Abstract
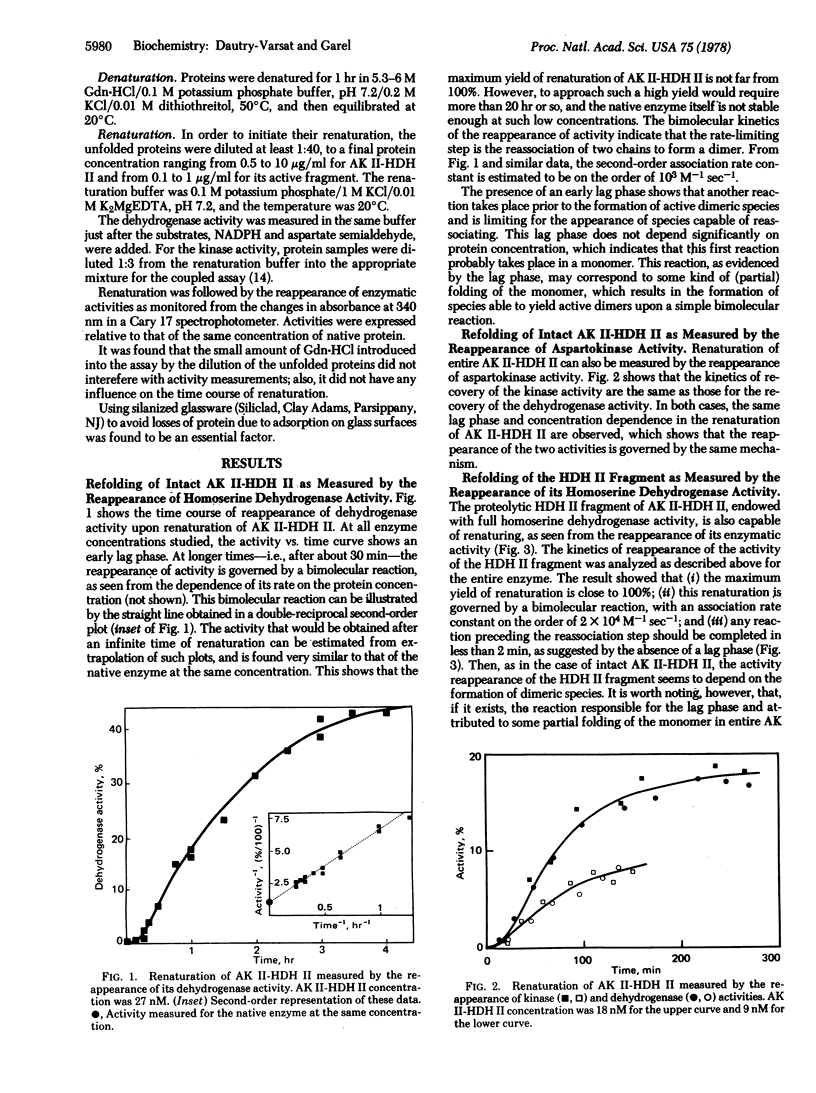
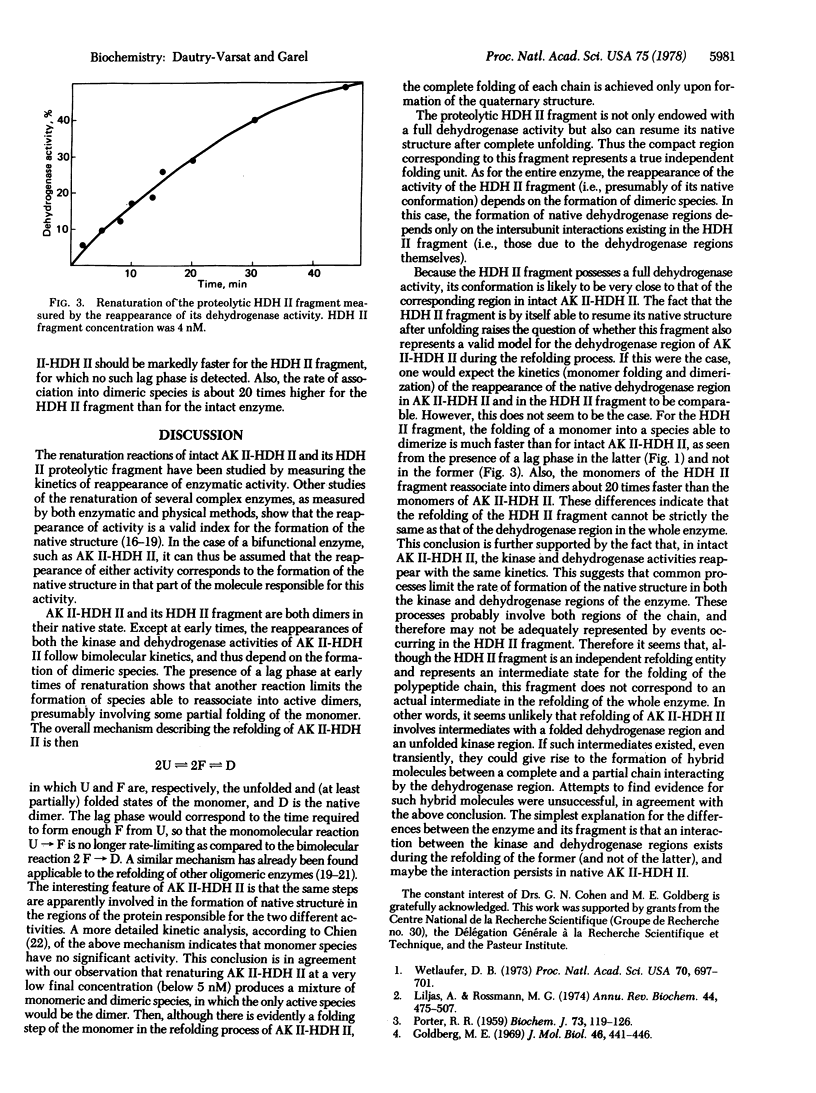
The renaturation of the bifunctional enzyme aspartokinase II-homoserine dehydrogenase II has been studied by using the reappearance of its two activities. The same kinetics of renaturation are obtained for the dehydrogenase (EC 1.1.1.3) and the kinase activity (EC 2.7.2.4). The mechanism of refolding of the enzyme apparently involves two steps, a folding step occurring within a monomer and a subsequent dimerization step. The reappearance of the two activities depends on this dimerization step, suggesting that monomeric species are inactive. A proteolytic fragment possessing full dehydrogenase activity is shown to be able to renature, as judged by the recovery of its activity. In this case also, the refolding depends on the formation of dimeric species. However, the refolding of this fragment is much faster than that of the dehydrogenase region in the intact enzyme. These results suggest that, although the dehydrogenase region can refold by itself when isolated as a fragment, refolding of this same region in the whole protein involves interactions with the remainder of the protein.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLACK S., WRIGHT N. G. Aspartic beta-semialdehyde dehydrogenase and aspartic beta-semialdehyde. J Biol Chem. 1955 Mar;213(1):39–50. [PubMed] [Google Scholar]
- Dautry-Varsat A., Cohen G. N. Proteolysis of the bifunctional methionine-repressible aspartokinase II-homoserine dehydrogenase II of Escherichia coli K12. Production of an active homoserine dehydrogenase fragment. J Biol Chem. 1977 Nov 10;252(21):7685–7689. [PubMed] [Google Scholar]
- Engelhard M., Rudolph R., Jaenicke R. Equilibrium studies on the refolding and reactivation of rabbit-muscle aldolase after acid dissociation. Eur J Biochem. 1976 Aug 16;67(2):447–453. doi: 10.1111/j.1432-1033.1976.tb10709.x. [DOI] [PubMed] [Google Scholar]
- Falcoz-Kelly F., van Rapenbusch R., Cohen G. N. The methionine-repressible homoserine dehydrogenase and aspartokinase activities of Escherichia coli K 12. Preparation of the homogeneous protein catalyzing the two activities. Molecular weight of the native enzyme and of its subunits. Eur J Biochem. 1969 Mar;8(1):146–152. doi: 10.1111/j.1432-1033.1969.tb00507.x. [DOI] [PubMed] [Google Scholar]
- Goldberg M. E. Tertiary structure of Escherichia coli beta-D-galactosidase. J Mol Biol. 1969 Dec 28;46(3):441–446. doi: 10.1016/0022-2836(69)90187-9. [DOI] [PubMed] [Google Scholar]
- Högberg-Raibaud A., Goldberg M. E. Preparation and characterization of a modified form of beta2 subunit of Escherichia coli tryptophan synthetase suitable for investigating protein folding. Proc Natl Acad Sci U S A. 1977 Feb;74(2):442–446. doi: 10.1073/pnas.74.2.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenicke R. Reassociation and reactivation of lactic dehydrogenase from the unfolded subunits. Eur J Biochem. 1974 Jul 1;46(1):149–155. doi: 10.1111/j.1432-1033.1974.tb03607.x. [DOI] [PubMed] [Google Scholar]
- PORTER R. R. The hydrolysis of rabbit y-globulin and antibodies with crystalline papain. Biochem J. 1959 Sep;73:119–126. doi: 10.1042/bj0730119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patte J. C., Le Bras G., Cohen G. N. Regulation by methionine of the synthesis of a third aspartokinase and of a second homoserine dehydrogenase in Escherichia coli K 12. Biochim Biophys Acta. 1967 Mar 22;136(2):245–247. doi: 10.1016/0304-4165(67)90069-4. [DOI] [PubMed] [Google Scholar]
- Platt T., Files J. G., Weber K. Lac repressor. Specific proteolytic destruction of the NH 2 -terminal region and loss of the deoxyribonucleic acid-binding activity. J Biol Chem. 1973 Jan 10;248(1):110–121. [PubMed] [Google Scholar]
- Rudolph R., Heider I., Westhof E., Jaenicke R. Mechanism of refolding and reactivation of lactic dehydrogenase from pig heart after dissociation in various solvent media. Biochemistry. 1977 Jul 26;16(15):3384–3390. doi: 10.1021/bi00634a015. [DOI] [PubMed] [Google Scholar]
- Rudolph R., Westhof E., Jaenicke R. Kinetic analysis of the reactivation of rabbit muscle aldolase after denaturation with guanidine-HCL. FEBS Lett. 1977 Feb 1;73(2):204–206. doi: 10.1016/0014-5793(77)80981-2. [DOI] [PubMed] [Google Scholar]
- Setlow P., Kornberg A. Deoxyribonucleic acid polymerase: two distinct enzymes in one polypeptide. II. A proteolytic fragment containing the 5' leads to 3' exonuclease function. Restoration of intact enzyme functions from the two proteolytic fragments. J Biol Chem. 1972 Jan 10;247(1):232–240. [PubMed] [Google Scholar]
- Teipel J. W., Koshland D. E., Jr Kinetic aspects of conformational changes in proteins. I. Rate of regain of enzyme activity from denatured proteins. Biochemistry. 1971 Mar 2;10(5):792–798. doi: 10.1021/bi00781a011. [DOI] [PubMed] [Google Scholar]
- Teipel J. W., Koshland D. E., Jr Kineticsspects of conformational changes in proteins. II. Structural changes in renaturation of denatured proteins. Biochemistry. 1971 Mar 2;10(5):798–805. doi: 10.1021/bi00781a012. [DOI] [PubMed] [Google Scholar]
- Véron M., Falcoz-Kelly F., Cohen G. N. The threonine-sensitive homoserine dehydrogenase and aspartokinase activities of Escherichia coli K12. The two catalytic activities are carried by two independent regions of the polypeptide chain. Eur J Biochem. 1972 Aug 4;28(4):520–527. doi: 10.1111/j.1432-1033.1972.tb01939.x. [DOI] [PubMed] [Google Scholar]
- Wampler D. E., Westhead E. W. Two aspartokinases from Escherichia coli. Nature of the inhibition and molecular changes accompanying reversible inactivation. Biochemistry. 1968 May;7(5):1661–1671. doi: 10.1021/bi00845a007. [DOI] [PubMed] [Google Scholar]
- Wetlaufer D. B. Nucleation, rapid folding, and globular intrachain regions in proteins. Proc Natl Acad Sci U S A. 1973 Mar;70(3):697–701. doi: 10.1073/pnas.70.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]


