Abstract
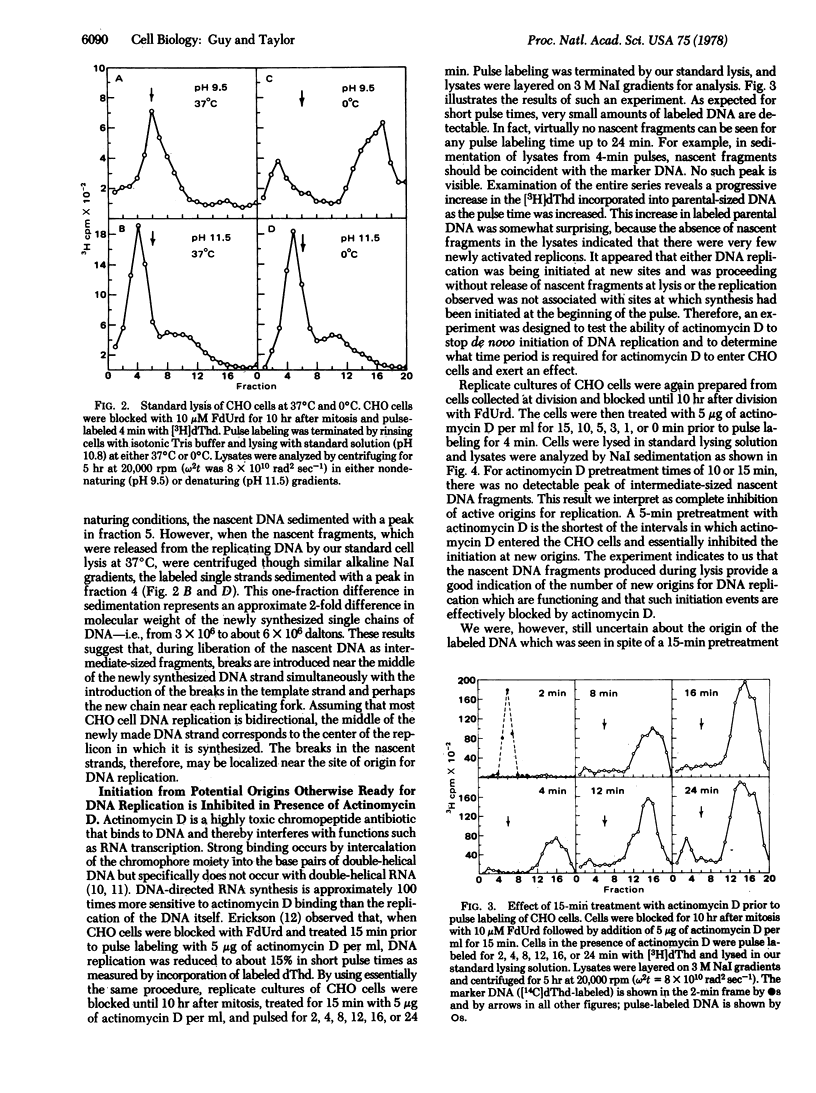
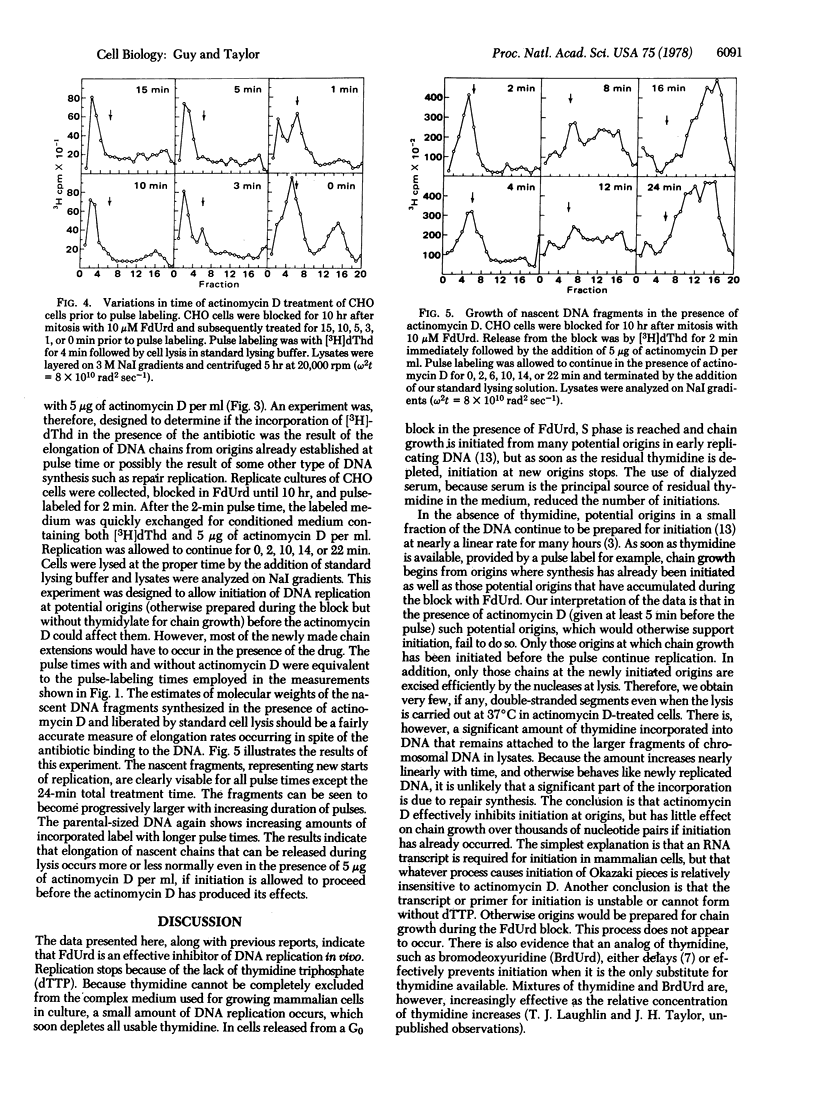
Mammalian (Chinese hamster ovary) cells were synchronized in the division cycle and blocked at the beginning of S phase with fluorodeoxyuridine. Traces of thymidine in the medium allowed cells to enter S phase and initiate DNA replication at some origins. For many hours after the traces of thymidine were depleted new sites for DNA replication accumulated in a small fraction of the DNA. However, these potential origins became active in bidirectional replication only when cells were released by adding [3H]thymidine to the medium. Lysis at 37 degrees C released most of the pulse-labeled DNA as linear double-stranded segments larger than Okazaki fragments and smaller than the unreplicated parental DNA. Release of the newly replicated DNA involves breakage of the template chains at or near each fork. The size of the fragments increased linearly with time of pulse labeling, but the efficiency of their release decreased. The excision could be prevented by lysis at 0 degrees C. When cells were treated with actinomycin D for 3--5 min before release, the new origins failed to function, but chain growth continued from those sites at which initiation had taken place before depletion of thymidine. We interpret these results to indicate that initiation at origins requires an actinomycin D-sensitive step, presumably RNA transcription, while chain elongation, which involves the formation of Okazaki pieces, is relatively insensitive to actinomycin D during growth over long intervals.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Goldberg I. H., Friedman P. A. Antibiotics and nucleic acids. Annu Rev Biochem. 1971;40:775–810. doi: 10.1146/annurev.bi.40.070171.004015. [DOI] [PubMed] [Google Scholar]
- Hozier J. C., Taylor J. H. Length distributions of single-stranded DNA in Chinese hamster ovary cells. J Mol Biol. 1975 Apr 5;93(2):181–201. doi: 10.1016/0022-2836(75)90127-8. [DOI] [PubMed] [Google Scholar]
- Kurek M. P., Taylor J. H. Replication of DNA in mammalian chromosomes. IV. Partial characterization of DNA fragments released from replicating DNA of CHO cells. Exp Cell Res. 1977 Jan;104(1):7–14. doi: 10.1016/0014-4827(77)90062-3. [DOI] [PubMed] [Google Scholar]
- Taylor J. H., Adams A. G., Kurek M. P. Replication of DNA in mammalian chromosomes. II. Kinetics of 3H-thymidine incorporation and the isolation and partial characterization of labeled subunits at the growing point. Chromosoma. 1973 Apr 27;41(4):361–384. doi: 10.1007/BF00396495. [DOI] [PubMed] [Google Scholar]
- Taylor J. H. Increase in DNA replication sites in cells held at the beginning of S phase. Chromosoma. 1977 Jul 18;62(4):291–300. doi: 10.1007/BF00327029. [DOI] [PubMed] [Google Scholar]
- Taylor J. H., Myers T. L., Cunningham H. L. Programmed synthesis of deoxyribonucleic acid during the cell cycle. In Vitro. 1971 Jan-Feb;6(4):309–321. doi: 10.1007/BF02625945. [DOI] [PubMed] [Google Scholar]
- Taylor J. H. Replication of DNA in mammalian chromosomes: isolation of replicating segments. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1083–1087. doi: 10.1073/pnas.70.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. H., Wu M., Erickson L. C., Kurek M. P. Replication of DNA in mammalian chromosomes. III. Size and RNA content of Okazaki fragments. Chromosoma. 1975 Nov 24;53(2):175–189. doi: 10.1007/BF00333044. [DOI] [PubMed] [Google Scholar]


