Abstract
β2-microglobulin (β2-m) has become the focus of intense scrutiny since the discovery of its undesirable roles promoting osteomimicry and cancer progression. β2-m is a well-known housekeeping protein that forms complexes with the heavy chain of major histocompatibility complex class I molecules, which are heterodimeric cell surface proteins that present antigenic peptides to cytotoxic T cells. On recognition of foreign peptide antigens on cell surfaces, T cells actively bind and lyse antigen-presenting cancer cells. In addition to its roles in tumor immunity, β2-m has two different functions in cancer cells, either tumor promoting or tumor suppressing, in cancer cell context-dependent manner. Our studies have demonstrated that β2-m is involved extensively in the functional regulation of growth, survival, apoptosis, and even metastasis of cancer cells. We found that β2-m is a soluble growth factor and a pleiotropic signaling molecule which interacts with its receptor, hemochromatosis protein, to modulate epithelial-to-mesenchymal transition (EMT) through iron-responsive pathways. Specific antibodies against β2-m have remarkable tumoricidal activity in cancer, through β2-m action on iron flux, alterations of intracellular reactive oxygen species, DNA damage and repair enzyme activities, β-catenin activation and cadherin switching, and tumor responsiveness to hypoxia. These novel functions of β2-m and β2-m signaling may be common to several solid tumors including human lung, breast, renal, and prostate cancers. Our experimental results could lead to the development of a novel class of antibody-based pharmaceutical agents for cancer growth control. In this review, we briefly summarize the recent data regarding β2-m as a promising new cancer therapeutic target and discuss antagonizing this therapeutic target with antibody therapy for the treatment of localized and disseminated cancers.
Keywords: Anti-β2-m antibody, apoptosis, β2-microglobulin (β2-m), osteomimicry.
INTRODUCTION
β2-microglobulin (β2-m), a well-known housekeeping gene, is a nonglycosylated protein with a low molecular weight of 12-kDa. This 99-amino acid residue protein is synthesized by all nucleated cells. It has been identified as a major structural component of amyloid fibrils deposited in dialysis-related amyloidosis, a common and serious complication in patients receiving hemodialysis for more than 10 years [1, 2]. β2-m forms a small invariable light chain subunit of the major histocompatibility complex (MHC) class I antigen, also known as human leukocyte antigen (HLA), on the cell surface of nucleated cells. β2-m is an important structural protein in the regulation of host immune recognition of self and non-self antigens by CD8+ T lymphocytes and for immunoglobulin transport and iron metabolism [3-6]. Some animal studies have reported that even when no MHC class I antigens could be detected on cells and the animals were grossly deficient in CD4- CD8+ T cells, which normally mediate cytotoxic T cell function, the homozygotes appeared normal [7, 8]. The best characterized function of β2-m is its interaction with and stabilizing of the tertiary structure of the MHC class I α-chain for presenting antigenic peptides from intracellular proteins to cytotoxic T lymphocytes, although the specific roles of β2-m in this process are not yet understood [9]. On recognizing foreign peptide antigens on cell surfaces, T cells actively bind and degrade the antigen-presenting cells with a large multicatalytic proteolytic particle, the proteasome [10-12]. β2-m is also a light chain of the neonatal Fc receptor, one of the HLA class I-associated antigens that maintains the turnover time and functions of albumin and IgG by preventing their digestion by lysosomal enzymes in the body [13]. In addition to the roles of β2-m in immunity, several other β2-m functions with clinical relevance have been elucidated, including the regulation of survival, proliferation, metastasis, and even apoptosis of cancer cells [14, 15]. The cell-associated form of the β2-m subunit does not contribute directly to the binding interface, and therefore can be exchanged with circulating β2-m, which is present at low levels in serum, urine, and other body fluids under physiologic conditions [16]. Elevated β2-m has been observed in patients with renal failure and autoimmune and infectious diseases [17, 18]. Furthermore, increased synthesis and release of β2-m occurs in several malignant diseases including multiple myeloma, lymphoma, and solid tumors as indicated by elevated serum or urine β2-m concentrations [19-26]. Also, the level of β2-m is one of the most important independent prognostic factors and survival predictors for some cancers [22, 26-29].
β2-m has been reported by our laboratory and others to be a growth-stimulating factor and cell signaling molecule in several types of cancer cells [30-33]. We reported that β2-m played multiple roles in cancer development and mediates tumorigenesis, angio-genesis, and osteomimicry [30, 31, 34]. β2-m is also known to promote the growth and survival of stromal cells, such as mesenchymal stem cells (MSCs), osteoblasts, and osteoclasts supporting cancer bone metastasis [31, 35-37]. The unique niche of β2-m in cancer bone metastasis can be explained by its double roles. First, β2-m directly acts on cancer cells to increase their growth, survival and aggressiveness through the induction of epithelial-to-mesenchymal transition (EMT). Second, β2-m also has direct action on bone cells to establish a prometastatic niche and enhance bone metastasis. These distinctive roles may make the β2-m-mediated cell signaling network a highly desirable therapeutic target. To uncover the β2-m-meditated intracellular signaling network, we identified the hemochromatosis (HFE) protein as a β2-m receptor [34]. HFE is a non-classical MHC class I molecule that complexes with β2-m. The β2-m/HFE complex protects against the influx and accumulation of intracellular iron and negatively regulates intracellular iron concentration in cancer cells, mediated by β2-m/HFE complex interaction with transferrin receptor (TFR) [38, 39]. We also found that lower levels of intracellular iron concentration caused by the β2-m/HFE complex activate hypoxia inducible factor-1 (HIF-1α) and induce overexpression of its downstream target genes in cancer cells, driving EMT and promoting cancer lethal bone and soft tissue metastases [34]. This cell signaling network is highly conserved in human prostate, kidney, lung, and breast cancer cells, with activation of β2-m/HFE complex resulting in the induction of EMT, the lethal progression of these cancers to host bone and soft tissues and ultimately the demise of the host.
In this review, we focused specifically on important findings concerning previously unrecognized roles of β2-m as a growth factor and cell signaling molecule. We will discuss potential β2-m targeting as a novel therapeutic approach for the treatment of lethal progression of human cancer bone and soft tissue metastases.
MHC CLASS I AND β2-MICROGLOBULIN
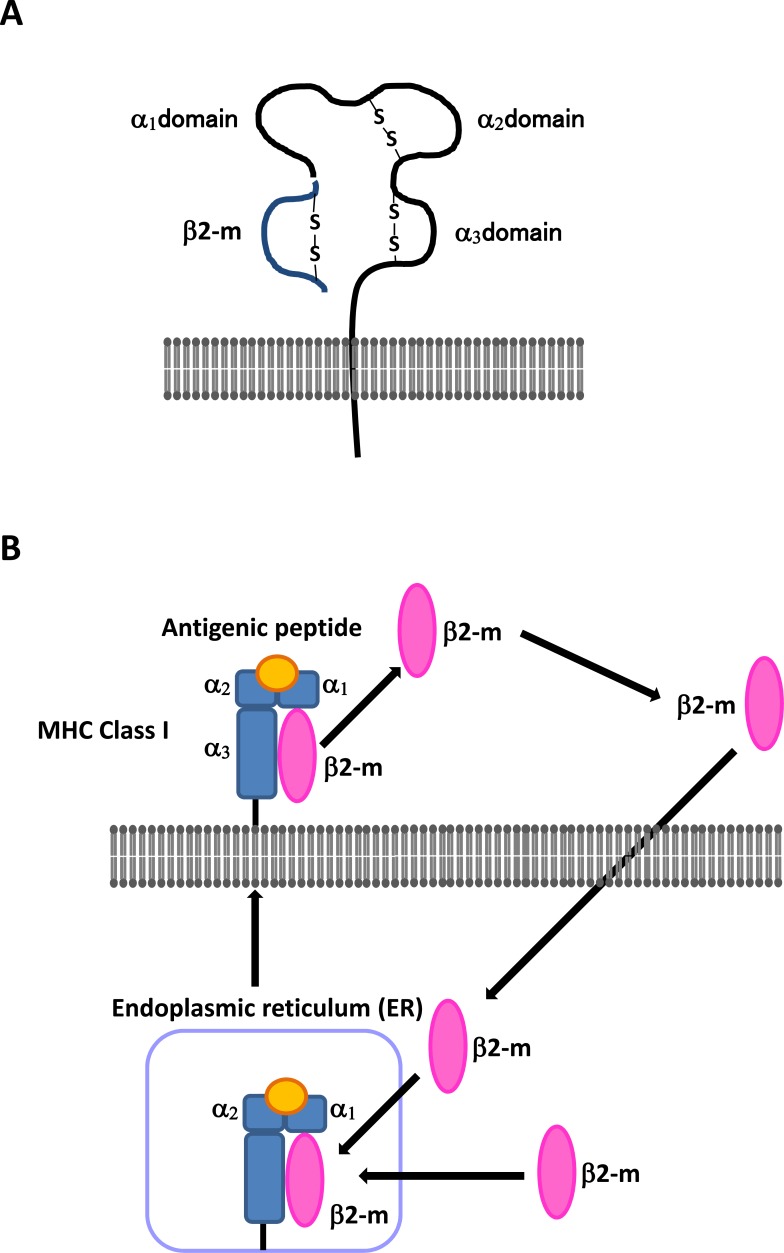
MHC class I molecules are heterodimeric cell surface proteins that present processed antigenic peptides from pathogen-infected or transformed cells to CD8+ T lymphocytes for host immune surveillance [3, 4]. The antigenic peptide-presenting cells are degraded by the proteasome but are still recognized by cytotoxic T lymphocytes [10-12]. The MHC class I complex consists of a 45-kDa heavy chain, called the α chain, containing α1, α2, and α3 domains, the 12-kDa light chain β2-m, and a transmembrane domain with a short cytoplasmic domain (Fig. 1A). The α1and α2 domains of the α chain are polymorphic and form a peptide-binding groove. The α3 domain is an immunoglobulin (Ig)-like domain. β2-m forms a protein-building subunit of the MHC class I molecule, and its association with the MHC class I α chain is required for transport of the complex to the cell surface (Fig. 1A and B) [40]. After production of the antigenic peptide in the cytosol, it is transported across the endoplasmic reticulum (ER) membrane in an ATP-dependent manner. The MHC class I heavy chain and β2-m are co-translationally translocated into the ER, where their assembly may be facilitated by the sequential association of the heavy chain with chaperone proteins. After the MHC class I molecule binds an antigenic peptide, it is released to the cell surface where cytotoxic T lymphocytes recognize its peptides as originating from foreign proteins. The cell-associated form of β2-m is not anchored on the cell membrane, and therefore β2-m exhibits dissociation and equilibrium exchange with circulating soluble β2-m in the extracellular fluid where it is present at low levels in urine, serum, and other body fluids under physiologically relevant conditions, but is elevated with renal tubular failure, autoimmune disease, chronic infectious disease, and malignancies [16-26, 41].
Fig. (1).
(A) The structure of the MHC class I complex. The MHC class I complex consists of the heavy chain containing α1, α2, and α3 domains, and the light chain called β2-m. (B) The production and transport of β2-m. The cell-associated form of β2-m is not anchored on the cell surface membrane, and β2-m exhibits dissociation and equilibrium exchange with circulating soluble β2-m.
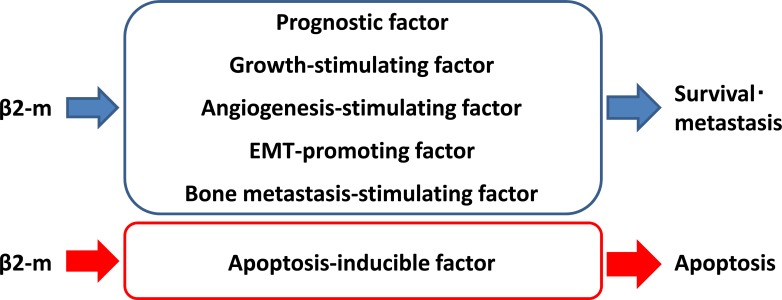
Along with its roles in host immune surveillance, β2-m functions as a regulator of cell survival, proliferation, invasion, migration, angiogenesis, apoptosis, and even metastasis in cancer cells [22, 26-31, 34, 42-45]. Recent reports from our laboratory and others have assigned fascinating biological functions to β2-m as a growth-stimulating factor and a cell signaling molecule, a new androgen and androgen receptor (AR) target gene, and importantly a new therapeutic target for both liquid and solid tumors [30-33, 46]. The unique functions of β2-m in cancer cells are briefly recapitulated in Fig. (2) where cell surface, circulating, or intracellular β2-m is regarded as a growth- angiogenesis-, EMT-, and bone metastasis-promoting factor and a prognostic indicator in solid tumor cells. Targeting the β2-m-HFE complex or β2-m-mediated signaling pathways could ultimately trigger strong tumoricidal effects on localized and metastatic cancer cells and is therefore a promising and novel cancer treatment.
Fig. (2).
Schematic diagram of β2-m functions in cancer cells. β2-m is a regulator of cancer cell survival and metastasis. β2-m is an excellent prognostic indicator of both liquid and solid malignancies. β2-m as shown to have growth-stimulating, angiogenesis-stimulating, EMT-promoting, and bone metastasis-stimulating activities in cancer cells.
β2-M AS A BIOMARKER FOR CANCERS
Catabolism of β2-m following its dissociation from the MHC class I heavy chain occurs predominantly in the proximal tubules in the kidney. Glomerular-filtered β2-m is reabsorbed by megalin, an endocytic receptor, in proximal tubule cells, where it is metabolized [47, 48]. Free β2-m protein is present at low levels in urine, serum, and other body fluids under normal conditions. A rise in β2-m concentration indicates abnormal conditions associated with various disorders [16-26]. Multiple lines of evidence show that β2-m is involved in cancer and other human malignancies. Increased synthesis and release of β2-m, as indicated by elevated serum, plasma, or urine β2-m concentration, occurs in several malignant diseases, including prostate cancer, lung cancer, breast cancer, renal cell carcinoma (RCC), gastrointestinal and nasopharyngeal cancers, hepatocellular carcinoma, ovarian cancer, multiple myeloma, and lymphocytic malignancies such as non-Hodgkin’s lymphoma, in addition to autoimmune and chronic infectious diseases [19, 21-29, 49, 50]. Furthermore, a number of studies have demonstrated that the level of serum β2-m is one of the most important independent prognostic factors and survival predictors for some tumors including RCC, multiple myeloma, and T-cell leukemia [22, 24, 28]. Overexpression of β2-m in tissue specimens assessed by immunohistochemical staining is also associated with tumor progression and poor prognosis in colorectal cancer and esophageal cancer [51, 52]. Analysis of β2-m in the first voided urine indicates that urine β2-m levels are elevated with a high frequency in patients with prostate cancer compared to matched healthy subjects and correlate inversely with patient survival [25]. Moreover, β2-m urine levels are higher in advanced prostate cancer patients with bone and visceral metastasis compared to those with local/regional disease [25]. Gross et al. reported that serum β2-m levels are also elevated in patients with metastatic, androgen-independent prostate cancer, and β2-m is more specific for androgen stimulation than prostate-specific antigen (PSA) in a cell culture model [53]. PSA, the most widely used serum marker for prostate cancer diagnosis and evaluation of treatment, is an androgen-regulated secreted protein, but the validity of PSA for predicting tumor burden and survival remains controversial, partly because PSA originates from both normal, benign and malignant prostate epithelial cells. β2-m could be a complementary biomarker that could refine the prognostic value of PSA in human prostate cancer.
In all malignancies, the best investigated aspect of β2-m as a predictive biomarker is for lymphoproliferative disorders, especially multiple myeloma. The International Staging System (ISS) stratifies multiple myeloma patients into three stages based on the combination of serum β2-m with albumin because serum β2-m and albumin are powerful predictors of survival [54-56]. Serum β2-m is an extremely useful marker in patients with multiple myeloma not only for initial stratification but for follow-up of patients with standard therapy or autotransplantation. The prognostic value of pretreatment serum β2-m levels has been reported for multiple myeloma, suggesting an excellent correlation between serum β2-m levels and tumor burden in multiple myeloma patients [28]. A high serum β2-m level is an established predictor of poor survival in patients undergoing chemotherapy and an independent predictor of both overall and event-free survival after stem cell transplantation in multiple myeloma patients [27, 57]. Studies have also shown that a low serum β2-m concentration is a positive predictor of complete response among patients treated with stem cell transplantation therapy [57].
Additionally serum β2-m measurements also provide prognostic information on tumor burden in solid tumors. While serum β2-m levels predict positively the progression of metastatic, androgen-independent prostate cancer, low β2-m mRNA expression is a strong predictive indicator of lymph node metastasis and/or poor survival in colorectal cancer patients [58]. The reason for this is that cancer cells often downregulate MHC class I antigens, and the loss of these antigens allows tumors to escape recognition by the immune system. For example, RCC cells can escape elimination by cytotoxic T cells through: 1) reduction of MHC antigen expression, and 2) prevention of MHC antigen processing by lymphocytes [59, 60]. Interestingly, unlike multiple myeloma and prostate cancer, the decrease or loss of β2-m expression has been associated with the development of drug resistance and loss of estrogen receptors in breast carcinoma cell lines [61]. In summary, it is likely that β2-m is a dual positive or negative prognostic and therapeutic response indicator for solid malignancies in a cancer type-specific manner. The potential significance of β2-m as a biomarker predicting cancer progression and its expression under tight regulation by tumor-host microenvironment warrant further investigation.
FUNCTIONS OF β2-M IN CANCER CELLS
Regulation of Cancer Cell Growth by β2-m
Because serum, urine, and tissue levels of β2-m are elevated in many cancers, it is critical to understand how β2-m regulates cancer cell proliferation [30, 31, 33, 62]. β2-m from prostate or bone stromal cell conditioned media has growth-stimulatory activity on prostate cancer cells and shows antagonistic activity toward transforming growth factor-β1 (TGF-β1)-induced growth inhibitory actions in human PC-3 prostatic carcinoma cells and rat PS-1 stromal cells [62]. Our laboratory has described a host of unexpected roles for β2-m in cancer promotion using a wide range of experimental approaches [30, 31, 33]. We have reported that β2-m acts like a prototypical oncogenic factor capable of stimulating the growth and progression of various cancers [30, 31, 33]. Overexpression of β2-m promotes tumor growth and enhances migration and invasion of prostate cancer, breast cancer, lung cancer, and renal cancer cells by the induction of EMT. We found that genetically stably-expressed β2-m in human prostate, breast, lung and renal cancer cells confers growth, survival, angiogenesis and bone metastatic potential leading to poorer prognosis for these solid tumor malignancies in experimental mouse models [34].
Roles of β2-m in Signaling Pathways in Cancer Cells
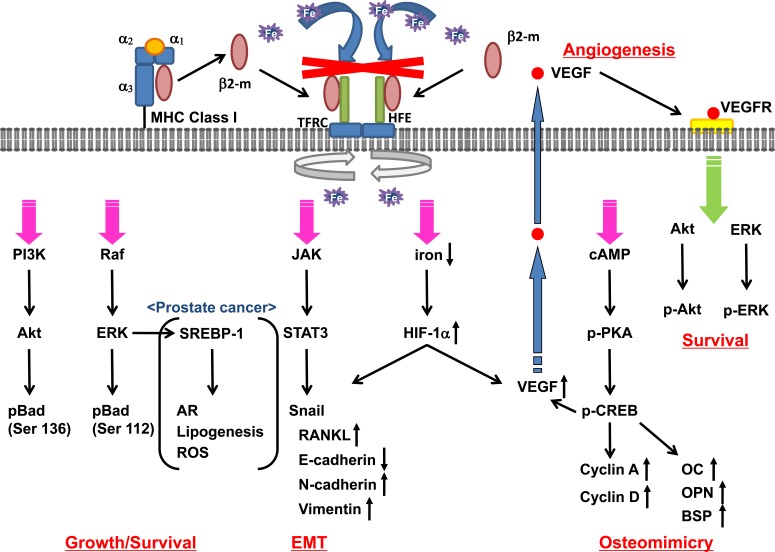
β2-m is known to present MHC class I molecules on cell surface by stabilizing these complexes. β2-m is also a crucial cell growth and survival signaling molecule in cancer cells. In the first report in our series, β2-m was identified by protein purification and mass spectrometry in the conditioned media of both prostate cancer and prostate and bone stromal cells as a protein capable of stimulating osteomimicry via the expression of bone-restricted proteins by prostate cancer cells in vitro. β2-m enhanced osteocalcin (OC) and bone sialoprotein (BSP) gene expression by activating cyclic AMP (cAMP)-dependent protein kinase A (PKA) activity, which phosphorylates cAMP-responsive element-binding protein (CREB). This activation induced tumor growth in mouse bone via increased phosphorylated CREB (pCREB) and the expression of CREB-target genes including OC, BSP, cyclin A, cyclin D1, and the potent angiogenic factor, vascular endothelial growth factor (VEGF) [30]. Similar results were observed in a renal carcinoma cell model by forced expression of β2-m in a human renal carcinoma SN12C cell line. β2-m overexpressed SN12C cells underwent EMT with enhanced ability to invade and migrate, eventually supporting the lethal progression of cancer to bone and soft tissues in mice. β2-m expressing cancer cells exhibited increased bone turnover and generated mixed osteolytic and osteoblastic responses, though displaying a predominantly osteolytic phenotype with significant bone destruction in renal carcinoma cells (31, 34). These reports also indicated that β2-m activated both β2-m/PKA/CREB signaling and its convergent cell survival signaling network, phosphatidylinositol 3-kinase (PI3K)/Akt and extracellular signal-regulated kinase (ERK). Furthermore, we found that recombinant β2-m protein could phosphorylate the Bcl-xL/Bcl-2-associated death-promoting protein, Bad, at Ser136 and Ser112 via activated PI3K/Akt and ERK signaling pathways in renal carcinoma SN12C cells [33]. Using a robust human prostate cancer EMT progression model, Zhau et al. [63] from our group also showed that β2-m stably transfected ARCaPE prostate cancer cells had consistently activated STAT3, Snail, LIV-1, and overexpressed LIV-1 and receptor activator of nuclear factor kappa B ligand (RANKL) protein, with all of the transfected cells expressing markers indicative of EMT and morphologic transition to fibroblast-like cells. β2-m-overexpressing cancer cells including prostate cancer, breast cancer, lung cancer, and renal carcinoma, underwent a cadherin switch in which cells expressed decreased E-cadherin but enhanced N-cadherin and vimentin in harvested tumor tissue specimens and in intratibial tumor tissue sections [34]. These results support the concept that EMT occurs subsequent to β2-m expression and that this phenotype is stable in vivo. We first assumed that β2-m could activate cAMP-dependent PKA activity by binding to and activating the seven-transmembrane G protein-coupled receptor (GPCR) or a yet to be identified β2-m receptor. β2-m has been reported to interact with both classical and non-classical members of MHC class I [40, 64]. Josson et al. reported that HFE protein, a non-classical MHC class I member, interacts with β2-m to modulate iron homeostasis and govern EMT in cancer cells. The β2-m/HFE complex plays a key role in regulating iron homeostasis, mediated by its interaction with the TF-TFR complex, or TFR complex. β2-m protected against the influx and accumulation of intracellular iron, and lower levels of intracellular iron activated HIF-1α and its target genes including EMT markers and VEGF. In this report, knocking down either the HFE protein or β2-m resulted in MET, a reversal of EMT, in prostate cancer cells with supportive morphological, biochemical, and behavioral characteristics. Thus, β2-m/HFE interactions are important for β2-m-mediated EMT and cell survival [34]. β2-m also regulates the expression of hormone/growth factor receptors, such as, AR, epidermal growth factor receptor, insulin receptor, and insulin-like growth factor receptor, on cell surfaces, suggesting that β2-m-mediated signaling may be transmitted through these receptors [46, 65-69].
It is well-known that aberrant androgen signaling mediated by AR, a ligand-activated transcription factor and survival factor, plays a key role in regulating prostate cancer growth and survival [70]. Gross et al. reported that β2-m is a downstream androgen target gene under the control of AR in a human prostate cancer cell line [53]. Interestingly, Huang et al. showed a reciprocal relationship between β2-m and AR in which β2-m regulates downstream AR and PSA expression directly in AR-positive prostate cancer cells [69]. This further provided a molecular link between the β2-m intracellular signaling axis mediated by ERK and sterol regulatory element-binding protein-1 (SREBP-1), involving lipogenic signaling, which collectively regulates AR expression and function. These results in aggregate suggest that multiple β2-m downstream signaling pathways, including AR signaling, SREBP-1/lipogenesis and lipid raft-mediated signaling, and reactive oxygen species (ROS) and its regulated cell signaling network, all converge with the β2-m-mediated downstream cell signaling network [69, 71]. A proposed β2-m-mediated multiple molecular signaling network promoting human cancer cell survival, EMT, angiogenesis, and osteomimicry is depicted in Fig. (3).
Fig. (3).
Proposed molecular mechanism whereby β2-m affects cancer growth and progression. The β2-m/HFE complex negatively regulates iron uptake through TFR complex and activates the PI3K/Akt, Raf/ERK, JAK/STAT3, cAMP/PKA/CREB, and HIF-1α signaling pathways, with increased cell growth/survival, angiogenesis, EMT and osteomimicry. β2-m activates both β2-m/PKA/CREB signaling and its convergent cell survival signaling network, PI3K/Akt and ERK, through VEGF-VEGFR signaling resulting in phosphorylation of Bad at Ser136 and Ser112. β2-m/PKA/CREB signaling also activates CREB-target gene expression including OC, OPN, BSP, cyclin A, and cyclin D leading to osteomimicry. β2-m/JAK/STAT3 signaling promotes the induction of EMT with increase of RANKL, N-cadherin, LIV-1 and vimentin expression and decrease of E-cadherin expression through Snail activation. Activated β2-m signaling also increases SREBP-1 transcription factor activity resulting in enhanced AR expression, lipogenesis, and ROS in prostate cancer cells.
ROLES OF β2-M IN DEVELOPING CANCER BONE METASTASIS
Roles of β2-m in Interaction between Cancer Cells and Bone Microenvironment
Bone is the second most common site of cancer metastasis. Advanced-stage cancer patients develop bone metastases with or without hormonal therapy, radiation therapy, chemotherapy, and immunotherapy, and there is no effective treatment currently available. To develop new approaches targeting advanced cancer bone metastasis, we need to understand the reciprocal interactions between cancer and bone cells at the molecular level. Bone is constantly being remodeled, which involves the synthesis of bone matrices by osteoblasts coordinated with increased bone resorption by osteoclasts [72-74]. Bone homeostasis generally depends on the dynamic equilibrium between osteoblasts and osteoclasts and the numerous cytokines and chemokines that mediate the “crosstalk” between cancer and bone cells [72-74]. Once in the bone, cancer cells alter the balance between bone formation and bone resorption, often by increasing osteoclast activity, leading to increased bone resorption and crippling bone damage [72-74]. In the case of prostate cancer, however, increased formation of new but weak “woven” bone or osteoblastic reaction was noted. These predominant mechanisms of cancer bone metastasis rely on cytokines and growth factors produced by metastatic cancer cells. One of the unique features of bone metastatic cancer cells is their property of osteomimicry, or ability to mimic the gene expression and behaviors of bone cells by synthesizing bone-like proteins. Some of the factors expressed by cancer cells that are involved in osteomimicry include OC, osteopontin (OPN), osteonectin/SPARC, RANKL, BSP, tumor necrosis factors (TNF)-α and –β, parathyroid hormone related protein (PTHrP), interleukin (IL)-11, and CTGF [75, 76]. Circulating soluble β2-m has been identified by our laboratory as a crucial autocrine and paracrine growth-stimulating factor that allows cancer cells to metastasize, or gain access to bone and colonize the skeleton. This action of β2-m is mainly due to its ability to stimulate the expression of RANKL or LIV-1, which was confirmed by the overexpression or knockdown of these genes in human prostate cancer cell lines, with either increased or decreased prostate cancer bone metastasis, respectively [77, 78]. We have reported that β2-m activates PKA signaling, mediated by CREB with increased expression of CREB target genes, including OC and BSP, in prostate cancer [30]. This activation could also enhance angiogenesis with increased VEGF expression and facilitate the recruitment of osteoclasts to the site of metastatic cancer colonization in bone. Previous studies have supported the association of increased EMT with the ability of cancer cells to migrate, invade, and metastasize to the skeleton. β2-m also regulates cancer bone metastasis and confers cancer lethality through EMT by downregulation of E-cadherin and upregulation of N-cadherin, vimentin, and RANKL in solid tumors [34, 63].
Cancer bone metastasis requires strong interaction between cancer cells and bone microenvironments. It has been well documented that β2-m regulates bone cell metabolism and plays a role in the development of bony destruction in dialysis-related amyloidosis [79]. In an experimental model, subcutaneous injection of β2-m induced bone resorption and purified human β2-m induced calcium efflux mediated in part by IL-1β, but not by prostaglandins [35]. β2-m also induces cyclooxygenase-2 (COX-2) expression in human synovial cells and stimulates the synthesis and secretion of IL-6 and RANKL, both potent bone-resorbing cytokines promoting osteoclastogenesis [36]. In addition to bone metabolism, β2-m can directly or indirectly initiate the inflammatory process in synovial cells, which may be involved in cancer development and progression. We have reported that β2-m enhances cancer cell mediated osteolysis by increasing osteoclast activity in mouse bones implanted with β2-m overexpressing cancer cell clones, including prostate cancer, breast cancer, lung cancer, and renal cancer, which confirmed that β2-m can stimulate osteoclastogenesis [34]. RANKL (associated with cancer cells or osteoblasts) and its TNF-family receptor RANK (associated with osteoclasts) are essential regulators of bone remodeling and bone metastasis in cancer [80]. Thus, increased bone turnover through increased RANKL and RANK interaction results in activation of osteoclasto-genesis. We have reported that β2-m-transfected cancer clones consistently show activated STAT3, Snail, LIV-1, and RANKL with all of the transfected cells expressing indicative EMT markers [63].
Regulation of MSCs Growth by β2-m
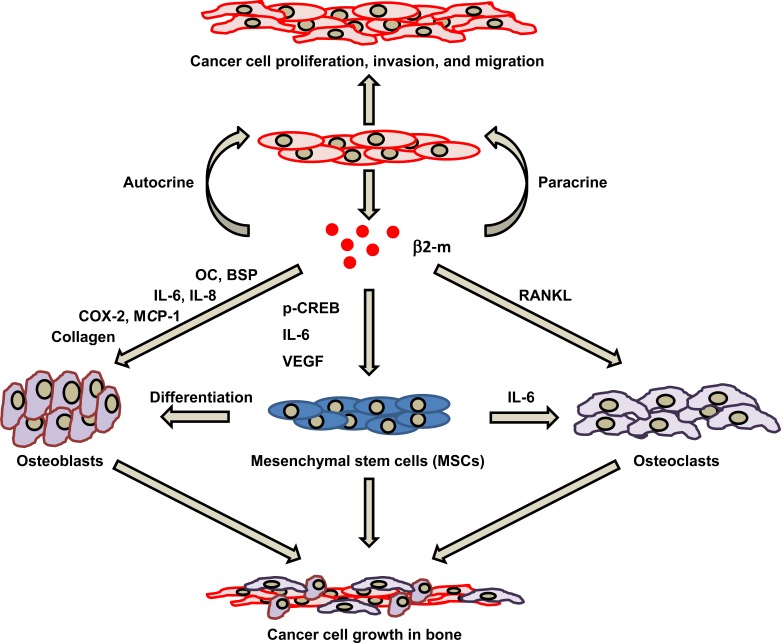
Over the last decade, a great deal of attention has been directed toward the role of bone marrow-derived MSCs in cancer progression [81]. Bone marrow-derived MSCs with migratory, invasive, and self-renewal potential have been proposed to give rise to a variety of mesenchymal cells such as osteoblasts, chondrocytes, adipocytes, fibroblasts, and muscle cells [82, 83]. The biology and roles of MSCs in carcinogenesis and cancer progression are more complicated than those of their hematopoietic counterparts. Upon recruitment into a tumor, MSCs could exist in a special location, fuse with adult stem cells, and participate in cancer cell growth and colonization at metastatic bone sites [81, 84]. It is reasonable to suppose that an intimate reciprocal interplay exists between bone marrow-derived MSCs and cancer cells in bone because bone metastatic cancer cells have close contact with bone marrow stromal cells. MSCs, which have great proliferative and multi-potentiality, produce growth factors and cytokines in a niche supporting the expansion of cancer cells in an osteolysis-promoting microenvironment. Shi et al. reported that MSCs interact with prostate cancer cells to promote cancer cell growth, migration, and invasion through the induction of osteomimicry and EMT [85]. In contrast, the proliferation of MSCs is influenced by growth-stimulating factors secreted by cancer cells in a microenvironment in which MSCs become tumor-associated fibroblasts. Possibly β2-m regulates the growth and migration of MSCs [86]. β2-m secreted by cancer cells induces the growth of MSCs, whereas anti-β2-m antibody or siRNA for β2-m blocks the expansion of MSCs. β2-m was found to increase the proliferation of MSCs through increased pCREB and upregulation of IL-6 and VEGF [87, 88]. VEGF, an important factor involved in the development of tumor blood supply, can also substitute for pro-osteoclastogenic cytokine macrophage colony-stimulating factor and upregulate RANK expression in osteoclast precursors, thus promoting osteoclasto-genesis. We have reported that β2-m-overexpressing cancer cells in bone tumors develop osteolytic lesions with a large number of osteoclasts, although osteoblastic foci could be co-present [34]. That is, β2-m may play a role in mediating the “vicious cycle” interaction between MSCs and cancer cells with accelerating osteoclastogenesis. The possible roles of β2-m in the regulation of osteoblasts, osteoclasts, and MSCs are summarized in Fig. (4).
Fig. (4).
The β2-m-mediated association between cancer cells and bone stromal cells in developing cancer bone metastasis. β2-m functions as an autocrine and/or paracrine growth-stimulating factor, inducing several cytokines in cancer cells, allowing MSCs to develop enhanced osteoclastogenesis or osteoblastogenesis. First, β2-m produced by cancer cells stimulates their proliferation, invasion, and migration through autocrine and/or paracrine mechanisms. Second, cytokines including those produced by β2-m stimulated cancer cells and regulated the “vicious cycle” interaction between cancer cells and cells in cancer microenvironments, such as MSCs, osteoblasts, and osteoclasts during bone metastasis.
β2-M AS A NEGATIVE GROWTH REGULATOR
The ability of β2-m to act either as a positive or negative cell growth regulator is cell context-dependent. We have assessed the growth stimulatory role of β2-m in various solid tumor malignancies; however, β2-m also appears to play a role as a negative growth regulator in hematological malignancies, principally myeloid and lymphoid leukemia cell lines. The mechanisms underlying β2-m-induced apoptosis appear complicated. Mori et al. have reported that recombinant human β2-m acts on both T-leukemic cells and myeloid leukemic cells to induce apoptosis, which activates caspase-1 and -3 [89]. β2-m-induced apoptosis is not mediated by either the FasL/Fas or the TNF-α/TNFR systems and is also independent of IL-8-induced apoptosis. Anti-β2-m monoclonal antibody completely blocked both the suppression of cell proliferation and the induction of apoptosis by β2-m in K562 and CCRF-CEM cells. Min et al. reported that β2-m suppressed the growth of both primary myeloma cells isolated from patients and myeloma cell lines (ARK-RS, ARP-1, RPMI-8226, U266, ARH-77, and IM-9) by induction of apoptosis and cell cycle arrest [43]. β2-m-induced apoptosis was dependent on activation of a caspase cascade, was inhibited by IL-6, and was not mediated by death receptors. Furthermore, β2-m-induced cell growth arrest was accompanied by downregulation of cyclin A and cyclin D2. Exogenous β2-m induced apoptosis in CCRF-HSB-2 human lymphoblastic leukemia cells through an unknown caspase-dependent mechanism that was independent of mitochondrial permeability transition pore formation. β2-m also significantly enhanced the production of ROS, and the antioxidant N-acetylcysteine (NAC) inhibited β2-m-induced apoptosis, providing evidence that β2-m-induced apoptosis in CCRF-HSB-2 cells was ROS-dependent [45]. Moreover, exogenous β2-m induces apoptosis through a caspase-dependent mitochondrial pathway in HL-60 leukemia and vincristine-resistant HL-60/VCR cells and through a Bcl-2-associated X protein (Bax)-independent, non-mitochondrial, caspase-dependent pathway in doxorubicin-resistant HL-60/ADR cells [90]. Interestingly, β2-m increased the sensitivity of MCF-7 cells to doxorubicin, and a decrease or loss of β2-m expression by antisense RNA was involved in the acquisition of doxorubicin resistance [61]. As mentioned above, the activities of β2-m as an apoptosis inducing factor or as a determinant of chemosensitivity are extremely variable. Further studies are required to determine the precise molecular mechanisms by which β2-m regulates the elimination of tumor cells.
β2-M AS A PROMISING TARGET IN CANCER THERAPY
Given the elevated β2-m expression in many tumor types and its functional importance as a signal transducing molecule in the survival of cancer cells, it is not surprising that β2-m and β2-m-mediated signaling are considered to be attractive targets for therapeutic intervention in human malignancies. Several targeting strategies have been explored thus far. Of these, sequence-specific siRNA and antibodies have garnered the most attention. Huang et al. in our group first reported that β2-m siRNA inhibited the growth of C4-2B cells, an androgen-independent human prostate cancer cell line of LNCaP lineage [30]. They also validated the effect of β2-m siRNA on human prostate tumor growth, both in subcutaneous bone powder xenografts and in mouse skeleton. β2-m siRNA injection eliminated tumors inoculated in bone as well as preexisting tumors grown as bone powder xenografts, via induction of apoptosis. Similar results were found using siRNA in a RCC system, demonstrating that β2-m siRNA reduced cell proliferation by induction of apoptosis through activation of the initiator caspases [31]. In addition to the growth inhibitory effect of β2-m siRNA, inhibition of β2-m expression negatively affected invasion and migration of renal carcinoma cells. Interestingly, inhibition of β2-m by β2-m siRNA can reverse EMT with stable morphologic MET, which is accompanied by increased E-cadherin and decreased vimentin expression in prostate cancer cells. In these cancer cells, knocking down HFE by HFE shRNA lentiviral constructs results in decreased expression of vimentin and increased expression of E-cadherin with downregulation of β2-m, thus reducing the β2-m/HFE complexes [34]. It is also noteworthy that polyclonal and monoclonal antibodies specific for cell surface β2-m inhibited growth and induced apoptosis in prostate cancer and renal carcinoma cells [33, 46]. Analysis of signaling pathways downstream from antibody ligation of β2-m indicated that anti-β2-m antibodies inhibit the phosphorylation of Akt and ERK and activate c-jun N-terminal kinase (JNK), resulting in the induction of Bcl-2 phosphorylation and decreased phosphorylation of Bad. In addition, we reported that β2-m downstream signaling regulates AR and PSA expression, and reprograms fat metabolism through SREBP-1, while interrupting β2-m-mediated signaling with anti-β2-m antibodies inhibits prostate cancer cell growth and progression and induces apoptosis via the downregulation of AR and lipogenesis signaling [46, 69].
Near-simultaneously, Yang et al. demonstrated that monoclonal antibodies against β2-m induced apoptosis of hematologic malignant cells including β2-m-expressing multiple myeloma, Burkitt lymphoma, mantle cell lymphoma, and T-cell and myelogenous leukemia cell lines as well as primary tumor cells isolated from patients with myeloma, both in vitro and in xenograft mouse models [32]. The monoclonal antibodies induced apoptosis via the relocation of MHC class I molecules to lipid rafts and the activation of Lyn and the signal-transducing enzyme phospholipase C-γ2, leading to activated JNK and Akt and ERK inhibition, compromised mitochondrial integrity, cytochrome c release, and activation of the caspase-9 cascade. The lipid raft appears to mediate signal transduction by excluding cytokine-activated receptors and their downstream mediators. In fact, anti-β2-m monoclonal antibodies excluded IL-6 and insulin-like growth factor-1 (IGF-1) receptors and their substrates from the lipid rafts by recruiting MHC class I molecules into the rafts. Thus, the β2-m monoclonal antibodies induced apoptosis by abrogating the survival signaling mediated by IL-6- or IGF-1-mediated Janus kinase/STAT3 (JAK/STAT3), Akt, and ERK signaling pathways in multiple myeloma cells [91]. Similar biochemical mechanisms are affected in solid as well as liquid tumors, suggesting that the activation of a general and conserved cell signaling network mediated by β2-m could be the underlying mechanism of anti-β2-m antibody-induced cytotoxicity in these tumor models.
Encouraging studies have suggested that MHC molecules are targets for cancer therapy. Indeed human HLA-DR-specific monoclonal antibodies can efficiently induce apoptosis in malignant lymphoid cells, but the expression of HLA-DR on normal hematopoietic cells is a potential safety concern [92]. Likewise, β2-m monoclonal antibodies may influence normal hematopoietic cells because these cells also express β2-m on their cell surfaces. So far, the exact mechanisms by which β2-m antibodies display different effects on cancer cells versus normal cells remain unclear. However, anti-β2-m monoclonal antibodies appear to be favorably selective for tumor cells, in part because of the presence of higher levels of TF-TFR complex facilitating active iron transport in tumor but not normal cells when treated with anti-β2-m monoclonal antibodies (Josson et al. unpublished results). In contrast, the growth of normal cells, including T and B lymphocytes and CD-34-positive bone marrow stem cells, is insensitive to blockade by anti-β2-m monoclonal antibodies [32]. One technical note should not be ignored: commercial preparations of anti-β2-m monoclonal antibodies often contain a low concentration of sodium azide (0.1%) to avoid the growth of microorganisms. We found some prostate cancer cell lines, such as LNCaP and C4-2 and C4-2B are sensitive to the growth inhibitory effects of sodium azide in the anti-β2-m monoclonal antibody preparations while other prostate cancer cell lines, such as ARCaPE and ARCaPM, are not. It is crucial to evaluate the effectiveness of anti-β2-m monoclonal antibodies in the absence of sodium azide for mechanistic studies using cultured cell lines and studies of its therapeutic effects in animal models of local cancer growth and their distant metastases.
CONCLUSIONS
In summary, due to elevated expression in many tumor types, potent oncogenic activity, and contribution to pro-survival signaling pathways, β2-m and the β2-m-mediated cell signaling network have shown promise as a novel target for drug development and cancer control. While the biology of β2-m and its antagonism by anti-β2-m antibodies appears highly cell context-dependent, we characterized the effects of anti-β2-m antibodies in a series of human solid tumors in a preclinical setting and observed that antibodies targeting β2-m exhibited tumoricidal activity through interference with the β2-m-HFE complex regulating iron flux, and the redox state of cancer cells. Because of the differential function of the TF-TFR complex in cancer versus normal cells, anti-β2-m antibodies had a favorable growth-inhibitory effect on cancer cells without damaging normal cells and tissues, suggesting they are attractive and safe therapeutic agents for clinical translation. Continued research will generate additional insights into the functions of β2-m in cancer progression, including its involvement in cell signaling networks and apoptotic cell death, and thereby open additional avenues for therapeutic development and new intervention strategies in cancer.
ACKNOWLEDGEMENTS
This work was supported by a Grant-in-Aid for Scientific Research (C) 24592397 (T.Nomura) from the Japan Society for the Promotion of Science.
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflict of interest.
REFERENCES
- 1.Bjorkman PJ, Parham P. Structure function and diversity of class I major histocompatibility complex molecules. Annu. Rev. Biochem. 1990;59:253–288. doi: 10.1146/annurev.bi.59.070190.001345. [DOI] [PubMed] [Google Scholar]
- 2.Saito A, Gejyo F. Current clinical aspects of dialysis-related amyloidosis in chronic dialysis patients. Ther. Apher. Dial. 2006;10(4):316–320. doi: 10.1111/j.1744-9987.2006.00383.x. [DOI] [PubMed] [Google Scholar]
- 3.Yewdell JW, Reits E, Neefjes J. Making sense of mass destruction: quantitating MHC class I antigen presentation. Nat. Rev. Immunol. 2003;3(12):952–961. doi: 10.1038/nri1250. [DOI] [PubMed] [Google Scholar]
- 4.Buus S. MHC restricted antigen presentation and T cell recognition. Dan. Med. Bull. 1994;41(3):345–358. [PubMed] [Google Scholar]
- 5.Christianson GJ, Brooks W, Vekasi S, Manolfi EA, Niles J, Roopenian SL, Roths JB, Rothlein R, Roopenian DC. Beta 2-microglobulin-deficient mice are protected from hyper-gammaglobulinemia and have defective antibody responses because of increased IgG catabolism. J. Immunol. 1997;159(10):4781–4792. [PubMed] [Google Scholar]
- 6.Feder JN, Penny DM, Irrinki A, Lee VK, Lebrón JA, Watson N, Tsuchihashi Z, Sigal E, Bjorkman PJ, Schatzman RC. The hemochromatosis gene product complexes with the transferrin receptor and lowers its affinity for ligand binding. Proc. Natl. Acad. Sci. USA. 1998;95(4):1472–1477. doi: 10.1073/pnas.95.4.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koller BH, Marrack P, Kappler JW, Smithies O. Normal development of mice deficient in beta 2M, MHC class I proteins, and CD8+ T cells. Science. 1990;248(4960):1227–1230. doi: 10.1126/science.2112266. [DOI] [PubMed] [Google Scholar]
- 8.Zijlstra M, Bix M, Simister NE, Loring JM, Raulet DH, Jaenisch R. Beta 2 microglobulin deficient mice lack CD4-8+ cytolytic T cells. Nature. 1990;344(6268):742–746. doi: 10.1038/344742a0. [DOI] [PubMed] [Google Scholar]
- 9.Bjorkman PJ, Burmeister WP. Structures of two classes of MHC molecules elucidated: crucial differences and similarities. Curr. Opin. Struct. Biol. 1994;4(6):852–856. doi: 10.1016/0959-440x(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 10.Ploegh HL, Orr HT, Strominger JL. Major histicompatibility antigens: The human (HLA-A -B -C) and murine (H-2K, H-2D) class I molecules. Cell. 1981;24(2):287–299. doi: 10.1016/0092-8674(81)90318-4. [DOI] [PubMed] [Google Scholar]
- 11.Parham P, Ohta T. Population biology of antigen presentation by MHC class I molecules. Science. 1996;272(5258):67–74. doi: 10.1126/science.272.5258.67. [DOI] [PubMed] [Google Scholar]
- 12.Salter RD, Benjamin RJ, Wesley PK, Buxton SE, Garrett TP, Clayberger C, Krensky AM, Norment AM, Littman DR, Parham P. A binding site for the T-cell co-receptor CD8 on the alpha 3 domain of HLA-A2. Nature. 1990;345(6270):41–46. doi: 10.1038/345041a0. [DOI] [PubMed] [Google Scholar]
- 13.Rojas R, Apodaca G. Immunoglobulin transport across polarized epithelial cells. Nat. Rev. Mol. Cell Biol. 2002;3(12):944–955. doi: 10.1038/nrm972. [DOI] [PubMed] [Google Scholar]
- 14.Shi C, Zhu Y, Su Y, Chung LW, Cheng T. Beta2-microglobulin: emerging as a promising cancer therapeutic target. Drug Discov. Today. 2009;14(1-2):25–30. doi: 10.1016/j.drudis.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Yang J, Yi Q. Killing tumor cells through their surface beta(2)-microglobulin or major histocompatibility complex class I molecules. Cancer. 2010;116(7):1638–1645. doi: 10.1002/cncr.24953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strominger JL. Human histocompatibility proteins. Immunol. Rev. 2002;185:69–77. doi: 10.1034/j.1600-065x.2002.18508.x. [DOI] [PubMed] [Google Scholar]
- 17.Cheung AK, Greene T, Leypoldt JK, Yan G, Allon M, Delmez J, Levey AS, Levin NW, Rocco M V, Schulman G, Eknoyan G. HEMO Study Group.Association between serum beta2-microglobulin level and infectious mortality in hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2008;3(1):69–77. doi: 10.2215/CJN.02340607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Onishi S, Ikenoya K, Matsumoto K, Kamata Y, Nagashima T, Kamimura T, Iwamoto M, Minota S. Urinary beta2-microglobulin as a sensitive marker for haemophagocytic syndrome associated with collagen vascular disease. Rheumatology (Oxford): 2008;47(11):1730–1732. doi: 10.1093/rheumatology/ken361. [DOI] [PubMed] [Google Scholar]
- 19.Bunning RA, Haworth SL, Cooper EH. Serum beta-2-microglobulin levels in urological cancer. J. Urol. 1979;121(5):624–625. doi: 10.1016/s0022-5347(17)56910-4. [DOI] [PubMed] [Google Scholar]
- 20.Cooper EH, Plesner T. Beta-2-microglobulin review: its relevance in clinical oncology. Med. Pediatr. Oncol. 1980;8(4):323–334. doi: 10.1002/mpo.2950080403. [DOI] [PubMed] [Google Scholar]
- 21.Nissen MH, Bjerrum OJ, Plesner T, Winken M, Rørth M. Modification of beta-2-microglobulin in sera from patients with small cell lung cancer: evidence for involvement of a serine protease. Clin. Exp. Immunol. 1987;67(2):425–432. [PMC free article] [PubMed] [Google Scholar]
- 22.Sadamori N, Mine M, Hakaniya S, Ichiba M, Kawachi T, Itoyama T, Nakamura H, Tomonaga M, Hayashi K. Clinical significance of beta 2-microglobulin in serum of adult T cell leukemia. Leukemia. 1995;9(4):594–597. [PubMed] [Google Scholar]
- 23.Klein T, Levin I, Niska A, Koren R, Gal R, Schachter J, Kfir B, Narinski R, Warchaizer S, Klein B. Correlation between tumour and serum beta 2m expression in patients with breast cancer. Eur. J. Immunogenet. 1996;23(6):417–423. doi: 10.1111/j.1744-313x.1996.tb00132.x. [DOI] [PubMed] [Google Scholar]
- 24.Rasmuson T, Grankvist K, Ljungberg B. Serum beta 2-microglobulin and prognosis of patients with renal cell carcinoma. Acta Oncol. 1996;35(4):479–482. doi: 10.3109/02841869609109926. [DOI] [PubMed] [Google Scholar]
- 25.Abdul M, Banks M, Hoosein N. Urinary markers for prostate cancer. Int. J. Oncol. 1996;8(4):735–739. doi: 10.3892/ijo.8.4.735. [DOI] [PubMed] [Google Scholar]
- 26.Molica S, Levato D, Cascavilla N, Levato L, Musto P. Clinico-prognostic implications of simultaneous increased serum levels of soluble CD23 and beta2-microglobulin in B-cell chronic lymphocytic leukemia. Eur. J. Haematol. 1999;62(2):117–122. doi: 10.1111/j.1600-0609.1999.tb01731.x. [DOI] [PubMed] [Google Scholar]
- 27.Bataille R, Durie BG, Grenier J. Serum beta2 microglobulin and survival duration in multiple myeloma: A simple reliable marker for staging. Br. J. Haematol. 1983;55(3):439–447. doi: 10.1111/j.1365-2141.1983.tb02158.x. [DOI] [PubMed] [Google Scholar]
- 28.Durie BG, Stock-Novack D, Salmon SE, Finley P, Beckord J, Crowley J, Coltman CA. Prognostic value of pretreatment serum beta 2 microglobulin in myeloma: A Southwest Oncology Group Study. Blood. 1990;75(4):823–830. [PubMed] [Google Scholar]
- 29.Rajkumar SV, Greipp PR. Prognostic factors in multiple myeloma. Hematol. Oncol. Clin. North Am. 1999;13(6):1295–1314. doi: 10.1016/s0889-8588(05)70128-3. [DOI] [PubMed] [Google Scholar]
- 30.Huang W-C, Wu D, Xie Z, Zhau HE, Nomura T, Zayzafoon M, Pohl J, Hsieh C-L, Weitzmann M N, Farach-Carson MC, Chung LWK. Beta2-microglobulin is a signaling and growth-promoting factor for human prostate cancer bone metastasis. Cancer Res. 2006;66(18):9108–9116. doi: 10.1158/0008-5472.CAN-06-1996. [DOI] [PubMed] [Google Scholar]
- 31.Nomura T, Huang W-C, Zhau HE, Wu D, Xie Z, Mimata H, Zayzafoon M, Young AN, Marshall FF, Weitzmann MN, Chung LWK. Beta2-microglobulin promotes the growth of human renal cell carcinoma through the activation of the protein kinase A, cyclic AMP-resposive element-binding protein, and vascular endothelial growth factor axis. Clin. Cancer Res. 2006;12(24):7294–7305. doi: 10.1158/1078-0432.CCR-06-2060. [DOI] [PubMed] [Google Scholar]
- 32.Yang J, Qian J, Wezeman M, Wang S, Lin P, Wang M, Yaccoby S, Kwak LW, Barlogie B, Yi Q. Targeting beta2-microglobulin for induction of tumor apoptosis in human hematological malignancies. Cancer Cell. 2006;10(4):295–307. doi: 10.1016/j.ccr.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 33.Nomura T, Huang W-C, Seo S, Zhau HE, Mimata H, Chung LWK. Targeting beta2-microglobulin mediated signaling as a novel therapeutic approach for human renal cell carcinoma. J. Urol. 2007;178(1):292–300. doi: 10.1016/j.juro.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 34.Josson S, Nomura T, Lin J-T, Huang W-C, Wu D, Zhau HE, Zayzafoon M, Weitzmann MN, Gururajan M, Chung LWK. Beta2-microglobulin induces epithelial to mesenchymal transition and confers cancer lethality and bone metastasis in human cancer cells. Cancer Res. 2011;71(7):2600–2610. doi: 10.1158/0008-5472.CAN-10-3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moe SM, Hack BK, Cummings SA, Sprague SM. Role of IL-1 beta and prostaglandins in beta 2-microglobulin-induced bone mineral dissolution. Kidney Int. 1995;47(2):587–591. doi: 10.1038/ki.1995.74. [DOI] [PubMed] [Google Scholar]
- 36.Balint E, Marshall CF, Sprague SM. Role of interleukin-6 in beta2-microglobulin-induced mineral dissolution. Kidney Int. 2000;57(4):1599–1607. doi: 10.1046/j.1523-1755.2000.00004.x. [DOI] [PubMed] [Google Scholar]
- 37.Zhu Y, Su Y, Cheng T, Chung LW, Shi C. Beta2-microglobulin as a potential factor for the expansion of mesenchymal stem cells. Biotechnol. Lett. 2009;31(9):1361–1365. doi: 10.1007/s10529-009-0027-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salter-Cid L, Peterson PA, Yang Y. The major histocompatibility complex-encoded HFE in iron homeostasis and immune function. Immunol. Res. 2000;22(1):43–59. doi: 10.1385/IR:22:1:43. [DOI] [PubMed] [Google Scholar]
- 39.Enns CA. Pumping iron: The strange partnership of the hemo-chromatosis protein a class I MHC homolog with the transferrin receptor. Traffic. 2001;2(3):167–174. doi: 10.1034/j.1600-0854.2001.020303.x. [DOI] [PubMed] [Google Scholar]
- 40.Hansen TH, Connolly JM, Gould KG, Fremont DH. Basic and translational applications of engineered MHC class I proteins. Trends Immunol. 2010;31(10):363–369. doi: 10.1016/j.it.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hyafil F, Strominger JL. Dissociation and exchange of the beta 2-microglobulin subunit of HLA-A and HLA-B antigens. Proc. Natl. Acad. Sci. USA. 1979;76(11):5864–5868. doi: 10.1073/pnas.76.11.5834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mori M, Terui Y, Tanaka M, Tomizuka H, Mishima Y, Ikeda M, Kasahara T, Uwai M, Ueda M, Inoue R, Itoh T, Yamada M, Hayasawa H, Furukawa Y, Ishizaka Y, Ozawa K, Hatake K. Antitumor effect of beta2-microglobulin in leukemic cell-bearing mice via apoptosis-inducing activity: activation of caspase-3 and nuclear factor-kappaB. Cancer Res. 2001;61(11):4414–4417. [PubMed] [Google Scholar]
- 43.Min R, Li Z, Epstein J, Barlogie B, Yi Q. Beta(2)-microglobulin as a negative growth regulator of myeloma cells. Br. J. Haematol. 2002;118(2):495–505. doi: 10.1046/j.1365-2141.2002.03635.x. [DOI] [PubMed] [Google Scholar]
- 44.Wu CH, Gordon JD, Zhong X, Safa AR. Mechanism of beta 2-microglobulin-induced apoptosis in the K562 leukemia cell line, defective in major histocompatibility class 1. Anticancer Res. 2002;22(5):2613–2621. [PubMed] [Google Scholar]
- 45.Gordon J, Wu CH, Rastegar M, Safa AR. Beta2-microglobulin induces caspase-dependent apoptosis in the CCRF-HSB-2 human leukemia cell line independently of the caspase-3, -8 and -9 pathways but through increased reactive oxygen species. Int. J. Cancer. 2003;103(3):316–327. doi: 10.1002/ijc.10828. [DOI] [PubMed] [Google Scholar]
- 46.Huang W-C, Havel JJ, Zhau HE, Qian WP, Lue HW, Chu CY, Nomura T, Chung LW. Beta2-microglobulin signaling blockade inhibited androgen receptor axis and caused apoptosis in human prostate cancer cells. Clin. Camcer Res. 2008;14(17):5341–5347. doi: 10.1158/1078-0432.CCR-08-0793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Birn H, Willnow TE, Nielsen R, Norden AG, Bönsch C, Moestrup SK, Nexø E, Christensen EI. Megalin is essential for renal proximal tubule reabsorption and accumulation of transcobalamin-B (12). Am. J. Physiol. Renal Physiol. 2002;282(3):F408–F416. doi: 10.1152/ajprenal.00206.2000. [DOI] [PubMed] [Google Scholar]
- 48.Christensen EI, Nielsen R. Role of megalin and cubilin in renal physiology and pathophysiology. Rev. Physiol. Biochem. Pharmacol. 2007;158:1–22. doi: 10.1007/112_0604. [DOI] [PubMed] [Google Scholar]
- 49.Staab HJ, Anderer FA, Hiesche K, Wehrie E, Rodatz W. Is serum beta 2-microglobulin a tumor marker in gastrointestinal cancer?. Clin. Chim. Acta. 1980;106(3):309–317. doi: 10.1016/0009-8981(80)90315-0. [DOI] [PubMed] [Google Scholar]
- 50.Hällgren R, Nou E, Lundqvist G. Serum beta 2-microglobulin in patients with brochial carcinoma and controls. Cancer. 1980;45(4):780–785. doi: 10.1002/1097-0142(19800215)45:4<780::aid-cncr2820450428>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 51.Bossard C, Bézieau S, Matysiak-Budnik T, Volteau C, Laboisse CL, Jotereau F, Mosnier JF. HLA/ß2 microglobulin over-expression in colorectal cancer is associated with recruitment of inhibitory immune cells and tumor progression. Int. J. Cancer. 2012;131(4):855–963. doi: 10.1002/ijc.26453. [DOI] [PubMed] [Google Scholar]
- 52.Tanaka K, Tsuchikawa T, Miyamoto M, Maki T, Ichinokawa M, Kubota KC, Shichinohe T, Hirano S, Ferrone S, Dosaka-Akita H, Matsuno Y, Kondo S. Down-regulation of human leukocyte antigen class I heavy chain in tumors is associated with poor prognosis in advanced esophageal cancer patients. Int. J. Oncol. 2012;40(4):965–974. doi: 10.3892/ijo.2011.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gross M, Top I, Laux I, Katz J, Curran J, Tindell C, Agus D. Beta-2-microglobulin is an androgen-regulated secreted protein elevated in serum of patients with advanced prostate cancer. Clin. Cancer Res. 2007;13(7):1979–1986. doi: 10.1158/1078-0432.CCR-06-1156. [DOI] [PubMed] [Google Scholar]
- 54.Greipp PR, San Miguel J, Durie BG, Crowley JJ, Barlogie B, Bladé J, Boccadoro M, Child JA, Avet-Loiseau H, Kyle RA, Lahuerta JJ, Ludwig H, Morgan G, Powles R, Shimizu K, Shustik C, Sonneveld P, Tosi P, Turesson International staging system for multiple myeloma. J. Clin. Oncol. 2005;23(15):3412–3420. doi: 10.1200/JCO.2005.04.242. [DOI] [PubMed] [Google Scholar]
- 55.Kyrtsonis MC, Maltezas D, Tzenou T, Koulieris E, Bradwell AR. Staging systems and prognostic factors as a guide to therapeutic decisions in multiple myeloma. Semin. Hematol. 2009;46(2):110–117. doi: 10.1053/j.seminhematol.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 56.Zhang L, Qi JY, Qi PJ, Wang YF, Zou DH, Yao HJ, An G, Yi SH, Li Q, Qiu LG. Comparison among immunologically different subtypes of 595 untreated multiple myeloma patients in northern China. Clin. Lymphoma Myeloma Leuk. 2010;10(3):197–204. doi: 10.3816/CLML.2010.n.031. [DOI] [PubMed] [Google Scholar]
- 57.Rajkumar SV, Fonseca R, Lacy MQ, Witzig TE, Lust JA, Greipp PR, Therneau TM, Kyle RA, Litzow MR, Gertz MA. Beta2-microglobulin and bone marrow plasma cell involvement predict complete responders among patients undergoing blood cell transplantation for myeloma. Bone Marrow Transplant. 1999;23(12):1261–1266. doi: 10.1038/sj.bmt.1701787. [DOI] [PubMed] [Google Scholar]
- 58.Shrout J, Yousefzadeh M, Dodd A, Kirven K, Blum C, Graham A, Benjamin K, Hoda R, Krishna M, Romano M, Wallace M, Garrett-Mayer E, Mitas M. Beta(2)microglobulin mRNA expression levels are prognostic for lymph node metastasis in colorectal cancer patients. Br. J. Cancer. 2008;98(12):1999–2005. doi: 10.1038/sj.bjc.6604399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Selinger B, Höhne A, Knuth A, Bernhard H, Ehring B, Tampé R, Huber C. Reduced membrane major histocompatibility complex class I density and stability in a subset of human renal cell carcinoma with low TAP and LMP expression. Clin. Cancer Res. 1996;2(8):1427–1433. [PubMed] [Google Scholar]
- 60.Kallfelz M, Jung D, Hilmes C, Knuth A, Jaeger E, Huber C, Selinger B. Induction of immunogenicity of a human renal-cell carcinoma cell line by TAP1-gene transfer. Int. J. Cancer. 1999;81(1):125–133. doi: 10.1002/(sici)1097-0215(19990331)81:1<125::aid-ijc21>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 61.Ogretmen B, McCauley MD, Safa AR. Molecular mechanisms of loss of beta 2-microglobulin expression in drug-resistant breast cancer sublines and its involvement in drug resistance. Biochemistry. 1998;37(33):11679–11691. doi: 10.1021/bi980573c. [DOI] [PubMed] [Google Scholar]
- 62.Rowley DR, Dang TD, McBride L, Gerdes MJ, Lu B, Larsen M. Beta-2 microglobulin is mitogenic to PC-3 prostatic carcinoma cells and antagonistic to transforming growth factor beta 1 action. Cancer Res. 1995;55(4):781–786. [PubMed] [Google Scholar]
- 63.Zhau HE, Odero-Marah V, Lue HW, Nomura T, Wang R, Chu G, Liu Z R, Zhou BP, Huang WC, Chung LW. Epithelial to mesenchymal transition (EMT) in human prostate cancer: Lessons learned from ARCaP model. Clin. Exp. Metastasis. 2008;25(6):601–610. doi: 10.1007/s10585-008-9183-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Eichner T, Radford SE. Understanding the complex mechanisms of ß2-microglobulin amyloid assembly. FEBS J. 2011;278(20):3868–3883. doi: 10.1111/j.1742-4658.2011.08186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Centrella M, McCarthy TL, Canalis E. Beta 2-microglobulin enhances insulin-like growth factor I receptor levels and synthesis in bone cell cultures. J. Biol. Chem. 1989;264(31):18268–18271. [PubMed] [Google Scholar]
- 66.Stagsted J, Olsson L, Holman GD, Cushman SW, Satoh S. Inhibition of internalization of glucose transporters and IGF-II receptors.Mechanism of action of MHC class I-derived peptides which augment the insulin response in rat adipose cells. J. Biol. Chem. 1993;268(30):22809–22813. [PubMed] [Google Scholar]
- 67.Stagsted J, Ziebe S, Satoh S, Holman GD, Cushman SW, Olsson L. Insulinomimetic effect on glucose transport by epidermal growth factor when combined with a major histocompatibility complex class I-derived peptide. J. Biol. Chem. 1993;268(3):1770–1774. [PubMed] [Google Scholar]
- 68.Ramalingam TS, Chakrabarti A, Edidin M. Interaction of class I human leukocyte antigen (HLA-I) molecules with insulin receptors and its effect on the insulin-signaling cascade. Mol. Biol. Cell. 1997;8(12):2463–2474. doi: 10.1091/mbc.8.12.2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huang WC, Zhau HE, Chung LW. Androgen receptor survival signaling is blocked by anti-beta2-microglobulin monoclonal antibody via a MAPK/lipogenic pathway in human prostate cancer cells. J. Biol. Chem. 2010;285(11):7947–7956. doi: 10.1074/jbc.M109.092759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Scher HI, Sawyers CL. Biology of progressive, castration-resistant prostate cancer: directed therapies targeting the androgen-receptor signaling axis. J. Clin. Oncol. 2005;23(32):8253–8261. doi: 10.1200/JCO.2005.03.4777. [DOI] [PubMed] [Google Scholar]
- 71.Huang WC, Li X, Liu J, Lin J, Chung LW. Activation of androgen receptor, lipogenesis, and oxidative stress converged by SREBP-1 is responsible for regulating growth and progression of prostate cancer cells. Mol. Cancer Res. 2012;10(1):133–142. doi: 10.1158/1541-7786.MCR-11-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Karsenty G, Wagner EF. Reaching a genetic and molecular understandings of skeletal development. Dev. Cell. 2002;2(4):389–406. doi: 10.1016/s1534-5807(02)00157-0. [DOI] [PubMed] [Google Scholar]
- 73.Theill LE, Boyle WJ, Penninger JM. RANK-L and RANK: T cells bone loss and mammalian evolution. Annu. Rev. Immunol. 2002;20:795–823. doi: 10.1146/annurev.immunol.20.100301.064753. [DOI] [PubMed] [Google Scholar]
- 74.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423(6937):337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 75.Koeneman KS, Yeung F, Chung LW. Osteomimetic properties of prostate cancer cells: A hypothesis supporting the predilection of prostate cancer metastasis and growth in the bone environment. Prostate. 1999;39(4):246–261. doi: 10.1002/(sici)1097-0045(19990601)39:4<246::aid-pros5>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 76.Chung LW, Huang WC, Sung SY, Wu D, Odero-Marah V, Nomura T, Shigemura K, Miyagi T, Seo S, Shi C, Molitierno J, Elmore J, Anderson C, Isotani S, Edlund M, Hsieh CL, Wang R, Shehata B, Zhau HE. Stromal-epithelial interaction in prostate cancer progression. Clin. Genitourin. Cancer. 2006;5(2):162–170. doi: 10.3816/CGC.2006.n.034. [DOI] [PubMed] [Google Scholar]
- 77.Lue HW, Yang X, Wang R, Qian W, Xu RZ, Lyles R, Osunkoya AO, Zhou BP, Vessella RL, Zayzafoon M, Liu ZR, Zhau HE, Chung LW. LIV-1 promotes prostate cancer epithelial-to-mesenchymal transition and metastasis through HB-EGF shedding and EGFR-mediated ERK signaling. PLoS One. 2011;6(11):e27720. doi: 10.1371/journal.pone.0027720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hu P, Chu GC, Zhu G, Yang H, Luthringer D, Prins G, Habib F, Wang Y, Wang R, Chung LW, Zhau HE. Multiplexed quantum dot labeling of activated c-Met signaling in castration-resistant human prostate cancer. PLo One. 2011;6(12):e28670. doi: 10.1371/journal.pone.0028670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Balint E, Sprague SM. Beta2-microglobulin and bone cell metabolism. Nephrol. Dial. Transplant. 2001;16(6):1108–1111. doi: 10.1093/ndt/16.6.1108. [DOI] [PubMed] [Google Scholar]
- 80.Jones DH, Nakashima T, Sanchez OH, Kozieradzki I, Komarova SV, Sarosi I, Morony S, Rubin E, Sarao R, Hojilla CV, Komnenovic V, Schreiber M, Dixon SJ, Sims SM, Khokha R, Wada T, Penninger JM. Regulation of cancer cell migration and bone metastasis by RANKL. Nature. 2006;440(7084):692–696. doi: 10.1038/nature04524. [DOI] [PubMed] [Google Scholar]
- 81.Lazennec G, Jorgensen C. Concise review: adult multipotent stromal cells and cancer: risk or benefit?. Stem Cells. 2008;26(6):1387–1394. doi: 10.1634/stemcells.2007-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Voermans C, Van Heese WP, De Jong I, Gerritsen WR, Van Der Schoot CE. Migratory behavior of leukemic cells from acute myeloid leukemia patients. Leukemia. 2002;16(4):650–657. doi: 10.1038/sj.leu.2402431. [DOI] [PubMed] [Google Scholar]
- 83.Tabatabai G, Bähr O, Möhle R, Eyüpoglu IY, Boehmler AM, Wischhusen J, Rieger J, Blumcke I, Weller M, Wick W. Lessons from the bone marrow: how malignant glioma cells attract adult haematopoietic progenitor cells. Brain. 2005;128(Pt 9):2200–2211. doi: 10.1093/brain/awh563. [DOI] [PubMed] [Google Scholar]
- 84.Wang R, Sun X, Wang CY, Hu P, Chu CY, Liu S, Zhau HE, Chung LW. Spontaneous cancer-stromal cell fusion as a mechanism of prostate cancer androgen-independent progression. PLoS One. 2012;7(8):e42653. doi: 10.1371/journal.pone.0042653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shi C, Zhu Y, Huang WC, Zhau HE, Wang R, Odero-Marah V, Chung LWK. Bi-directional interactions of bone marrow mesenchymal stem cells with human prostate cancer cells. J. Urol. 2007;177(4 Suppl):92. [Google Scholar]
- 86.Zhu Y, Su Y, Cheng T, Chung LWK, Shi C. Beta2-microglobulin as a potential factor for the expansion of mesenchymal stem cells. Biotechnol. Lett. 2009;31(9):1361–1365. doi: 10.1007/s10529-009-0027-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhu Y, Shi C. Beta2-microglobulin a novel factor for the expansion of mesenchymal stem cells. J. Biotechnol. 2008;136(Suppl ):S177. doi: 10.1007/s10529-009-0027-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shi C, Zhu Y, Chung LWK, Su Y, Cheng T. PC4 is a novel oncogenic gene for mesenchymal stem cell transformation and mediates the reciprocal actions between mesenchymal stem cells and prostate cancer cells. Exp. Hematol. 2008;36:S82–S83. [Google Scholar]
- 89.Mori M, Terui Y, Ikeda M, Tomizuka H, Uwai M, Kasahara T, Kubota N, Itoh T, Mishima Y, Douzono-Tanaka M, Yamada M, Shimamura S, Kikuchi J, Furukawa Y, Ishizaka Y, Ikeda K, Mano H, Ozawa K, Hatake K. Beta(2)-microglobulin identified as an apoptosis-inducing factor and its characterization. Blood. 1999;94(8):2744–2753. [PubMed] [Google Scholar]
- 90.Wu CH, Rastegar M, Gordon J, Safa AR. Beta(2)-microglobulin induces apoptosis in HL-60 human leukemia cell line and its multidrug resistant variants overexpressing MRP1 but lacking Bax or overexpressing P-glycoprotein. Oncogene. 2001;20(48):7006–7020. doi: 10.1038/sj.onc.1204893. [DOI] [PubMed] [Google Scholar]
- 91.Yang J, Zhang X, Wang J, Qian J, Zhang L, Wang M, Kwak LW, Yi Q. Anti beta2-microglobulin monoclonal antibodies induce apoptosis in myeloma cells by recruiting MHC class I to and excluding growth and survival cytokine receptors from lipid rafts. Blood. 2007;110(8):3028–3035. doi: 10.1182/blood-2007-06-094417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nazy ZA, Hubner B, L?hning C, Rauchenberger R, Reiffert S, Thomassen-Wolf E, Zahn S, Leyer S, Schier EM, Zahradnik A, Brunner C, Lobenwein K, Rattel B, Stanglmaier M, Hallek M, Wing M, Anderson S, Dunn M, Kretzschmar T, Tesar M. Fully human HLA-DR-specific monoclonal antibodies efficiently induce programmed death of malignant lymphoid cells. Nat. Med. 2002;8(8):801–807. doi: 10.1038/nm736. [DOI] [PubMed] [Google Scholar]