Abstract
Cystic fibrosis (CF) is a life-shortening, recessive, multiorgan genetic disorder caused by the loss of CF transmembrane conductance regulator (CFTR) chloride channel function found in many types of epithelia. Animal models that recapitulate the human disease phenotype are critical to understanding pathophysiology in CF and developing therapies. CFTR knockout ferrets manifest many of the phenotypes observed in the human disease, including lung infections, pancreatic disease and diabetes, liver disease, malnutrition, and meconium ileus. In the present study, we have characterized abnormalities in the bioelectric properties of the trachea, stomach, intestine, and gallbladder of newborn CF ferrets. Short-circuit current (ISC) analysis of CF and wild-type (WT) tracheas revealed the following similarities and differences: (1) amiloride-sensitive sodium currents were similar between genotypes; (2) responses to 4,4′-diisothiocyano-2,2′-stilbene disulphonic acid were 3.3-fold greater in CF animals, suggesting elevated baseline chloride transport through non-CFTR channels in a subset of CF animals; and (3) a lack of 3-isobutyl-1-methylxanthine (IBMX)/forskolin–stimulated and N-(2-Naphthalenyl)-((3,5-dibromo-2,4-dihydroxyphenyl)methylene)glycine hydrazide (GlyH-101)–inhibited currents in CF animals due to the lack of CFTR. CFTR mRNA was present throughout all levels of the WT ferret and IBMX/forskolin–inducible ISC was only observed in WT animals. However, despite the lack of CFTR function in the knockout ferret, the luminal pH of the CF ferret gallbladder, stomach, and intestines was not significantly changed relative to WT. The WT stomach and gallbladder exhibited significantly enhanced IBMX/forskolin ISC responses and inhibition by GlyH-101 relative to CF samples. These findings demonstrate that multiple organs affected by disease in the CF ferret have bioelectric abnormalities consistent with the lack of cAMP-mediated chloride transport.
Keywords: cystic fibrosis, animal model, ferret, intestine, trachea
Clinical Relevance
Cystic fibrosis (CF) is a lethal multiorgan disease that affects anion transport in multiple epithelia throughout the body. This study provides the first characterization of bioelectric defects observed in four organs of a new CF ferret model.
Cystic fibrosis (CF) is the most common life-threatening, autosomal recessive, genetic disorder among Caucasians, occurring in approximately 1 in 3,500 births. Mutations to the CF transmembrane conductance regulator (CFTR) gene and the resulting protein product cause CF (1). The most common disease causing mutation is the deletion of phenylalanine 508 (∆F508) occurring in at least one allele of roughly 90% of patients with CF (2). CFTR acts primarily as an epithelial chloride channel that aids in homeostatic transport of ions and water in many organs throughout the body. Disruption of this channel leads to impaired chloride and bicarbonate secretion that causes dehydrated viscous mucus in many epithelia of the human body. These abnormalities are thought to be the underlying cause of the development of chronic lung disease, the primary cause of mortality in patients with CF. The gastrointestinal tract, gallbladder, pancreas, liver, reproductive tract, and sweat ducts are other organs significantly affected in patients with CF (2–4).
Animal models that accurately reproduce the CF human disease phenotype are critical to understanding the disease process and developing therapies. To date, there are three reported species of CF animal models, including the mouse, pig, and ferret (5–8). Characterization of each of these models has clearly demonstrated that species-specific differences in organ physiology and CFTR biology influence the extent of pathologic and electrophysiologic changes observed when CFTR is deleted or mutated (8–11). CF ferrets have been shown to develop intestinal complications, diabetes, pancreatic disease, absence of the vas deferens, growth retardation, liver disease, and an elevated risk of developing lung infections (9, 11–13).
The airways have been, and will continue to be, a focus of CF research, as lung disease is the main cause of patient morbidity and mortality (2, 3). Bioelectric properties of the trachea are the most studied among the different CF animal models. CFTR in tracheal epithelium has been shown to be the primary cAMP-inducible chloride channel in humans, pigs, and ferrets (12, 14). However, mouse trachea has an alternative cAMP-inducible chloride channel in addition to CFTR—possibly one of the reasons that CF mouse models fail to develop spontaneous chronic lung disease like that seen in the other three species (5, 15).
There are two major controversial hypotheses regarding the initial CFTR-dependent mechanism that leads to the development of CF lung disease (9). The first hypothesis generally states that CFTR negatively regulates the activity of epithelial sodium channels (ENaCs) (16, 17); removal of this negative regulation, due to the lack of CFTR, is thought to lead to hyperabsorption of Na+ ions—causing dehydration of the mucous lining the airways and failure to clear and kill bacteria. The second hypothesis states that the lack of Cl− and HCO3− movement through CFTR, not Na+ hyperabsorption, directly leads to impaired innate immunity in the airway. Recent analysis of the CFTR−/− pig model demonstrated that Na+ hyperabsorption does not occur in the newborn CF porcine airway epithelia, but that the primary defect involves the loss of cAMP-inducible Cl− and HCO3− permeability (14).
Intestinal complications are the most consistently observed phenotype among various CF species. Similar to humans with CF, the CF mouse intestinal epithelia largely lack electrogenic cAMP-inducible currents (5, 18–20), and generally have intestinal pathology due to the absence of the alternative cAMP-inducible Cl− channel seen in the trachea (5, 21). Intestines from infants with CF have been shown to have enhanced electrogenic Na+ absorption that is sensitive to amiloride (20); however, studies using human CF and non-CF rectal biopsies have failed to demonstrate significant differences in amiloride-sensitive ENaC current (22, 23). The majority of studies on mouse intestinal epithelia report minimal amiloride-sensitive electrogenic responses (5, 24). However, enhanced amiloride-sensitive electrogenic Na+ absorption in the colon of CF versus non-CF mouse intestine was observed when animals were maintained on a low-sodium diet (24). Another source of electrogenic absorption of Na+ in the intestine is through the apical Na+–glucose transporter (SLGT1). This transporter has been shown in some studies to have increased activity in human CF tissues relative to controls (18, 19), whereas another report showed no difference between genotypes (20). Furthermore, the activity of this channel is generally accepted to be higher in the small intestine than in the colon (25–27). Electrophysiologic characterization of the ferret and pig CF proximal to distal intestinal epithelia has yet to be reported.
The stomach produces large amounts of HCl, generated by parietal cells lining the gastric mucosa that promotes the digestive process. It is well accepted that the process of acid generation depends upon the secretion of protons by the H+/K+-ATPase, channel-dependent secretion of Cl−, and the recycling of K+ (28). CFTR is hypothesized to be one of the pathways for Cl− secretion, because CFTR mutations and inhibitors reduce gastric secretion (29). Short-circuit current (ISC) studies of murine gastric mucosa have been shown to be sensitive to cAMP induction and subsequent inhibition of the basolateral Cl− source with bumetanide—suggestive of CFTR activity (30). Studies in CF ferrets and pigs would be helpful in evaluating the importance of CFTR in the production of gastric HCl.
Gallbladder abnormalities, including cholelithiasis (gallstones) and microgallbladder, occur in 15–30% of patients with CF (31, 32). The pathology of the CF gallbladder between human, ferret, pig, and mouse is highly variable (9). The ferret gallbladder is grossly and histologically indistinguishable from non-CF animals (12). All CF pigs are born with microgallbladder, and their gallbladders are often filled with a thick mucus relative to non-CF controls (33). Gross pathology in the CF mouse gallbladder is minimal, but CF gallbladders lack cAMP-inducible Cl− and liquid secretion observed in wild-type (WT) controls, demonstrating that CFTR is the main pathway for liquid secretion in the mouse gallbladder (8). High levels of CFTR mRNA have been shown to exist in the human and mouse gallbladder epithelium (5, 34, 35). In addition, non-CF human gallbladder epithelia have been shown to produce cAMP-inducible electrogenic Cl− secretion that is absent in CF tissues (36, 37). Functional studies on ferret and pig CF and normal gallbladder epithelia have yet to be reported.
In this study, we evaluated the bioelectric properties of the newborn CF and WT ferret trachea, intestine, stomach, and gallbladder. The major goal of this study was to define changes in electrogenic movement of sodium and chloride in these various epithelia between genotypes. All CF organs tested retained significantly reduced cAMP-induced chloride currents relative to WT controls. CFTR mRNA was present throughout all levels of the WT intestine without any significant regional trends in expression. Interestingly, CF ferret intestines produced significantly greater electrogenic sodium absorption in response to application of apical glucose. In the trachea, changes in sodium currents in response to amiloride were not different between genotypes; however, some CF animals demonstrated much greater changes in 4,4′-diisothiocyano-2,2′-stilbene disulphonic acid (DIDS)–responsive chloride currents, suggesting that the activity of non-CFTR chloride channels may be elevated in a subset of CF animals. Finally, studies evaluating the pH of the gastrointestinal tract demonstrated no significant differences in lumenal pH of the stomach, gallbladder, and intestine between genotypes. These studies characterizing bioelectric abnormalities in multiple types of epithelia from the CF ferret model provide useful information on which to better understand CF pathophysiology in each of these organs.
Materials and Methods
Animal Usage and Tissue Harvest
All experimentation involving ferrets was performed using protocols approved by the Institutional Animal Care and Use Committees of the University of Iowa. Details of genotyping are described elsewhere (12). The animals used in this report ranged from 4 to 24 hours of age. In all electrophysiologic experiments, CF (CFTR−/−) and WT (CFTR+/+) samples were evaluated. The intestine was quickly excised, flushed with warm Ringer’s solution, and divided into four equal-length segments (R1, R2, R3, and R4) before ISC measurement or snap freezing in liquid nitrogen for RNA analysis. The stomach, gallbladder, and trachea were also harvested and opened longitudinally for electrophysiologic measurements.
Quantitative PCR
CFTR and GAPDH transcripts levels were used to compare the relative abundance of CFTR message throughout the four regions of the ferret intestine. Frozen pulverized intestinal tissue was ground in a mortar and pestle under liquid nitrogen; 100 mg of the homogenized tissue powder was used for preparation of total RNA using TRIzol reagent (Invitrogen, Carlsbad, CA). First-strand cDNA was synthesized using 2 μg of total RNA, the SuperScript III First-Strand Synthesis System (Invitrogen), and GAPDH and CFTR primers. GAPDH (302 bp amplicon): forward, 5′-AGCAATGCCTCCTGTACCACCA-3′; reverse 5′-CGGCAGGTCAGATCCACAACAG-3′. CFTR (307 bp amplicon): forward 5′-TGGTGCTCCTGTCCTGAAAGATATC-3′; reverse 5′-CCTTCTCTGCAAACTTGGAGATGTC-3′. Quantitative RT-PCR was performed using the iCycler iQ5 (Bio-Rad Laboratories, Hercules, CA) with Power SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA). The average Ct values for duplicate PCR runs were used to calculate the relative abundance of CFTR/glyceraldehyde 3-phosphate dehydrogenase (GAPDH) transcripts for each sample using the ∆Ct method (relative abundance of CFTR/GAPDH transcripts = 2−(average CFTR:Ct − average GAPDH:Ct)).
Ussing Chamber Analysis
Freshly harvested newborn ferret WT and CF trachea, stomach, intestine, and gallbladder were opened longitudinally and mounted in a P2300 Ussing chamber system using the P2307–2mm sliders (Physiologic Instruments, San Diego, CA). All tissues were harvested into Ringer’s solution containing 1 μM indomethacin, and this level of indomethacin was maintained throughout the experiment to eliminate stimulation of cAMP production by endogenous prostaglandins (22, 38). ISC and resistances (R) were measured using a VCC MC8 Multichannel voltage/current clamp (Physiologic Instruments) as previously reported (10, 39). For more details on electrophysiologic measurements see the online supplement.
Luminal pH Measurements
Luminal pH measurements of the stomach, gallbladder, and four regions of the intestine were made using a 500-μm pH electrode, 100-μm reference electrode, and pH/mV Meter Measuring System and software (Unisense, Aarhus, Denmark). For more details see the online supplement.
Statistical Analysis
Changes in ISC after each drug were analyzed by a mixed-effects model, where genotype (and region for intestine) was a fixed effect and litter and tissue were random effects to account for the relatedness in animals from the same litter and multiple tissues from the same animal. Isc analysis was conducted using PROC MIXED in SAS version 9.3 (SAS Institute, Cary, NC). Intestinal mRNA comparisons were made by simple repeated measures in SPSS version 18 (Chicago, IL), with Bonferroni’s post hoc comparisons. The pH comparisons were made by MANOVA in SPSS version 18.
Results
CFTR Knockout Ferret Tracheal Epithelia Demonstrate Abnormalities in cAMP-Inducible and DIDS-Sensitive Anion Currents Relative to WT Controls
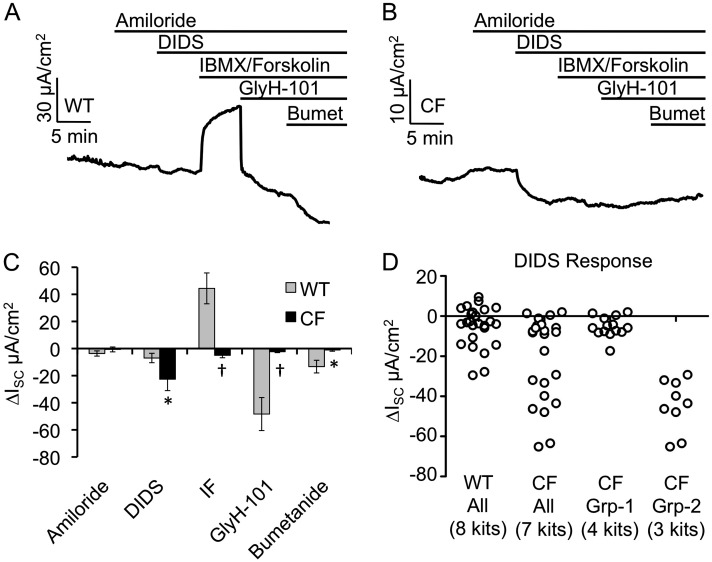
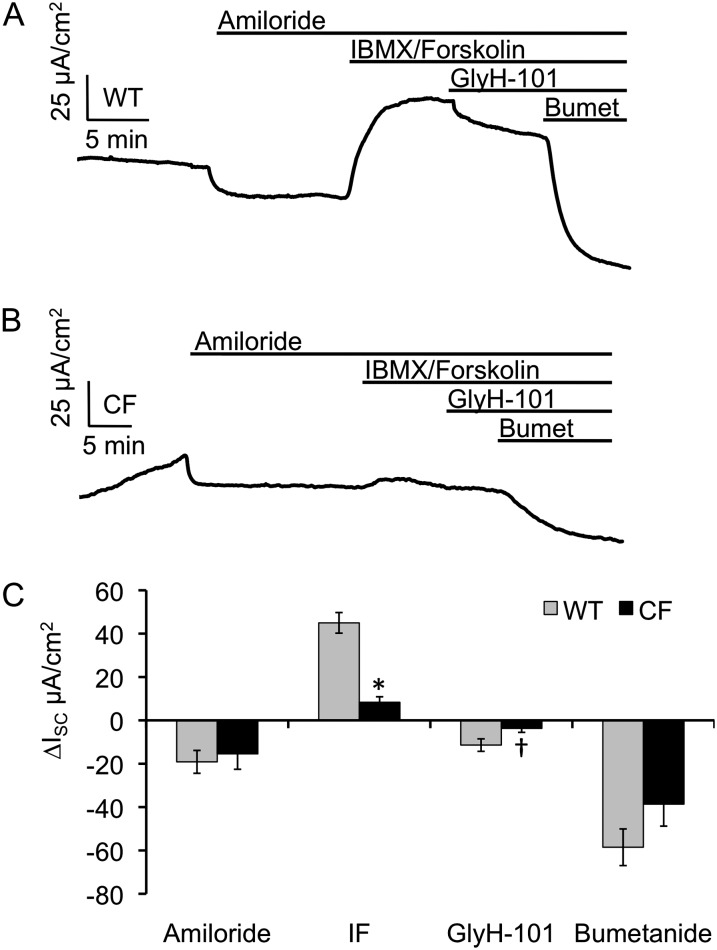
ISC of CF and WT newborn tracheal epithelia were measured after the sequential addition of amiloride, DIDS, 3-isobutyl-1-methylxanthine (IBMX), and forskolin, N-(2-Naphthalenyl)-((3,5-dibromo-2,4-dihydroxyphenyl)methylene)glycine hydrazide (GlyH-101), and bumetanide (Figures 1A and 1B and Table E1 in the online supplement). The WT ISC responses to each of these compounds were remarkably similar to previously published data using polarized cultured adult ferret tracheal epithelia (40). Amiloride was used to inhibit ENaCs and resulted in ISC changes of −3.65 (±1.93) and −0.64 (±1.8) μA/cm2 for WT and CF, respectively (Figure 1C). This nonsignificant difference between genotypes is consistent with other reports evaluating amiloride-sensitive transepithelial potential difference in a ferret tracheal xenograft model (10–12). The addition of DIDS, an inhibitor of the majority of non-CFTR electrogenic epithelial chloride channels, inhibited transepithelial current to a significantly greater extent in CF relative to WT tracheas (P = 0.0401; Figure 1C and Table E1). Interestingly, the variance of the DIDS response was significantly greater (fivefold) for CF animals relative to the WT littermates (P < 0.05 by F test) (Figure 1D). Evaluation of the distribution of ΔISC DIDS responses for each tissue and animal evaluated (Figure E1) demonstrated that the observed increased variance in CF animals was attributable to higher responses in a subset of animals. This variability between CF animals might be explained by heritable modifier genes that act to compensate for the loss of CFTR in CF airways through the hyperactivation of a compensatory chloride channel. Of note, we evaluated the addition of UTP in the presence of amiloride, which gave no significant genotypic differences in current or the variance of current—in the presence of 100 μM amiloride, changes in tracheal ISC in response to 100 μM UTP were 5.3 (±2.1) μA/cm2 for CF animals (n = 7 animals) and 6.4 (±1.3) μA/cm2 for non-CF animals (n = 6 animals). This observation suggests that purinergic P2Y2 receptor–stimulated Ca+2-activated chloride channels may not be responsible for the enhanced electrogenic anion transport observed in CF ferret tracheas.
Figure 1.
Bioelectric characterization of the cystic fibrosis (CF) and wild-type (WT) neonatal ferret trachea. Newborn ferret CF and WT tracheas were mounted in Ussing chambers and short-circuit current (ISC) measured with sequential addition of amiloride, 4,4′-diisothiocyano-2,2′-stilbene disulphonic acid (DIDS), 3-isobutyl-1-methylxanthine (IBMX)/forskolin, N-(2-Naphthalenyl)-((3,5-dibromo-2,4-dihydroxyphenyl)methylene)glycine hydrazide (GlyH-101), and bumetanide (Bumet). (A and B) Representative ISC tracings for (A) WT and (B) CF tracheas. (C) Quantification of the change in ISC for each of the indicated drugs. Values depict the mean ± SEM for n = 8 WT kits (25 measurements) and n = 7 CF kits (23 measurements). The average values for multiple tissues from each animal were used to calculate the mean and SEM. (D) Vertical scatter plot of the individual DIDS ∆ISC measurements from data shown in (C) with the CF animals also split into two groups based on differences in responses. Marked comparisons demonstrate significant differences between WT and CF as determined by a mixed model repeated measures analysis (*P < 0.05, †P < 0.003). IF, IBMX/forskolin.
As expected, the addition of cAMP agonists (IBMX and forskolin) induced ISC to a significantly greater extent (P = 0.0008) in WT than in CF tracheal tissues (Figure 1C). The subsequent inhibition of the cAMP-induced CFTR-dependent ISC with GlyH-101 was also greater in WT samples (P = 0.0029), consistent with the greater response to IBMX/forskolin. Inhibition of the basolateral Na-K-Cl (NKCC1) cotransporter with bumetanide also led to significantly different responses between genotypes (P = 0.0276), with little or no change in CF animals. Interestingly the effect of summing the GlyH-101 and bumetanide responses for WT animals resulted in a net change in ISC of 61.7 μA/cm2, which was significantly greater than IBMX/forskolin–inducible ISC of 44.4 μA/cm2. This difference in current of 17.3 μA/cm2 supports previous findings seen in ferret airway epithelial cultures, and suggests that there is substantial basal CFTR activation in the WT ferret trachea (40).
CFTR Is Expressed throughout the Ferret Intestine
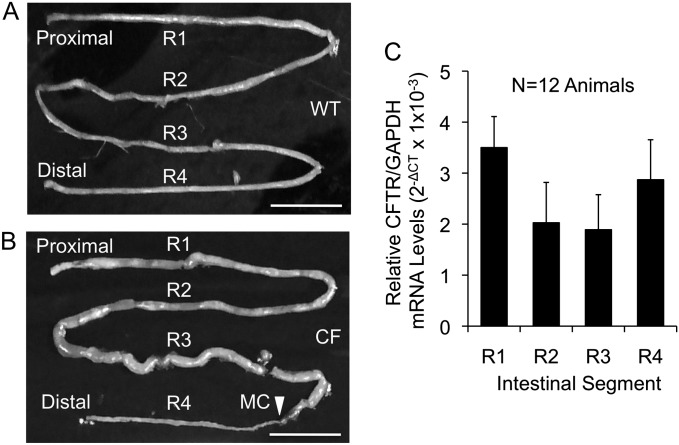
One of the roles of CFTR in the intestine is to aid in the hydration of the luminal contents by promoting secretion of chloride and bicarbonate, primarily from the intestinal crypts of Lieberkühn. Unlike humans, mice, and pigs, the ferret intestinal tract lacks a cecum and ileocolic valve (41). The relatively short ferret intestine is unique in that there are no gross anatomical landmarks by which to differentiate regions of the small intestine and colon at birth (42). Therefore, with the goal of evaluating the distribution of CFTR expression throughout the ferret intestinal tract, we divided the intestine equally by length into four segments (R1, R2, R3, and R4), as shown for WT and CF intestines in Figures 2A and 2B. These segments were then used to evaluate CFTR mRNA expression (Figure 2C). Ferret CFTR and GAPDH mRNA levels were analyzed by quantitative RT-PCR, and the relative CFTR levels are shown as 2−ΔCT values in Figure 2C. No significant differences in CFTR mRNA were observed along the length of the WT ferret intestine. These findings are different from CFTR mRNA in situ hybridization studies on the human gut suggesting that CFTR is more highly expressed in the small intestine relative to the colon (34).
Figure 2.
Ferret intestinal CF transmembrane conductance regulator (CFTR) expression is observed through all regions of the ferret intestine. (A and B) Gross images of newborn WT and CF intestines and methods for region assignments. Each intestine was divided into four equal length regions (R1–R4) as indicated. The arrowhead (B) indicates the start of microcolon (MC) in the CF example. Scale bars represent 2 cm. (C) Tissue from midportion of each of the indicated regions was used for RNA analysis. Quantitative RT PCR was performed on RNA to determine the relative abundance of ferret CFTR mRNA normalized to GAPDH mRNA levels. Data represent the mean ± SEM 2−ΔCT (n = 12 animals for each genotype). There was no statistical difference between regions by simple repeated measures analysis.
Proximal to Distal Characterization of the Bioelectric Properties of WT and CF Ferret Intestine
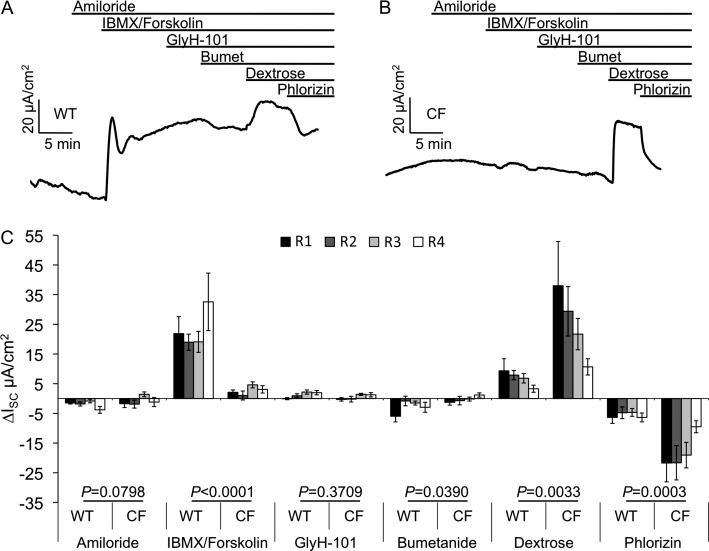
To evaluate electrophysiologic changes in the intestine caused by the lack of CFTR, we performed ISC measurements on R1–R4 regions of CF and WT newborn ferret intestine (Figure 3 and Tables E2 and E3). Changes in ISC after amiloride treatment, to block ENaC-mediated sodium transport, were minimal between the different intestinal segments and between genotypes. As expected, the addition of the cAMP agonists, IBMX/forskolin, significantly (P < 0.0001) increased ISC in WT relative to the corresponding CF tissues, which lacked substantial responses. Consistent with CFTR mRNA levels not changing significantly between intestinal segments (Figure 2C), cAMP-inducible ISC demonstrated no significant regional trends (P = 0.2303). Surprisingly, unlike WT tracheas, addition of the CFTR inhibitor, GlyH-101, failed to inhibit cAMP-inducible ISC in WT intestine, suggesting organ-specific differences in the efficacy of this CFTR inhibitor (Figure 3C). Others have also reported similar findings in the intestine of other species when using GlyH-101 (43, 44). We also did not observe inhibition of cAMP-mediated anion currents in response to CFTRinh172, 5-nitro-2(3-phenylpropyl-amino)benzoate (NPPB) and glibenclamide (data not shown). As in human intestine (19, 20), ferret intestine also demonstrated a lack of electrogenic response to DIDS, suggesting low or absent levels of electrogenic calcium-mediated chloride transport throughout the intestine (data not shown). Bumetanide, an inhibitor of the basolateral NKCC1 cotransporter, also marginally inhibited cAMP-inducible ISC in WT intestine, and had no effect on the CF intestine (Figure 3C). Finally, 20 mM dextrose, followed by 100 μM phlorizin, was added to the mucosal bath to test tissue viability. Addition of dextrose generates an electrogenic Na+ absorption via apically located SGLT1 channel. SGLT1 expression in other species has been shown to be the highest in the small intestine and significantly lower in the colon (25–27). In agreement with other species, we observed a proximal-to-distal gradient (R1 > R4) of dextrose-stimulated ISC in both CF and WT intestines (P = 0.0115). Interestingly the dextrose-stimulated currents were significantly higher in CF intestines relative to WT (P = 0.0033; Figure 3C and Tables E2 and E3). Overall, these differences in electrogenic ion transport between CF and WT intestine, most notably IBMX/forskolin response (P < 0.0001), demonstrate that the CF ferret intestine has significantly altered electrogenic ion transport.
Figure 3.
Proximal to distal characterization of the bioelectric properties of WT and CF ferret intestine. Newborn WT and CF ferret intestine was divided into four regions and mounted in Ussing chambers. ISC was then measured after the sequential addition of amiloride, IBMX/forskolin, GlyH-101, bumetanide, dextrose, and phlorizin. (A and B) Representative ISC tracings for WT and CF intestines, respectively. (C) Quantification of the ∆ISC for each of the intestinal regions (R1, black; R2, dark gray; R3, light gray; and R4, white). Data represent the mean ± SEM (WT: n = 8 kits with 11–13 measurements; CF: n = 9 kits with 10–12 measurements). The average values for multiple tissues from each animal were used to calculate the mean and SEM. P values for comparisons between genotypes, as determined by mixed model repeated measures analysis, are indicated above each treatment.
Gastric, Intestinal, and Gallbladder Luminal pH Is Not Altered in Newborn CF Ferrets
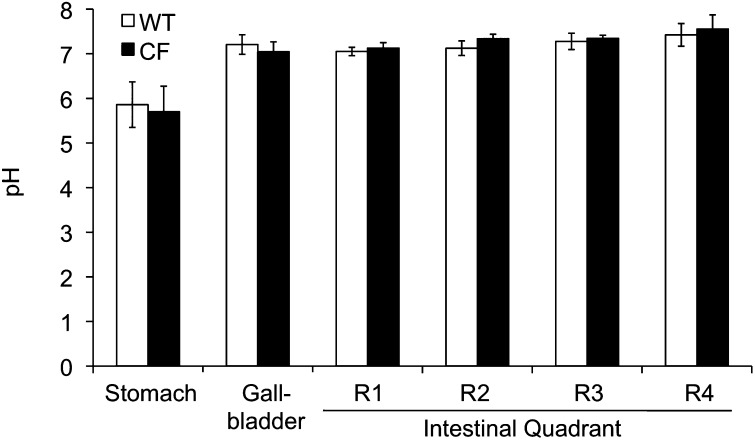
In CFTR-knockout (KO) ferrets, growth retardation can be partially alleviated by oral administration of omeprazole (a proton pump inhibitor). We therefore hypothesized that there may be a CFTR-dependent change in gastrointestinal pH that is important for nutrient absorption (12). To test this hypothesis, we evaluated how the lack of CFTR influenced the luminal pH of the stomach, gallbladder, and gastrointestinal tract. Results from these studies demonstrated that pH was not significantly different between genotypes for the intestine, stomach, or gallbladder (Figure 4). These results differ from those from other species tested, where duodenal pH is significantly lower in humans with CF (45) and CFTR-KO mice (46, 47). It should be noted, however, that the pH of the gastrointestinal tract is significantly influenced by feeding, and this was not controlled for in the current studies.
Figure 4.
The pH of the stomach, gallbladder, and intestine of the CF ferret is not different relative to WT animals. The luminal pH of the stomach, gallbladder, and intestine were measured immediately after killing of WT (open bars) and CF (solid bars) newborn kits. These measurements were made using a pH and reference electrode from Unisense, as described in the Materials and Methods section. The chart represents the quantification of the pH of the stomach, gallbladder, and four quadrants of the intestine. Data represent the mean ± SEM pH from seven CF and eight WT kits with one measurement per animal. There were no significant differences between genotypes for any of these organs.
Bioelectric Characterization of the Newborn Ferret Stomach and Gallbladder
Next, we sought to measure ISC on the newborn ferret stomach using the same ISC protocol employed with the intestine (Figure 5 and Table E4). There was no significant difference among genotypes in the amiloride response. However, as expected, the change in ISC after IBMX/forskolin treatment was significantly greater in WT animals relative to the CF animals (P < 0.0001). WT animals also showed increased inhibition by GlyH-101 in comparison to CF animals (P = 0.0227); however, GlyH-101 was only able to inhibit 25% of the cAMP-induced ISC response. Furthermore, unlike the intestine, there was an equally significant decrease in ISC for both genotypes upon inhibition of NKCC1 with bumetanide, with no difference between genotypes (P = 0.1030). This suggests that there are likely non-CFTR Cl− channels that control a major portion of electrogenic current in the ferret stomach.
Figure 5.
Bioelectric characterization of the CF and WT neonatal ferret stomach. Newborn WT and CF ferret stomach was mounted in Ussing chambers and ISC was measured after sequential addition of amiloride, IBMX/forskolin, GlyH-101, and bumetanide. (A and B) Representative ISC tracings for WT and CF stomach respectively. (C) Quantification of the ∆ISC of WT (gray) and CF (black) stomachs. Data represent the mean ± SEM (WT: n = 8 kits; CF: n = 6 kits) with one measurement per animal. Marked comparisons demonstrate significant differences between genotypes as determined by mixed model repeated measures analysis (*P < 0.0001, †P = 0.0227).
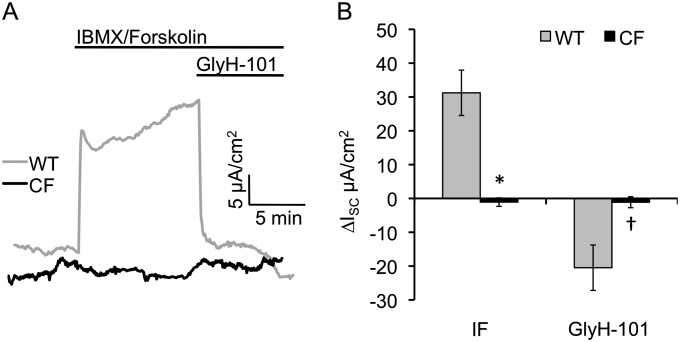
Many patients with CF will develop complications of the gallbladder due to viscous bile and the development of gallstones that are known to increase the risk of pancreatitis (31, 36). The newborn CF ferret gallbladder is histologically indistinguishable from the non-CF littermates (12). Given the lack of pathology in the newborn CF ferret gallbladder, we hypothesized that alternative, non-CFTR cAMP-inducible pathways for electrogenic chloride movement might exist in the gallbladder. Contrary to this hypothesis, IBMX/forskolin induced significantly larger Cl− currents in WT relative to CF gallbladder samples (P = 0.0003). Furthermore, GlyH-101 inhibited IBMX/forskolin–induced Cl− currents in the gallbladder of WT, but not CF, animals (P = 0.0136; Figure 6 and Table E5). Although there are no early histopathologic differences between newborn CF and WT ferret gallbladders, these data demonstrate that CFTR is functional in the ferret gallbladder, and that older CF ferrets may still be predisposed to gallbladder disease.
Figure 6.
Bioelectric characterization of the CF and WT neonatal ferret gallbladder. Newborn WT and CF ferret gallbladder was mounted in Ussing chambers and ISC was measured after sequential addition of amiloride, IBMX/forskolin, GlyH-101, and bumetanide. (A) Representative ISC tracings for WT (gray) and CF (black) gallbladder respectively. (B) Quantification of the ∆ISC response in the gallbladders of WT (gray) and CF (black). Data represent the mean ± SEM (WT: n = 13 kits; CF: n = 14 kits) with one measurement per animal. Marked comparisons demonstrate significant differences between genotypes as determined by mixed model repeated measures analysis (*P = 0.0003, †P = 0.0136).
Discussion
ISC analysis of the trachea, stomach, intestine, and gallbladder functionally confirmed the expected lack of CFTR in neonatal CF ferret organs, as evident by the lack of cAMP-inducible changes in current as seen in the WT controls. Interestingly, the inhibition of these currents with GlyH-101 was organ specific. Previous findings have shown that species-specific differences exist in the sensitivities of human, pig, ferret, and mouse CFTR to different CFTR inhibitors using cultured primary polarized airway epithelia (40). In the ferret, GlyH-101 was most effective at inhibiting cAMP-mediated electrogenic chloride secretion in the airway epithelium (40). In WT trachea, GlyH-101 inhibited all of the cAMP-mediated chloride current assigned to CFTR based on the lack of current in CF animals. This is in agreement with other studies on CFTR using newborn ferret tracheal xenograft transepithelial potential difference measurements and ectopic expression studies of ferret CFTR in polarized CF human airway epithelia (10–12). The percent GlyH-101–sensitive inhibition of the IBMX/forskolin–stimulated currents was less or absent in the gallbladder (66%), stomach (25%), and intestine (0%). Furthermore, IBMX/forskolin–stimulated current in the WT intestine was also nonresponsive to bumetanide, CFTRinh172, NPPB, and glibenclamide (Figure 3 and data not shown). One possible explanation for these observations is that apically applied compounds, such as GlyH-101, do not easily obtain access to the CFTR pore deep within the intestinal crypts of Lieberkühn and potentially the gastric glands due to the high level of fluid secretion. In the case of serosally applied bumetanide, we hypothesize that the muscular layers likely prevented bumetanide access to the NKCC1 transporter. Given the small size of the newborn ferret intestine, it was not possible to strip the muscular layer from the intestinal epithelium to test this hypothesis.
The increased variance of CF tracheal responses to DIDS, relative to WT, was another unexpected observation. The significantly higher variance was likely the result of two different groups of CF animals with normal and above-normal values (Figure 1D and Figure E1). One potential explanation for the broad range of DIDS responses in CF animals could be the existence of a modifier gene(s) that acts to increase apical localization of a DIDS-sensitive electrogenic anion channel in the absence of CFTR. This finding is consistent with enhanced Ca+2-activated chloride channel activity in tracheas of CFTR-KO mice (5, 15). Although such compensatory responses in CFTR-KO mice were uniform, the outbred nature of the ferret model and variable heritable penetrance of meconium ileus in CFTR-KO ferrets (12) is consistent with the existence of modifier genes that effect electrogenic anion transport in this outbred species. However, the lack of genotypic differences in tracheal currents produced after UTP stimulation appears to rule out purinergic P2Y2 receptor–stimulated Ca+2-activated chloride channels as a modifier influencing the variable DIDS response in CF ferrets.
Of all the CF models, the CF ferret appears to have the most severe defects in nutrition and growth. Oral administration of omeprazole (an inhibitor of H+/K+-ATPase) to CF ferrets improves weight gain (12), and also improves fat absorption in CF mice (48), suggesting that raising the gastrointestinal pH may be beneficial to growth. It was thus surprising that neither the stomach nor intestinal tract had reduced pH in the newborn CF ferrets. The chloride channel required for acid production by gastric glands is debated, with reports citing CFTR, CLC2, or an unknown channel as its source (28). Enhanced gastric acid secretion in patients with CF has been previously observed (49–51); however, these findings differ from studies in ΔF508-CFTR mice that exhibit reduced gastric acid secretion in comparison to control mice (52). Duodenal pH in humans with CF (45, 53) and CFTR-KO mice (46, 47) has also been shown to be lower than in non-CF controls. Although our studies demonstrated no differences in gastrointestinal pH in newborn CF ferrets, we cannot conclude that alterations in pH may occur with increased age and/or after feeding. Alternatively, the dissimilarity in findings between the various CF models may reflect species-specific differences between carnivores and omnivores and the manner in which food is digested in their gastrointestinal tract. A second potentially interesting finding relevant to defects in nutrient absorption was the observation that CF ferrets retained significantly higher (∼ fourfold) electrogenic Na+ absorption in response to glucose at all levels of the intestine, which was inhibited by phlorizin (an inhibitor of the SGLT1 transporter). This enhanced activity of the SGLT1 Na+/glucose transporter in CF could be caused by compensatory up-regulation of the channel to enhance glucose absorption by a dysfunctional intestine. Alternatively, hyperpolarization of the intestinal epithelium could occur in the absence of CFTR and create a greater electrogenic driving force for the absorption of Na+. Regardless of the mechanism for this observation, it could be particularly relevant to recent observations that CF ferrets have abnormal glucose tolerance and defects in insulin secretion (13).
In conclusion, previous reports have shown that the CF ferret develops many of the human disease phenotypes, including bacterial lung infections, CF-related diabetes, meconium ileus, malnourishment, pancreatic disease, and abnormalities of the vas deferens (9, 11–13). Comparative characterization of the bioelectric properties of epithelia from several organs of neonatal WT and CF ferrets has set the stage for a better understanding of the pathophysiologic mechanisms of CF disease in each of these organs.
Acknowledgments
Acknowledgments
The authors appreciate the expert advice received from Dr. Robert J. Bridges.
Footnotes
This work was supported by National Institutes of Health grants DK047967, DK096518, and HL108902 (J.F.E.), University of Iowa Center for Gene Therapy grant DK054759, and the Cystic Fibrosis Foundation.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2012-0433OC on June 19, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou JL, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- 2.Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med. 2005;352:1992–2001. doi: 10.1056/NEJMra043184. [DOI] [PubMed] [Google Scholar]
- 3.Rosenstein BJ, Zeitlin PL. Cystic fibrosis. Lancet. 1998;351:277–282. doi: 10.1016/S0140-6736(97)09174-5. [DOI] [PubMed] [Google Scholar]
- 4.Davis PB, Drumm M, Konstan MW. Cystic fibrosis. Am J Respir Crit Care Med. 1996;154:1229–1256. doi: 10.1164/ajrccm.154.5.8912731. [DOI] [PubMed] [Google Scholar]
- 5.Grubb BR, Boucher RC. Pathophysiology of gene-targeted mouse models for cystic fibrosis. Physiol Rev. 1999;79:S193–S214. doi: 10.1152/physrev.1999.79.1.S193. [DOI] [PubMed] [Google Scholar]
- 6.Rogers CS, Stoltz DA, Meyerholz DK, Ostedgaard LS, Rokhlina T, Taft PJ, Rogan MP, Pezzulo AA, Karp PH, Itani OA, et al. Disruption of the CFTR gene produces a model of cystic fibrosis in newborn pigs. Science. 2008;321:1837–1841. doi: 10.1126/science.1163600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun X, Yan Z, Yi Y, Li Z, Lei D, Rogers CS, Chen J, Zhang Y, Welsh MJ, Leno GH, et al. Adeno-associated virus–targeted disruption of the CFTR gene in cloned ferrets. J Clin Invest. 2008;118:1578–1583. doi: 10.1172/JCI34599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilke M, Buijs-Offerman RM, Aarbiou J, Colledge WH, Sheppard DN, Touqui L, Bot A, Jorna H, de Jonge HR, Scholte BJ. Mouse models of cystic fibrosis: phenotypic analysis and research applications. J Cyst Fibros. 2011;10:S152–S171. doi: 10.1016/S1569-1993(11)60020-9. [DOI] [PubMed] [Google Scholar]
- 9.Keiser NW, Engelhardt JF. New animal models of cystic fibrosis: what are they teaching us? Curr Opin Pulm Med. 2011;17:478–483. doi: 10.1097/MCP.0b013e32834b14c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fisher JT, Liu X, Yan Z, Luo M, Zhang Y, Zhou W, Lee BJ, Song Y, Guo C, Wang Y, et al. Comparative processing and function of human and ferret cystic fibrosis transmembrane conductance regulator. J Biol Chem. 2012;287:21673–21685. doi: 10.1074/jbc.M111.336537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fisher JT, Zhang Y, Engelhardt JF. Comparative biology of cystic fibrosis animal models. Methods Mol Biol. 2011;742:311–334. doi: 10.1007/978-1-61779-120-8_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun X, Sui H, Fisher JT, Yan Z, Liu X, Cho HJ, Joo NS, Zhang Y, Zhou W, Yi Y, et al. Disease phenotype of a ferret CFTR-knockout model of cystic fibrosis. J Clin Invest. 2010;120:3149–3160. doi: 10.1172/JCI43052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olivier AK, Yi Y, Sun X, Sui H, Liang B, Hu S, Xie W, Fisher JT, Keiser NW, Lei D, et al. Abnormal endocrine pancreas function at birth in cystic fibrosis ferrets. J Clin Invest. 2012;122:3755–3768. doi: 10.1172/JCI60610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen JH, Stoltz DA, Karp PH, Ernst SE, Pezzulo AA, Moninger TO, Rector MV, Reznikov LR, Launspach JL, Chaloner K, et al. Loss of anion transport without increased sodium absorption characterizes newborn porcine cystic fibrosis airway epithelia. Cell. 2010;143:911–923. doi: 10.1016/j.cell.2010.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grubb BR, Paradiso AM, Boucher RC. Anomalies in ion transport in cf mouse tracheal epithelium. Am J Physiol. 1994;267:C293–C300. doi: 10.1152/ajpcell.1994.267.1.C293. [DOI] [PubMed] [Google Scholar]
- 16.Boucher RC. Airway surface dehydration in cystic fibrosis: pathogenesis and therapy. Annu Rev Med. 2007;58:157–170. doi: 10.1146/annurev.med.58.071905.105316. [DOI] [PubMed] [Google Scholar]
- 17.Donaldson SH, Boucher RC. Sodium channels and cystic fibrosis. Chest. 2007;132:1631–1636. doi: 10.1378/chest.07-0288. [DOI] [PubMed] [Google Scholar]
- 18.O’Loughlin EV, Hunt DM, Gaskin KJ, Stiel D, Bruzuszcak IM, Martin HC, Bambach C, Smith R. Abnormal epithelial transport in cystic fibrosis jejunum. Am J Physiol. 1991;260:G758–G763. doi: 10.1152/ajpgi.1991.260.5.G758. [DOI] [PubMed] [Google Scholar]
- 19.Taylor CJ, Baxter PS, Hardcastle J, Hardcastle PT. Failure to induce secretion in jejunal biopsies from children with cystic fibrosis. Gut. 1988;29:957–962. doi: 10.1136/gut.29.7.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berschneider HM, Knowles MR, Azizkhan RG, Boucher RC, Tobey NA, Orlando RC, Powell DW. Altered intestinal chloride transport in cystic fibrosis. FASEB J. 1988;2:2625–2629. doi: 10.1096/fasebj.2.10.2838365. [DOI] [PubMed] [Google Scholar]
- 21.Clarke LL, Grubb BR, Yankaskas JR, Cotton CU, McKenzie A, Boucher RC. Relationship of a non–cystic fibrosis transmembrane conductance regulator–mediated chloride conductance to organ-level disease in CFTR(−/−) mice. Proc Natl Acad Sci USA. 1994;91:479–483. doi: 10.1073/pnas.91.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Veeze HJ, Halley DJ, Bijman J, de Jongste JC, de Jonge HR, Sinaasappel M. Determinants of mild clinical symptoms in cystic fibrosis patients: residual chloride secretion measured in rectal biopsies in relation to the genotype. J Clin Invest. 1994;93:461–466. doi: 10.1172/JCI116993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Veeze HJ, Sinaasappel M, Bijman J, Bouquet J, de Jonge HR. Ion transport abnormalities in rectal suction biopsies from children with cystic fibrosis. Gastroenterology. 1991;101:398–403. doi: 10.1016/0016-5085(91)90017-f. [DOI] [PubMed] [Google Scholar]
- 24.Grubb BR, Boucher RC. Enhanced colonic Na+ absorption in cystic fibrosis mice versus normal mice. Am J Physiol. 1997;272:G393–G400. doi: 10.1152/ajpgi.1997.272.2.G393. [DOI] [PubMed] [Google Scholar]
- 25.Garriga C, Rovira N, Moreto M, Planas JM. Expression of Na+-D-glucose cotransporter in brush-border membrane of the chicken intestine. Am J Physiol. 1999;276:R627–R631. doi: 10.1152/ajpregu.1999.276.2.R627. [DOI] [PubMed] [Google Scholar]
- 26.Lee WS, Kanai Y, Wells RG, Hediger MA. The high affinity Na+/glucose cotransporter: re-evaluation of function and distribution of expression. J Biol Chem. 1994;269:12032–12039. [PubMed] [Google Scholar]
- 27.Yoshikawa T, Inoue R, Matsumoto M, Yajima T, Ushida K, Iwanaga T. Comparative expression of hexose transporters (SGLT1, GLUT1, GLUT2 and GLUT5) throughout the mouse gastrointestinal tract. Histochem Cell Biol. 2011;135:183–194. doi: 10.1007/s00418-011-0779-1. [DOI] [PubMed] [Google Scholar]
- 28.Heitzmann D, Warth R. No potassium, no acid: K+ channels and gastric acid secretion. Physiology (Bethesda) 2007;22:335–341. doi: 10.1152/physiol.00016.2007. [DOI] [PubMed] [Google Scholar]
- 29.Schubert ML. Gastric secretion. Curr Opin Gastroenterol. 2010;26:598–603. doi: 10.1097/MOG.0b013e32833f2010. [DOI] [PubMed] [Google Scholar]
- 30.McDaniel N, Pace AJ, Spiegel S, Engelhardt R, Koller BH, Seidler U, Lytle C. Role of Na-K-2Cl cotransporter-1 in gastric secretion of nonacidic fluid and pepsinogen. Am J Physiol Gastrointest Liver Physiol. 2005;289:G550–G560. doi: 10.1152/ajpgi.00095.2005. [DOI] [PubMed] [Google Scholar]
- 31.Roy CC, Weber AM, Morin CL, Lepage G, Brisson G, Yousef I, Lasalle R. Hepatobiliary disease in cystic fibrosis: a survey of current issues and concepts. J Pediatr Gastroenterol Nutr. 1982;1:469–478. doi: 10.1097/00005176-198212000-00005. [DOI] [PubMed] [Google Scholar]
- 32.Wilschanski M, Durie PR. Patterns of GI disease in adulthood associated with mutations in the CFTR gene. Gut. 2007;56:1153–1163. doi: 10.1136/gut.2004.062786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meyerholz DK, Stoltz DA, Pezzulo AA, Welsh MJ. Pathology of gastrointestinal organs in a porcine model of cystic fibrosis. Am J Pathol. 2010;176:1377–1389. doi: 10.2353/ajpath.2010.090849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strong TV, Boehm K, Collins FS. Localization of cystic fibrosis transmembrane conductance regulator mRNA in the human gastrointestinal tract by in situ hybridization. J Clin Invest. 1994;93:347–354. doi: 10.1172/JCI116966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cohn JA, Strong TV, Picciotto MR, Nairn AC, Collins FS, Fitz JG. Localization of the cystic fibrosis transmembrane conductance regulator in human bile duct epithelial cells. Gastroenterology. 1993;105:1857–1864. doi: 10.1016/0016-5085(93)91085-v. [DOI] [PubMed] [Google Scholar]
- 36.Dray-Charier N, Paul A, Scoazec JY, Veissiere D, Mergey M, Capeau J, Soubrane O, Housset C. Expression of delta F508 cystic fibrosis transmembrane conductance regulator protein and related chloride transport properties in the gallbladder epithelium from cystic fibrosis patients. Hepatology. 1999;29:1624–1634. doi: 10.1002/hep.510290634. [DOI] [PubMed] [Google Scholar]
- 37.Chinet T, Fouassier L, Dray-Charier N, Imam-Ghali M, Morel H, Mergey M, Dousset B, Parc R, Paul A, Housset C. Regulation of electrogenic anion secretion in normal and cystic fibrosis gallbladder mucosa. Hepatology. 1999;29:5–13. doi: 10.1002/hep.510290142. [DOI] [PubMed] [Google Scholar]
- 38.Clarke LL, Argenzio RA. NaCl transport across equine proximal colon and the effect of endogenous prostanoids. Am J Physiol. 1990;259:G62–G69. doi: 10.1152/ajpgi.1990.259.1.G62. [DOI] [PubMed] [Google Scholar]
- 39.Xie W, Fisher JT, Lynch TJ, Luo M, Evans TI, Neff TL, Zhou W, Zhang Y, Ou Y, Bunnett NW, et al. CGRP induction in cystic fibrosis airways alters the submucosal gland progenitor cell niche in mice. J Clin Invest. 2011;121:3144–3158. doi: 10.1172/JCI41857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu X, Luo M, Zhang L, Ding W, Yan Z, Engelhardt JF. Bioelectric properties of chloride channels in human, pig, ferret, and mouse airway epithelia. Am J Respir Cell Mol Biol. 2007;36:313–323. doi: 10.1165/rcmb.2006-0286OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bell JA. Ferret nutrition. Vet Clin North Am Exot Anim Pract. 1999;2:169–192. doi: 10.1016/s1094-9194(17)30146-9. [DOI] [PubMed] [Google Scholar]
- 42.Bueno L, Fioramonti J, More J. Is there a functional large intestine in the ferret? Experientia. 1981;37:275–277. doi: 10.1007/BF01991652. [DOI] [PubMed] [Google Scholar]
- 43.Cehak A, Burmester M, Geburek F, Feige K, Breves G. Electrophysiological characterization of electrolyte and nutrient transport across the small intestine in horses. J Anim Physiol Anim Nutr (Berl) 2009;93:287–294. doi: 10.1111/j.1439-0396.2008.00882.x. [DOI] [PubMed] [Google Scholar]
- 44.Simpson JE, Walker NM, Supuran CT, Soleimani M, Clarke LL. Putative anion transporter-1 (Pat-1, Slc26a6) contributes to intracellular pH regulation during H+-dipeptide transport in duodenal villous epithelium. Am J Physiol Gastrointest Liver Physiol. 2010;298:G683–G691. doi: 10.1152/ajpgi.00293.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gelfond D, Ma C, Semler J, Borowitz D. Intestinal pH and gastrointestinal transit profiles in cystic fibrosis patients measured by wireless motility capsule. Dig Dis Sci. doi: 10.1007/s10620-012-2209-1. [DOI] [PubMed] [Google Scholar]
- 46.De Lisle RC, Isom KS, Ziemer D, Cotton CU. Changes in the exocrine pancreas secondary to altered small intestinal function in the CF mouse. Am J Physiol Gastrointest Liver Physiol. 2001;281:G899–G906. doi: 10.1152/ajpgi.2001.281.4.G899. [DOI] [PubMed] [Google Scholar]
- 47.Kaur S, Norkina O, Ziemer D, Samuelson LC, De Lisle RC. Acidic duodenal pH alters gene expression in the cystic fibrosis mouse pancreas. Am J Physiol Gastrointest Liver Physiol. 2004;287:G480–G490. doi: 10.1152/ajpgi.00035.2004. [DOI] [PubMed] [Google Scholar]
- 48.Bijvelds MJ, Bronsveld I, Havinga R, Sinaasappel M, de Jonge HR, Verkade HJ. Fat absorption in cystic fibrosis mice is impeded by defective lipolysis and post-lipolytic events. Am J Physiol Gastrointest Liver Physiol. 2005;288:G646–G653. doi: 10.1152/ajpgi.00295.2004. [DOI] [PubMed] [Google Scholar]
- 49.Cameron DJ, Pitcher-Wilmott R, Milla PJ, More J, Ghale GK, Matthew DJ, Harries JT. The effect of cimetidine on meal-stimulated gastric function and exogenous pancreatic enzymes in cystic fibrosis. Hum Nutr Clin Nutr. 1982;36:475–481. [PubMed] [Google Scholar]
- 50.Cox KL, Isenberg JN, Ament ME. Gastric acid hypersecretion in cystic fibrosis. J Pediatr Gastroenterol Nutr. 1982;1:559–565. doi: 10.1097/00005176-198212000-00019. [DOI] [PubMed] [Google Scholar]
- 51.Naimi D, Gueant JL, Hambaba L, Vidailhet M, Monin B, Nicolas JP. Gastric intrinsic factor hypersecretion stimulated by pentagastrin in cystic fibrosis. J Pediatr Gastroenterol Nutr. 1987;6:899–903. doi: 10.1097/00005176-198711000-00013. [DOI] [PubMed] [Google Scholar]
- 52.Sidani SM, Kirchhoff P, Socrates T, Stelter L, Ferreira E, Caputo C, Roberts KE, Bell RL, Egan ME, Geibel JP. DeltaF508 mutation results in impaired gastric acid secretion. J Biol Chem. 2007;282:6068–6074. doi: 10.1074/jbc.M608427200. [DOI] [PubMed] [Google Scholar]
- 53.Barraclough M, Taylor CJ. Twenty-four hour ambulatory gastric and duodenal pH profiles in cystic fibrosis: effect of duodenal hyperacidity on pancreatic enzyme function and fat absorption. J Pediatr Gastroenterol Nutr. 1996;23:45–50. doi: 10.1097/00005176-199607000-00009. [DOI] [PubMed] [Google Scholar]