FY, Nathan C, Radzioch D, Ding A. Secretory leukocyte protease inhibitor: a macrophage product induced by and antagonistic to bacterial lipopolysaccharide. Cell. 1997;88:417–426. doi: 10.1016/s0092-8674(00)81880-2. [DOI] [PubMed] [Google Scholar]
- Johnston C, Jiang W, Chu T, Levine B. Identification of genes involved in the host response to neurovirulent alphavirus infection. J Virol. 2001;75:10431–10445. doi: 10.1128/JVI.75.21.10431-10445.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamalainen A, Viswanathan J, Natunen T, Helisalmi S, Kauppinen T, Pikkarainen M, Pursiheimo JP, Alafuzoff I, Kivipelto M, Haapasalo A, Soininen H, Herukka SK, Hiltunen M. GRN variant rs5848 reduces plasma and brain levels of granulin in Alzheimer’s disease patients. J Alzheimers Dis. 2013;33:23–27. doi: 10.3233/JAD-2012-120946. [DOI] [PubMed] [Google Scholar]
- Kamat A, Lyons JL, Misra V, Uno H, Morgello S, Singer EJ, Gabuzda D. Monocyte Activation Markers in Cerebrospinal Fluid Associated With Impaired Neurocognitive Testing in Advanced HIV Infection. J Acquir Immune Defic Syndr. 2012;60:234–243. doi: 10.1097/QAI.0b013e318256f3bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao AW, Eisenhut RJ, Martens LH, Nakamura A, Huang A, Bagley JA, Zhou P, de LA, Neukomm LJ, Cabello J, Farese RV, Jr, Kenyon C. A neurodegenerative disease mutation that accelerates the clearance of apoptotic cells. Proc Natl Acad Sci U S A. 2011;108:4441–4446. doi: 10.1073/pnas.1100650108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul M. HIV-1 associated dementia: update on pathological mechanisms and therapeutic approaches. Curr Opin Neurol. 2009;22:315–320. doi: 10.1097/WCO.0b013e328329cf3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo DH, Park CY, Lee ES, Ro J, Oh SW. Progranulin as a prognostic biomarker for breast cancer recurrence in patients who had hormone receptor-positive tumors: a cohort study. PLoS ONE. 2012;7:e39880. doi: 10.1371/journal.pone.0039880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kure K, Lyman WD, Weidenheim KM, Dickson DW. Cellular localization of an HIV-1 antigen in subacute AIDS encephalitis using an improved double-labeling immunohistochemical method. Am J Pathol. 1990;136:1085–1092. [PMC free article] [PubMed] [Google Scholar]
- Li X, Massa PE, Hanidu A, Peet GW, Aro P, Savitt A, Mische S, Li J, Marcu KB. IKKalpha, IKKbeta, and NEMO/IKKgamma are each required for the NF-kappa B-mediated inflammatory response program. J Biol Chem. 2002;277:45129–45140. doi: 10.1074/jbc.M205165200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Brosnan CF, Dickson DW, Lee SC. Macrophage colony-stimulating factor mediates astrocyte-induced microglial ramification in human fetal central nervous system culture. Am J Pathol. 1994;145:48–53. [PMC free article] [PubMed] [Google Scholar]
- Malaspina A, Kaushik N, de BJ. Differential expression of 14 genes in amyotrophic lateral sclerosis spinal cord detected using gridded cDNA arrays. J Neurochem. 2001;77:132–145. doi: 10.1046/j.1471-4159.2001.t01-1-00231.x. [DOI] [PubMed] [Google Scholar]
- Martens LH, Zhang J, Barmada SJ, Zhou P, Kamiya S, Sun B, Min SW, Gan L, Finkbeiner S, Huang EJ, Farese RV., Jr Progranulin deficiency promotes neuroinflammation and neuron loss following toxin-induced injury. J Clin Invest. 2012;122:3955–3959. doi: 10.1172/JCI63113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArthur JC, Steiner J, Sacktor N, Nath A. Human immunodeficiency virus-associated neurocognitive disorders: Mind the gap. Ann Neurol. 2010;67:699–714. doi: 10.1002/ana.22053. [DOI] [PubMed] [Google Scholar]
- Moore JP, Dragic T. HIV. See a pocket, block it. Nature. 1999;401:759. doi: 10.1038/44504. [DOI] [PubMed] [Google Scholar]
- Okura H, Yamashita S, Ohama T, Saga A, Yamamoto-Kakuta A, Hamada Y, Sougawa N, Ohyama R, Sawa Y, Matsuyama A. HDL/apolipoprotein A-I binds to macrophage-derived progranulin and suppresses its conversion into proinflammatory granulins. J Atheroscler Thromb. 2010;17:568–577. doi: 10.5551/jat.3921. [DOI] [PubMed] [Google Scholar]
- Ong CH, He Z, Kriazhev L, Shan X, Palfree RG, Bateman A. Regulation of progranulin expression in myeloid cells. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1602–R1612. doi: 10.1152/ajpregu.00616.2005. [DOI] [PubMed] [Google Scholar]
- Park B, Buti L, Lee S, Matsuwaki T, Spooner E, Brinkmann MM, Nishihara M, Ploegh HL. Granulin is a soluble cofactor for toll-like receptor 9 signaling. Immunity. 2011;34:505–513. doi: 10.1016/j.immuni.2011.01.018. [DOI] [PubMed] [Google Scholar]
- Perry SW, Norman JP, Gelbard HA. Adjunctive therapies for HIV-1 associated neurologic disease. Neurotox Res. 2005;8:161–166. doi: 10.1007/BF03033827. [DOI] [PubMed] [Google Scholar]
- Petkau TL, Neal SJ, Orban PC, MacDonald JL, Hill AM, Lu G, Feldman HH, Mackenzie IR, Leavitt BR. Progranulin expression in the developing and adult murine brain. J Comp Neurol. 2010;518:3931–3947. doi: 10.1002/cne.22430. [DOI] [PubMed] [Google Scholar]
- Philips T, De ML, Thu HN, Weynants B, Vanacker P, Dhondt J, Sleegers K, Schelhaas HJ, Verbeek M, Vandenberghe R, Sciot R, Van BC, Lambrechts D, Van LF, Van Den BL, Robberecht W, Van DP. Microglial upregulation of progranulin as a marker of motor neuron degeneration. J Neuropathol Exp Neurol. 2010;69:1191–1200. doi: 10.1097/NEN.0b013e3181fc9aea. [DOI] [PubMed] [Google Scholar]
- Pickford F, Marcus J, Camargo LM, Xiao Q, Graham D, Mo JR, Burkhardt M, Kulkarni V, Crispino J, Hering H, Hutton M. Progranulin is a chemoattractant for microglia and stimulates their endocytic activity. Am J Pathol. 2011;178:284–295. doi: 10.1016/j.ajpath.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademakers R, et al. Common variation in the miR-659 binding-site of GRN is a major risk factor for TDP43-positive frontotemporal dementia. Hum Mol Genet. 2008;17:3631–3642. doi: 10.1093/hmg/ddn257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneemann M, Schoedon G, Hofer S, Blau N, Guerrero L, Schaffner A. Nitric oxide synthase is not a constituent of the antimicrobial armature of human mononuclear phagocytes. J Infect Dis. 1993;167:1358–1363. doi: 10.1093/infdis/167.6.1358. [DOI] [PubMed] [Google Scholar]
- Shoham N, Cohen L, Gazit A, Yaniv A. The Tat protein of the caprine arthritis encephalitis virus interacts with the Notch2 EGF-like repeats and the epithelin/granulin precursor. Intervirology. 2003;46:239–244. doi: 10.1159/000072434. [DOI] [PubMed] [Google Scholar]
- Si Q, Kim MO, Zhao ML, Landau NR, Goldstein H, Lee S. Vpr- and Nef-dependent induction of RANTES/CCL5 in microglial cells. Virology. 2002;301:342–353. doi: 10.1006/viro.2002.1613. [DOI] [PubMed] [Google Scholar]
- Sleegers K, Brouwers N, Maurer-Stroh S, van Es MA, Van DP, van Vught PW, van der ZJ, Serneels S, De PT, Van den BM, Cruts M, Schymkowitz J, De JP, Rousseau F, van den Berg LH, Robberecht W, Van BC. Progranulin genetic variability contributes to amyotrophic lateral sclerosis. Neurology. 2008;71:253–259. doi: 10.1212/01.wnl.0000289191.54852.75. [DOI] [PubMed] [Google Scholar]
- Sleegers K, Brouwers N, Van DP, Engelborghs S, Gijselinck I, van der ZJ, Peeters K, Mattheijssens M, Cruts M, Vandenberghe R, De Deyn PP, Robberecht W, Van BC. Serum biomarker for progranulin-associated frontotemporal lobar degeneration. Ann Neurol. 2009;65:603–609. doi: 10.1002/ana.21621. [DOI] [PubMed] [Google Scholar]
- Steinacker P, Fang L, Kuhle J, Petzold A, Tumani H, Ludolph AC, Otto M, Brettschneider J. Soluble beta-amyloid precursor protein is related to disease progression in amyotrophic lateral sclerosis. PLoS ONE. 2011;6:e23600. doi: 10.1371/journal.pone.0023600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh HS, Choi N, Tarassishin L, Lee SC. Regulation of Progranulin Expression in Human Microglia and Proteolysis of Progranulin by Matrix Metalloproteinase-12 (MMP-12) PLoS ONE. 2012;7:e35115. doi: 10.1371/journal.pone.0035115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh HS, Cosenza-Nashat M, Choi N, Zhao ML, Li JF, Pollard JW, Jirtle RL, Goldstein H, Lee SC. Insulin-like growth factor 2 receptor is an IFNgamma-inducible microglial protein that facilitates intracellular HIV replication: implications for HIV-induced neurocognitive disorders. Am J Pathol. 2010;177:2446–2458. doi: 10.2353/ajpath.2010.100399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh HS, Zhao ML, Derico L, Choi N, Lee SC. Insulin-like growth factor 1 and 2 (IGF1, IGF2) expression in human microglia: differential regulation by inflammatory mediators. J Neuroinflammation. 2013;10:37. doi: 10.1186/1742-2094-10-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taggart CC, Cryan SA, Weldon S, Gibbons A, Greene CM, Kelly E, Low TB, O’Neill SJ, McElvaney NG. Secretory leucoprotease inhibitor binds to NF-kappaB binding sites in monocytes and inhibits p65 binding. J Exp Med. 2005;202:1659–1668. doi: 10.1084/jem.20050768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka A, Tsukamoto H, Mitoma H, Kiyohara C, Ueda N, Ayano M, Ohta SI, Inoue Y, Arinobu Y, Niiro H, Horiuchi T, Akashi K. Serum progranulin levels are elevated in patients with systemic lupus erythematosus, reflecting disease activity. Arthritis Res Ther. 2012;14:R244. doi: 10.1186/ar4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Matsuwaki T, Yamanouchi K, Nishihara M. Exacerbated inflammatory responses related to activated microglia after traumatic brain injury in progranulin-deficient mice. Neuroscience. 2013;231:49–60. doi: 10.1016/j.neuroscience.2012.11.032. [DOI] [PubMed] [Google Scholar]
- Tang W, et al. The growth factor progranulin binds to TNF receptors and is therapeutic against inflammatory arthritis in mice. Science. 2011;332:478–484. doi: 10.1126/science.1199214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarassishin L, Bauman A, Suh HS, Lee SC. Anti-viral and anti-inflammatory mechanisms of the innate immune transcription factor interferon regulatory factor 3: relevance to human CNS diseases. J Neuroimmune Pharmacol. 2013;8:132–144. doi: 10.1007/s11481-012-9360-5. [DOI] [PubMed] [Google Scholar]
- Tkaczuk KR, Yue B, Zhan M, Tait N, Yarlagadda L, Dai H, Serrero G. Increased Circulating Level of the Survival Factor GP88 (Progranulin) in the Serum of Breast Cancer Patients When Compared to Healthy Subjects. Breast Cancer (Auckl ) 2011;5:155–162. doi: 10.4137/BCBCR.S7224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todoric J, Handisurya A, Perkmann T, Knapp B, Wagner O, Tura A, Pacini G, Esterbauer H, Kautzky-Willer A. Circulating progranulin levels in women with gestational diabetes mellitus and healthy controls during and after pregnancy. Eur J Endocrinol. 2012;167:561–567. doi: 10.1530/EJE-12-0060. [DOI] [PubMed] [Google Scholar]
- Tonjes A, Fasshauer M, Kratzsch J, Stumvoll M, Bluher M. Adipokine pattern in subjects with impaired fasting glucose and impaired glucose tolerance in comparison to normal glucose tolerance and diabetes. PLoS ONE. 2010;5:e13911. doi: 10.1371/journal.pone.0013911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinh DP, Brown KM, Jeang KT. Epithelin/granulin growth factors: extracellular cofactors for HIV-1 and HIV-2 Tat proteins. Biochem Biophys Res Commun. 1999;256:299–306. doi: 10.1006/bbrc.1999.0317. [DOI] [PubMed] [Google Scholar]
- Van DP, Van HA, Lambrechts D, Vanacker P, Bogaert E, van SJ, Carmeliet P, Van Den BL, Robberecht W. Progranulin functions as a neurotrophic factor to regulate neurite outgrowth and enhance neuronal survival. J Cell Biol. 2008;181:37–41. doi: 10.1083/jcb.200712039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van DV, et al. Common variants at 7p21 are associated with frontotemporal lobar degeneration with TDP-43 inclusions. Nat Genet. 2010;42:234–239. doi: 10.1038/ng.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercellino M, Grifoni S, Romagnolo A, Masera S, Mattioda A, Trebini C, Chiavazza C, Caligiana L, Capello E, Mancardi GL, Giobbe D, Mutani R, Giordana MT, Cavalla P. Progranulin expression in brain tissue and cerebrospinal fluid levels in multiple sclerosis. Mult Scler. 2011;17:1194–1201. doi: 10.1177/1352458511406164. [DOI] [PubMed] [Google Scholar]
- Wang J, Van DP, Cruchaga C, Gitcho MA, Vidal JM, Seijo-Martinez M, Wang L, Wu JY, Robberecht W, Goate A. Pathogenic cysteine mutations affect progranulin function and production of mature granulins. J Neurochem. 2010;112:1305–1315. doi: 10.1111/j.1471-4159.2009.06546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Li G, Yin J, Lin T, Zhang J. Progranulin overexpression predicts overall survival in patients with glioblastoma. Med Oncol. 2012;29:2423–2431. doi: 10.1007/s12032-011-0131-6. [DOI] [PubMed] [Google Scholar]
- Wang T, Gong N, Liu J, Kadiu I, Kraft-Terry SD, Mosley RL, Volsky DJ, Ciborowski P, Gendelman HE. Proteomic modeling for HIV-1 infected microglia-astrocyte crosstalk. PLoS ONE. 2008;3:e2507. doi: 10.1371/journal.pone.0002507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wex T, Kuester D, Schonberg C, Schindele D, Treiber G, Malfertheiner P. Mucosal Progranulin expression is induced by H. pylori, but independent of Secretory Leukocyte Protease Inhibitor (SLPI) expression. BMC Gastroenterol. 2011;11:63. doi: 10.1186/1471-230X-11-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wils H, Kleinberger G, Pereson S, Janssens J, Capell A, Van DD, Cuijt I, Joris G, De Deyn PP, Haass C, Van BC, Kumar-Singh S. Cellular ageing, increased mortality and FTLD-TDP-associated neuropathology in progranulin knockout mice. J Pathol. 2012 doi: 10.1002/path.4043. [DOI] [PubMed] [Google Scholar]
- Wynes MW, Riches DW. Induction of macrophage insulin-like growth factor-I expression by the Th2 cytokines IL–4 and IL-13. J Immunol. 2003;171:3550–3559. doi: 10.4049/jimmunol.171.7.3550. [DOI] [PubMed] [Google Scholar]
- Yin F, Banerjee R, Thomas B, Zhou P, Qian L, Jia T, Ma X, Ma Y, Iadecola C, Beal MF, Nathan C, Ding A. Exaggerated inflammation, impaired host defense, and neuropathology in progranulin-deficient mice. J Exp Med. 2010;207:117–128. doi: 10.1084/jem.20091568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn BS, Bang SI, Kloting N, Park JW, Lee N, Oh JE, Pi KB, Lee TH, Ruschke K, Fasshauer M, Stumvoll M, Bluher M. Serum progranulin concentrations may be associated with macrophage infiltration into omental adipose tissue. Diabetes. 2009;58:627–636. doi: 10.2337/db08-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Nathan C, Jin W, Sim D, Ashcroft GS, Wahl SM, Lacomis L, Erdjument-Bromage H, Tempst P, Wright CD, Ding A. Conversion of proepithelin to epithelins: roles of SLPI and elastase in host defense and wound repair. Cell. 2002;111:867–878. doi: 10.1016/s0092-8674(02)01141-8. [DOI] [PubMed] [Google Scholar]