Abstract
In HIV-1 infection, plasma viral load (VL) has dual implications for pathogenesis and public health. Based on well-known patterns of HIV-1 evolution and immune escape, we hypothesized that VL is an evolving quantitative trait that depends heavily on duration of infection (DOI), demographic features, human leukocyte antigen (HLA) genotypes and viral characteristics. Prospective data from 421 African seroconverters with at least four eligible visits did show relatively steady VL beyond 3 months of untreated infection, but host and viral factors independently associated with cross-sectional and longitudinal VL often varied by analytical approaches and sliding time windows. Specifically, the effects of age, HLA-B*53 and infecting HIV-1 subtypes (A1, C and others) on VL were either sporadic or highly sensitive to time windows. These observations were strengthened by the addition of 111 seroconverters with 2–3 eligible VL results, suggesting that DOI should be a critical parameter in epidemiological and clinical studies.
Keywords: Africa, HIV-1, subtype, HLA, statistical models, viral load
Introduction
As an informative trait specific for HIV-1 infection, plasma viral load (VL) is well known for its strong correlation with the probability of sexual transmission (Fideli et al., 2001; Quinn et al., 2000) and rate of disease progression (Mellors et al., 1995; Mellors et al., 2007). Even in individuals with uncertain duration of infection, a relatively steady set-point VL can last for years to serve as a proxy of host-virus equilibrium (Arnaout et al., 1999; Fiebig et al., 2003). Factors independently associated with set-point VL range from viral characteristics to multiple quantitative trait loci (QTLs) in the human genome (Prentice and Tang, 2012; Yue et al., 2013), especially genes in the human major histocompatibility complex (MHC) that encode human leukocyte antigens (HLA) (Apps et al., 2013; Fellay et al., 2009; Leslie et al., 2010).
The widely accepted assumption and interpretation of set-point VL can have various problems. For example, HIV-1 immune escape can frequently tip the initial equilibrium in favor of the virus (Crawford et al., 2009; Kawashima et al., 2009; Mellors et al., 1995), which may obscure the relationship between set-point VL and HIV-1 disease progression (Mellors et al., 2007; Rodriguez et al., 2006). A second issue relates to various statistical methods that either fully embrace the concept of set-point VL (Prentice and Tang, 2012) or provide two alternative strategies, i.e., mixed model for repeated measures (Prentice et al., 2013; Shrestha et al., 2009; Tang et al., 2011) and assessment of cumulative viral load burden (Arnaout et al., 1999; Cole et al., 2010; Mugavero et al., 2011). To establish the advantages and disadvantages of these analytical approaches, we have tested a central hypothesis that cross-sectional and longitudinal VL data are evolving outcomes that depend heavily on duration of infection DOI), demographic features, HLA genotypes and viral characteristics.
Results
Characteristics of 421 HIV-1 seroconverters (SCs) in the study population
For this study, sufficient prospective data were available for 421 SCs from Zambia (Lusaka and Copper Belt, n=164), Uganda (Entebbe and Masaka, n=110), Kenya (Kilifi and Nairobi, n=83) and Rwanda (Kigali, n=64) (Table 1). In each subgroup, males outnumbered females, especially in Kenya. All volunteers were relatively young at enrollment (mean age between 25.1 and 33.6 years by site). The estimated dates of infection (EDI) ranged from May 2005 to March 2011, being highly comparable across study sites. Based on a 1.3-kb fragment of the HIV-1 pol gene sequences (successful in 93.4% of SCs), subtypes A1 and C accounted for 74.2% of the total, while other subtypes (B or D) and recombinant forms were infrequent, precluding further stratification (Table 1).
Table 1.
Characteristics of 421 HIV-1 seroconverters enrolled from four African countries.
| Characteristicsa and HLA variants of major interest | Kenya | Rwanda | Uganda | Zambia |
|---|---|---|---|---|
| Number of seroconverters with sufficient data | 83 | 64 | 110 | 164 |
| Sex ratio (M/F) | 5.9 (71/12) | 1.4 (37/27) | 1.4 (65/45) | 1.3 (92/72) |
| Age: mean ± SD (yr) | 25.1 ± 4.4 | 31.8 ± 8.9 | 31.9 ± 8.3 | 33.6 ± 8.0 |
| Age ≥ 40 yr: no. (%) | 0 (0.0) | 11 (16.9) | 22 (20.0) | 34 (20.7) |
| Estimated dates of infection | ||||
| Earliest | Nov. 2005 | May 2005 | Sep. 2005 | June 2005 |
| Latest | Feb. 2011 | Jan. 2011 | Sep. 2010 | Mar. 2011 |
| Person-visits with eligible viral load (VL) data (3–24 months) | 617 | 486 | 862 | 1,189 |
| HIV-1 subtype:b no. (%) | ||||
| A1 | 58 (69.9) | 50 (78.1) | 41 (37.3) | 0 (0.0) |
| C | 8 (9.6) | 7 (10.9) | 5 (4.6) | 144 (87.8) |
| D | 8 (9.6) | 1 (1.5) | 52 (47.3) | 0 (0.0) |
| Others (B and recombinants) | 5 (6.0) | 3 (4.7) | 6 (5.5) | 2 (1.2) |
| Unknown (no viral sequencing) | 4 (4.8) | 3 (4.7) | 6 (5.5) | 18 (11.0) |
| Cross-sectional viral load (VL): mean ± SD (in log10) | ||||
| 3–6 months | 4.2 ± 1.0 | 4.1 ± 1.1 | 4.4 ± 1.0 | 4.5 ± 1.0 |
| 6–9 months | 4.4 ± 0.9 | 4.1 ± 1.0 | 4.3 ± 1.1 | 4.4 ± 0.9 |
| 9–12 months | 4.1 ± 1.0c | 4.1 ± 1.0c | 4.3 ± 1.0 | 4.5 ± 0.9 |
| 12–18 months | 4.2 ± 0.8 | 4.1 ± 1.0c | 4.2 ± 1.2 | 4.5 ± 0.8 |
| 18–24 months | 4.0 ± 1.1c | 4.0 ± 1.1c | 4.1 ± 1.2c | 4.6 ± 0.8 |
| Repeated VL measurements (in log10) | ||||
| Year 1 geometric mean (3–12 months): mean ± SD | 4.3 ± 0.9 | 4.1 ± 1.0 | 4.3 ± 1.0 | 4.4 ± 0.9 |
| Number of VLs taken within year 1: median (IQR) | 3 (3–4) | 3 (3–5) | 3 (3–4) | 3 (3–4) |
| Year 2 geometric mean (13–24 months): mean ± SD | 4.1 ± 0.9c | 3.9 ± 1.1c | 4.2 ± 1.0 | 4.5 ± 0.7 |
| Number of VLs taken within year 2: median (IQR) | 4 (4–4) | 4 (4–4) | 4 (4–4) | 4 (3–4) |
| Viremia copy years (VCY) (3–24 months): mean ± SD | 4.7 ± 0.7c | 4.6 ± 0.8c | 4.7 ± 0.9 | 5.0 ± 0.6 |
| Number of VLs used for calculating VCY: median (IQR) | 7 (7–8) | 7 (7–8) | 7 (7–9) | 7 (6–8) |
| HLA-B*18 (B*18:01) | 5 (6.0) | 5 (7.8) | 10 (9.1) | 11 (6.7) |
| HLA-B*45 (B*45:01) | 18 (21.7) | 4 (6.3) | 15 (13.6) | 26 (15.9) |
| HLA-B*53 (B*53:01) | 14 (16.9) | 10 (15.6) | 22 (20.0) | 29 (17.7) |
| HLA-B*57 (mostly B*57:03) | 8 (9.6) | 6 (9.4) | 10 (9.1) | 17 (10.4) |
Non-standard abbreviations: IQR, interquartile range (25th to 75% percentile); SD, standard deviation of the mean.
The minor HIV-1 subtypes (B, D, and others) are combined for analysis. Unknown subtypes were assigned based on probabilities (see text).
Statistically significant differences when compared with data from Zambians.
As reported earlier (Prentice et al., 2013; Tang et al., 2011), HIV-1 subtypes A1 and C were predominant in Rwandan and Zambian SCs, respectively. Kenyan SCs differed from others in their lower age (25.1 ± 4.4 years), high male-to-female ratio (5.9), and prevalence of multiple infecting HIV-1 subtypes (A1, C, D and others) (6.0% to 69.9% per subtype). Ugandan SCs had the greatest frequency (52.8%) of non-A1 and non-C subtypes (mostly subtype D) (Table 1).
The spectrum of VLs and cumulative viremia (VCY) in 421 SCs during early HIV-1 infection
In the 3–24 months interval after EDI, VL was measured at a total of 3,154 person-visits. Cross-sectional VLs beyond 3 months of infection ranged from below detection (<2.60 log10 copies/mL) to 6.6 log10. The mean log10 VL in SCs from each country was stable, with little (≤0.40 log10) fluctuation between visit intervals, especially adjacent intervals (≤0.30 log10) (Table 1). Geometric mean VLs were also quite similar between year 1 and year 2 (≤0.30 log10). On the other hand, country-specific variations in VLs began to emerge during later visits (P <0.001 by ANOVA) (Table 1). Reflecting a strong collinearity between region (geography) and HIV-1 subtypes, Zambian SCs consistently had higher VLs than Kenyan and Rwandan SCs for the 18–24 months VL, year 2 geometric mean VL and cumulative viremia (P <0.05 in all six comparisons).
Evaluation of linear correlation between longitudinal and cross-sectional VL data
In 56 (8 × 7) pairwise comparisons of eight distinct outcomes of VL, the Pearson r values ranged from 0.51 to 0.92 before and after statistical adjustments for age, sex, country of origin, and duration of infection (P <0.0001 for all tests) (Table 2). For the seven cross-sectional VL measurements with slight variation in effective sample sizes (n = 381–419), the overall pattern persistently revealed that correlation was weak unless the tests involved cross-sectional results from two adjacent visits (e.g., 3–6 months versus 6–9 months and 9–12 months vs. 12–18 months). Even so, correlation that was strong enough to imply two mutually interchangeable outcomes (i.e., r2 >0.80) was rare, being detected on a single occasion. In this case, the 6–9 months set-point VL showed a strong collinearity with the geometric mean VL during the 3–12 months interval regardless of statistical adjustments (r = 0.92, r2 = 0.85, and P <0.0001). In addition, as far as the adjusted r values were concerned, country of origin as a covariate was interchangeable with HIV-1 subtype as a covariate.
Table 2.
Relationships of cross-sectional viral load (VL) and cumulative viremia (VCY) in early HIV-1 infection, as defined by Pearson correlation coefficients (r).a
| VL measurements (no. of subjects) | Cross-sectional (set-point) VL at five intervals | Repeated measures | VCY | |||||
|---|---|---|---|---|---|---|---|---|
| 3–6 mo | 6–9 mo | 9–12 mo | 12–18 mo | 18–24 mo | 3–12 mo | 13–24 mo | 3–24 mo | |
| 3–6 months (381) | 1.00 | 0.76 | 0.69 | 0.65 | 0.52 | 0.88 | 0.62 | 0.72 |
| 6–9 months (394) | 0.74 | 1.00 | 0.77 | 0.73 | 0.58 | 0.92b | 0.70 | 0.80 |
| 9–12 months (404) | 0.68 | 0.76 | 1.00 | 0.75 | 0.61 | 0.88 | 0.75 | 0.82 |
| 12–18 month (416) | 0.63 | 0.71 | 0.74 | 1.00 | 0.69 | 0.79 | 0.86 | 0.86 |
| 18–24 Month (408) | 0.51 | 0.57 | 0.61 | 0.68 | 1.00 | 0.64 | 0.90 | 0.78 |
| 3–12 months, geometric mean (419) | 0.88 | 0.92b | 0.88 | 0.77 | 0.63 | 1.00 | 0.77 | 0.87 |
| 13–24 months, geometric mean (418) | 0.61 | 0.70 | 0.74 | 0.85 | 0.89 | 0.76 | 1.00 | 0.90 |
| 3–24 months, VCY (421)b | 0.71 | 0.79 | 0.82 | 0.85 | 0.77 | 0.87 | 0.89 | 1.00 |
With log10-transformation before analysis, the r values below the diagonal are for the raw data, while r values above the diagonal are adjusted for age, sex, country of origin, and duration of infection (wherever applicable) (P <0.001 for all pairwise tests).
The R2 values exceed 0.80 in a single test only (shown in bold).
Identification of independent correlates of longitudinal viremia in 421 SCs
On the basis of adjusted statistical significance (P <0.05), multivariable models identified sex, duration of infection, infecting HIV-1 subtypes, and HLA-B genotypes as independent predictors of longitudinal VL outcomes (Table 3). These factors, along with age, accounted for about 5% of the overall variability in the VL dataset (P <0.0001). Ranking by univariable r2 values placed viral subtype C, HLA-B*57, and sex as the top three predictors. Alternatively, the top three predictors ranked by unadjusted VL differences (mean beta estimates) were HLA-B*57 (P <0.0001), HLA-B*18 (P = 0.018), and sex (P <0.0001) (Table 3).
Table 3.
Analyses of longitudinal viral load (VL) data (repeated measurements) in early HIV-1 infection (the 3–24 months interval after infection).
| Predictors (independent variables) | Effects by univariable modelsb | Multivariable modelc | |||
|---|---|---|---|---|---|
| Δ ± SE | P | R2 | Δ ± SE | P | |
| Age >40 years | −0.08 ± 0.11 | 0.494 | 0.000 | −0.14 ± 0.11 | 0.191 |
| Female sex | −0.36 ± 0.08 | <0.0001 | 0.038 | −0.37 ± 0.08 | <0.0001 |
| HIV-1 subtype Ca | 0.24 ± 0.08 | 0.004 | 0.028 | 0.38 ± 0.09 | <0.0001 |
| Other HIV-1 subtypesa | 0.13 ± 0.10 | 0.227 | 0.002 | 0.34 ± 0.11 | 0.002 |
| Duration of infection (quarterly) | −0.02 ± 0.00 | 0.009 | 0.007 | −0.02 ± 0.00 | 0.008 |
| HLA-B*18 | 0.37 ± 0.16 | 0.018 | 0.012 | 0.37 ± 0.15 | 0.013 |
| HLA-B*45 | 0.25 ± 0.12 | 0.028 | 0.008 | 0.25 ± 0.11 | 0.021 |
| HLA-B*57 | −0.53 ± 0.14 | 0.0001 | 0.016 | −0.47 ± 0.13 | 0.0003 |
| Overall summary statistics | NA | r2 =0.049, P <0.0001 | |||
HIV-1 subtype A is the reference group.
Top three predictors ranked by the effect size are underlined.
Based on simultaneous evaluation of all factors as shown. For consistency with reported approach (Tang et al., 2002b), age is kept as a covariate in all multivariable models (here and Table 3) regardless of statistical significance.
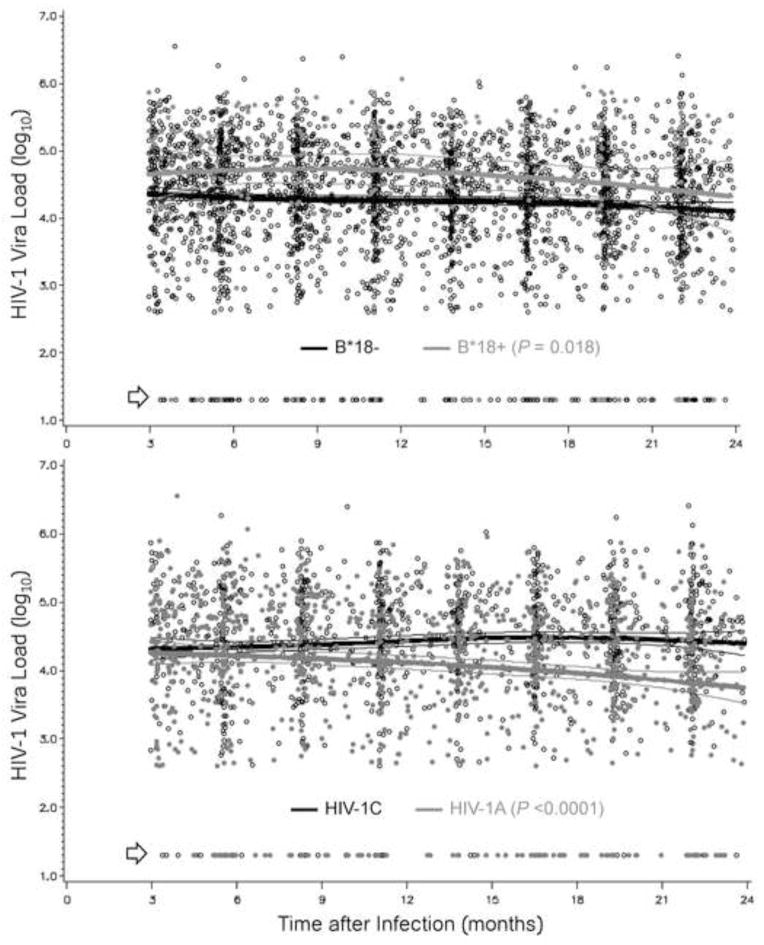
However, heterogeneity in HIV-1 viremia fluctuated over time when SCs defined by host and viral factors were compared. For example, clear separation in VL was seen between HLA-B*18 positive and HLA-B*18 negative SCs throughout the 3–24 months interval, but differences between HIV-1 subtypes A1 and C were not apparent in the 3–9 months period (Figure 1). Like HLA-B*18, B*45, B*57 and female sex also had highly persistent effects on VLs during the study intervals; these relationships were readily confirmed by analyses of cumulative viremia (data not shown).
Figure 1. Contrasting dynamics of HIV-1 viremia in African seroconverters defined by host and viral genotypes.
Prospective viral load measurements are compared between HLA-B*18-positive and HLA-B*18-negative subjects (top panel, 3,154 person-visits) and between two major HIV-1 subtypes, HIV-1C and HIV-1A (bottom panel, 2,306 person-visits). Thick and thin lines correspond to the expected mean (average) value and 95% confidence intervals for each stratum (see Table 3 for summary statistics based on mixed models). Arrows point to plasma viral load measurements that are <400 RNA copies/ml (transformed to 1.30 log10).
Viral and host factors associated with cross-sectional viremia: sensitivity analyses based on generalized linear regression models (GLMs)
Analyses of seven cross-sectional VL outcomes led to highly variable findings (Table 4). The number of independent predictors ranged from two (sex and HLA-B*45) for the 3–6 months VL to six (all but HIV subtype) for the 6–9 months VL. HLA-B*18 and B*45 as unfavorable factors were each detected in 4 out of 6 models, without a clear preference for early or later intervals (Table 4). HLA-B*57 was a favorable factor in 5 out of 6 models, with an effect size exceeding 0.30 log10 after the first 6 months of infection. Overall, host and viral factors explained 7.2% to 14.8% of variability in the six VL cross-sectional phenotypes (P <0.0001 in each test). Of note, analyses of GM VLs had little advantage over individual VLs.
Table 4.
Sensitivity analyses using cross-sectional viral load (VL) results in early HIV-1 infection (multivariable models)a.
| Predictors (independent variables) | Individual and joint effects on cross-sectional VLsd | Geometric meand | ||||
|---|---|---|---|---|---|---|
| 3–6 months | 6–9 months | 9–12 monthse | 12–18 months | 18–24 months | 13–24 months | |
| Age >40 years | −0.12 ± 0.1 | −0.29 ± 0.1* | −0.21 ± 0.1 | −0.18 ± 0.1 | −0.12 ± 0.1 | −0.13 ± 0.1 |
| Female gender | −0.38 ± 0.1*** | −0.50 ± 0.1*** | −0.32 ± 0.1** | −0.50 ± 0.1*** | −0.32 ± 0.1** | −0.36 ± 0.1*** |
| HIV-1 subtype Cb | 0.22 ± 0.1 | 0.20 ± 0.1 | 0.27 ± 0.1* | 0.38 ± 0.1*** | 0.66 ± 0.1*** | 0.51 ± 0.1*** |
| Other HIV-1 subtypesb | 0.26 ± 0.1 | 0.22 ± 0.1 | 0.31 ± 0.1* | 0.33 ± 0.1** | 0.39 ± 0.1** | 0.40 ± 0.1*** |
| Duration of infection (monthly) | −0.10 ± 0.1 | −0.11 ± 0.1* | 0.06 ± 0.1 | 0.04 ± 0.1 | −0.05 ± 0.0 | NA |
| HLA-B*18 | 0.27 ± 0.2 | 0.54 ± 0.2** | 0.48 ± 0.2** | 0.48 ± 0.2** | 0.25 ± 0.2 | 0.40 ± 0.2* |
| HLA-B*45 | 0.30 ± 0.1* | 0.32 ± 0.1* | 0.21 ± 0.1 | 0.11 ± 0.1 | 0.38 ± 0.1** | 0.22 ± 0.1 |
| HLA-B*57 | −0.20 ± 0.2 | −0.41 ± 0.2* | −0.58 ± 0.2*** | −0.57 ± 0.1*** | −0.46 ± 0.2** | −0.51 ± 0.1*** |
| Overall R2 for each modelc | 0.072 | 0.129 | 0.104 | 0.152 | 0.137 | 0.151 |
Individual predictors are defined in Table 3.
HIV-1 subtype A serves as the reference group.
Overall P <0.0001 for all tests.
The cross-sectional outcomes are defined in Table 1. Summary statistics correspond to mean difference (beta estimate) ± standard error of the mean. Statistical significance is shown at three levels (* for P <0.05; ** for <0.01, and *** for <0.001).
Estimates here are virtually identical to those based on the analysis of 3–12 months geometric mean VLs (overall r2 = 0.108).
Findings based on alternative approaches
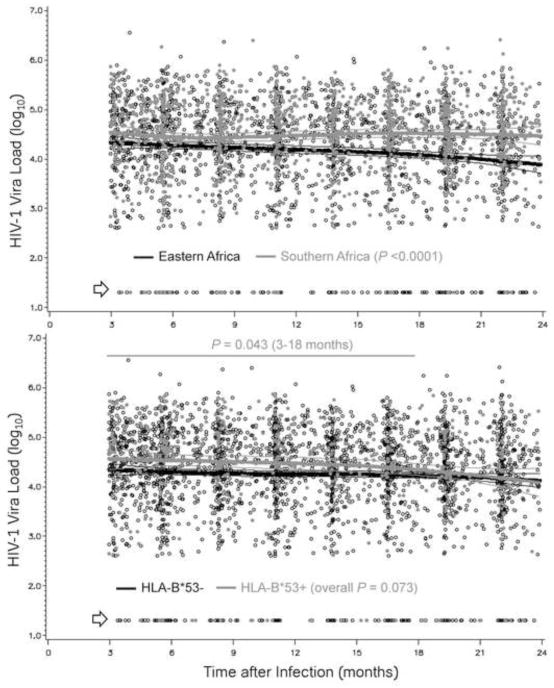
In alternative analyses, HIV-1 VL dynamics differed between eastern and southern Africa (P <0.0001) (Figure 2), which was consistent with differences seen with HIV-1 subtypes. In addition, analyses guided by LOESS curves revealed that HLA-B*53 had modest association with VL in the 3–18 months intervals (2,361 person-visit, P = 0.043) (Figure 2). VLs in the 75 subjects with HLA-B*53 had a unique pattern: a modest separation between B*53-positive and B*53-negative subjects (average Δ = 0.21 ± 0.1 log10) faded shortly after the 18 months mark (Figure 2). In the final round of data analyses for all 532 eligible SCs (Table 5), HLA-B*53 explained 1.1% of the overall variance in longitudinal VLs (P = 0.055 by univariable analysis), while the multivariable model confirmed its independent association with longitudinal VLs (adjusted P = 0.025).
Figure 2. Viremia in HIV-1 seroconverters stratified by geography and HLA-B*53.
Prospective viral load measurements (3,154 person-visits) are compared between Eastern Africa (Kenya, Rwandan and Uganda) and Southern Africa (Zambia) (top panel) and between HLA-B*53-positive and negative subgroups (bottom panel). Thick and thin lines correspond to the expected average value and 95% confidence intervals for each stratum (overall unadjusted P = 0.056). Arrow points to plasma viral load measurements that are <400 RNA copies/ml (transformed to 1.30 log10).
Table 5.
Sensitivity analyses using longitudinal viral load (VL) data from 532 seroconverters who have at least two VL data points during the 3–24 months interval after infection.
| Predictors (independent variables) | Effects by univariable modelsb | Multivariable modelc | |||
|---|---|---|---|---|---|
| Δ ± SE | P | R2 | Δ ± SE | P | |
| Age >40 years | −0.05 ± 0.10 | 0.611 | 0.000 | −0.11 ± 0.08 | 0.237 |
| Female sex | −0.35 ± 0.08 | <0.0001 | 0.025 | −0.38 ± 0.10 | <0.0001 |
| HIV-1 subtype Ca | 0.26 ± 0.08 | 0.001 | 0.020 | 0.37 ± 0.08 | <0.0001 |
| Other HIV-1 subtypesa | 0.10 ± 0.10 | 0.320 | 0.001 | 0.29 ± 0.10 | 0.003 |
| Duration of infection (monthly) | −0.01 ± 0.00 | 0.001 | 0.007 | −0.01 ± 0.00 | 0.001 |
| HLA-B*18 | 0.33 ± 0.15 | 0.025 | 0.007 | 0.36 ± 0.14 | 0.001 |
| HLA-B*45 | 0.28 ± 0.10 | 0.005 | 0.013 | 0.27 ± 0.10 | 0.006 |
| HLA-B*53 | 0.18 ± 0.10 | 0.055 | 0.011 | 0.20 ± 0.09 | 0.025 |
| HLA-B*57 | −0.50 ± 0.13 | <0.0001 | 0.012 | −0.46 ± 0.12 | <0.001 |
| Overall summary statistics | NA | R2 = 0.041, P <0.0001 | |||
HIV-1 subtype A is the reference group.
Top three predictors ranked by the effect size are underlined.
Based on simultaneous evaluation of all factors as shown. For consistency with reported approach (Tang et al., 2002b), age is kept as a covariate in all multivariable models (here and Table 3) regardless of statistical significance.
Additional observations on HLA variants
Among the four HLA variants independently associated with longitudinal VLs (Table 5), HLA-B*18, B*53 and B*57 had similar distribution in the four African countries (Table 1). Two thirds of subjects with B*57 had HLA-B*57:03, which had its own association with reduced viremia (data not shown). On the other hand, HLA-B*18, B*53 and B*45 were each represented by a single allele (B*18:01, B*53:01 and B*45:01, respectively) regardless of study sites. Other HLA alleles relevant to Africans, including A*29, A*36, A*74, B*13, B*14, B*35, B*39, B*42, B*51, B*58:01, B*58:02, B*81, C*04, C*18, and the A*30 + C*03 combination (Apps et al., 2013; Leslie et al., 2010; McLaren et al., 2012; Tang et al., 2010), had minimal impact on VL outcomes (data not shown). Alternative analyses of structurally defined HLA-A and HLA-B supertypes were inconclusive (data not shown).
Discussion
Through systematic analyses of prospective data from African seroconverters with early HIV-1 infection, our work demonstrated that cross-sectional VL outcomes change rapidly as the infection progresses. In other words, VLs separated by as little as three months can be considered as different outcomes (Table 2) with varying correlates (Table 4). For subgroups of SCs defined by host and viral factors (Table 4), three patterns of local regression curves (Figures 1–2) represent (i) steady and robust differences across visits, (ii) convergence followed by gradual divergence, and (iii) divergence followed by gradual convergence. Other patterns (e.g., wave-like and U-shaped) were less obvious, probably because the overall study interval (from 3 to 24 months after EDI) was relatively short.
In previous work based on 269 SCs (homosexual men in the U.S.) with semi-annual follow-up visits, VL trajectories (slopes) during the first three years after seroconversion correlated with AIDS-free time, while VLs in the three years immediately before AIDS diagnosis had no predictive values (Lyles et al., 1999). When stratified by host genotypes, SCs from the same study showed different patterns of VL dynamics in early infection as well (Tang et al., 2002a). While our new findings here are largely consistent with the reports from the Multicenter AIDS Cohort Study, three major advantages in study design and analytical approaches here can help strengthen the search for underlying mechanisms. First, frequent testing before and during seroconversion (at monthly to quarterly visits) has improved the precision in assigning EDI and DOI. Second, follow-up visits after seroconversion are frequent enough to facilitate the comparison of early virologic outcomes in sliding time windows (Table 4). Third, host and viral genotypes can be analyzed jointly in multivariable models. The two major HIV-1 subtypes, A1 and C, are widespread in sub-Saharan Africa (Osmanov et al., 2002; Tebit and Arts, 2011), with some evidence for disparity in evolutionary and pathophysiologic attributes when compared with other subtypes (Baeten et al., 2007; Kaleebu et al., 2001; Kiwanuka et al., 2008; Vasan et al., 2006). VL differences between HIV-1 subtypes A1 and C infection (Table 3 and Figure 1) are generally consistent with the contrasting rates of heterosexual HIV-1 transmission in Rwanda and Zambia where the respective subtypes predominate (Dunkle et al., 2008; Fideli et al., 2001).
Recent assessment of disease progression (time to severe immunodeficiency) has revealed the role of African HIV-1 subtypes in pathogenesis (Amornkul et al., 2013). However, given the strong collinearity between geography and HIV-1 subtypes (Table 1), a more definitive elucidation of host and viral factors in HIV-1 pathogenesis in Africa may require the assembly of a genetically homogeneous cohort with diverse HIV-1 subtypes and adequate follow-up. Such study is no longer feasible as new treatment guidelines recommended by the World Health Organization are expected to transform the test-and-treat concept into a global practice (Cohen et al., 2011; Montaner et al., 2010; Mugavero et al., 2012).
Among the three HLA-B variants independently and persistently associated with VL heterogeneity, HLA-B*57 is a well-known favorable factor, while HLA-B*18 and HLA-B*45 are consistently unfavorable in multiple studies (Apps et al., 2013; Lazaryan et al., 2011; Leslie et al., 2010; McLaren et al., 2012; Tang et al., 2010). Generalizable findings about these HLA-B variants, now in the context of sub-Saharan African populations, should benefit future epidemiologic and experimental studies, especially in the context of HIV-1 adaptation at the population level (Kawashima et al., 2009). HLA-B*18 has also been reported as partially protective against mother-to-child HIV-1 transmission in Kenyan infants (Farquhar et al., 2004), suggesting again that mechanisms for immune control of established HIV-1 infection can be quite distinct from those mediating acquisition of infection (Gao et al., 2010; Song et al., 2011; Tang et al., 2008).
HLA allelic products contribute to immune control of viral infection through both innate and adaptive immune pathways (Carrington, Martin, and van Bergen, 2008; Merino et al., 2013; Merino et al., 2012; Stewart et al., 2005). Alleles with early influences on HIV-1 infection tend to impose a strong selection pressure for viral immune escape mutations a phenomenon that has been repeatedly examined in individuals with HLA-B*57 and related alleles (Bansal et al., 2007; Crawford et al., 2009; Leslie et al., 2004; Novitsky et al., 2010; Wang et al., 2009). The unfavorable effect of HLA-B*18 on VL started early and remained stable (Figure 1), which may translate to a durable impact on HIV-1 pathogenesis. In settings where treatment priority is necessary, timely interventions directed to subjects carrying unfavorable HLA profile may maximize the benefits by preventing pathogenesis as well as transmission.
Our work also indicates that modest and relatively transient effects attributable to host factors like HLA-B*53 can easily escape detection when VLs in the first few months of infection are missed (Figure 2). As a common allele in Africans and African Americans, its relevance to HIV-1 infection has been highlighted in at least two recent studies (Apps et al., 2013; Lazaryan et al., 2011). Although VLs were only slightly elevated in Africans subjects with HLA-B*53 during the first 18 months of infection (Figure 2), the potential impact on HIV-1 reservoir and transmission rate may deserve some investigation.
Conclusions
Analyses of cross-sectional data often failed to identify host and viral factors (e.g., HLA-B*53 and HIV-1 subtypes) due to time-varying associations with VLs, even in early infection when complications by immune escape, co-infection and other forms of comorbidities should be minimal. For correlates (e.g, HLA-B*57) that can be readily identified using randomly chosen phenotypes, the magnitude of associations can vary from one interval to another. These observations continue to support our notion that DOI is an important parameter when cross-sectional VL results are assessed in clinical research (Prentice et al., 2013; Prentice and Tang, 2012; Tang et al., 2010). As early diagnosis of HIV-1 infection remains costly and difficult (Kozak et al., 2013), few studies can actually assess the timing of VL measurements in prevalent HIV-1 infection. One reasonable compromise is to down-play findings based solely on random sampling of cross-sectional data. Alternatively, immunologic and virologic techniques suitable for inferring DOI in seroprevalent infection (Cousins et al., 2013) may provide valuable information about DOI.
Materials and Methods
Study population
Recent HIV-1 seroconverters (SCs) were enrolled from Kenya, Rwanda, Uganda, and Zambia (Table 1) under a uniform study protocol developed and implemented by the International AIDS Vaccine Initiative (IAVI) (Amornkul et al., 2013). The procedures for written informed consent and research activities were approved by institutional review boards at all collaborating clinical research centers, with further compliance to human experimentation guidelines set forth by the United States Department of Health and Human Services.
Follow-up strategies before and after HIV-1 infection
Identification of SCs relied on frequent (monthly to quarterly) testing of HIV-1 seronegative subjects at high risk of HIV-1 infection through heterosexual (common) and homosexual (occasional) exposure, with the vast majority being partners of HIV-1 discordant couples and/or individuals diagnosed with sexually transmitted infections. As described in detail elsewhere (Amornkul et al., 2013; Karita et al., 2007; Prentice et al., 2013), the estimated dates of HIV-1 infection (EDI) were defined as one of the following: (i) the midpoint between the last seronegative and first positive HIV-1 antibody tests, (ii) two weeks before the first positive test for HIV-1 p24 antigen in plasma, (iii) 10 days before the first positive test for plasma viral load (VL) while being negative for both p24 and rapid HIV-1 antibody tests, and (iv) event date for the only high-risk exposure. Following confirmation of HIV-1 infection (detection of VL), clinical visits were scheduled monthly for the first three months after EDI and quarterly for the 3–24 months interval. Initiation of antiretroviral therapy (ART) followed national guidelines (Ngongo et al., 2012), and all visits and VL measurements after ART initiation were excluded. In all, 421 SCs (Table 1) were selected based on: (i) availability of more than 50 SCs from a single country, with biological specimens for DNA extraction and HLA class I genotyping, (ii) at least four time points of VL in the early chronic phase (3–24 months) of infection, with no gap greater than one year between two consecutive VL measurements, and (iii) no ART during the eligible study intervals. An additional 111 SCs with only 2–3 eligible VL measurements were available for secondary analyses. The remaining SCs excluded from analyses here included those enrolled from South Africa (n = 26) and a small group (n = 46) with limited follow-up (no more than a single VL for eligible visits).
HIV-1 viral load (VL) as a quantitative trait
Plasma VL (RNA copies/mL) was measured at a central location (Clinical Laboratory Services, Johannesburg, South Africa) using the Amplicor Monitor v1.5 assay (Roche Applied Science, Indianapolis, IN) and following good clinical laboratory practices (Amornkul et al., 2013). With a focus on visits beyond acute-phase (the first 3 months after EDI), eligible VLs in the 3–24 months intervals were first treated as the longitudinal outcome and then divided into cross-sectional outcomes corresponding to five visit intervals: 3.1 to 6.0 months, 6.1 to 9.0 months, 9.1 to 12.0 months, 12.1 to 18.0 months and 18.1 to 24.0 months (rounded to the nearest integer for tables and figures). Repeated measures in the 3.1–12.0 and 12.1–24.0 months intervals also allowed the calculation of geometric mean (GM) VLs. In addition, cumulative viremia as a time-varying measurement of VL in the 3.1–24.0 months after EDI was expressed as the number of virus copies per mL of plasma multiplied by time of follow-up (years) (Cole et al., 2010; Mugavero et al., 2011). A trapezoidal rule was used to approximate the integral representing the area under the curve for each participant’s longitudinal VL. Within each segment (time between two VL sampling dates), VL burden was the mean of the two VL measurements multiplied by the time interval between the sampling dates. Summation of the individual segments (3–24 months after EDI) gave rise to the viremia copy-year (VCY) outcome. For log10 transformation, all VLs below the lower limit of detection (LLD = 400 RNA copies/mL) were assumed to be 1.30 (half of log10400). In alternative analyses, setting VL below LLD to 2.30 log10 (200 copies/mL) did not change statistical models (data not shown).
Viral sequencing and human leukocyte antigen (HLA) class I genotyping
Methods for HIV-1 pol gene sequencing and HLA class I genotyping have been described elsewhere (Price et al., 2011; Tang et al., 2011). Viruses were grouped into subtypes (mostly A1, C and D) and recombinant forms (rare) (Table 1). Allelic variants at three HLA class I loci (HLA-A, HLA-B and HLA-C) were fully resolved to the 4-digit level. Data analyses focused on three prominent variants, HLA-B*18 (unfavorable), B*45 (unfavorable), and -B*57 (favorable), based on their confirmed effects on VL in native Africans and African-Americans (Apps et al., 2013; Leslie et al., 2010; Tang et al., 2010).
Statistical Analysis
Using software packages in SAS, version 9.2 (SAS Institute, Cary, NC), SCs were stratified by country of origin and tabulated for demographic data, laboratory findings (viral subtypes and VL) (Table 1) and distribution of major HLA variants of interest. Methods used for comparing baseline characteristics included (i) analysis of variance (ANOVA), (ii) t-test for quantitative variables with a normal distribution, and (iii) χ2 and Fisher exact tests for categorical variables. The extent of collinearity among eight VL outcomes was determined by Pearson’s correlation coefficients (r), and paired data with r ≥0.90 (r2 ≥0.80) were considered as mutually interchangeable (Craney and Surles, 2002). Subsequent association analyses, including mixed models, generalized linear models (GLMs) and local regression (LOESS) curves, aimed at identifying independent correlates of VL and assessing their relative effects on VL over time during the 3–24 months follow-up period. Based on evidence from earlier studies (Prentice et al., 2013; Tang et al., 2010), age, sex, duration of infection (DOI, measured monthly or quarterly), infecting viral subtypes and common HLA variants were tested as independent cofactors in univariable and multivariable models. The performance of individual models was gauged by their overall R2 values (corresponding to variability explained by factors in the model), while the performance of individual factors was ranked first by the regression beta (mean difference) and then by the R2 values from univariable models. Statistical significance was accepted at the level of P ≤0.05, but age was kept in all multivariable models regardless of statistical significance (for consistency with earlier work). Major findings from these models were subjected to three sets of sensitivity analyses: (i) replacing HIV-1 subtype with country of origin as the two covariates were highly collinear (Tang et al., 2011); (ii) including regional groupings (eastern versus southern Africa) as a covariate (Prentice et al., 2013); and (iii) adding the results from 101 eligible SCs with limited (2–3) VL data points to the overall model for repeated VL measurements.
Research Highlights.
In recent HIV-1 seroconverters, plasma viral load (VL) is a rapidly evolving outcome;
Host and viral factors show time-varying associations with longitudinal or cross-sectional VL;
Three types of VL trajectories are readily detected in analyses of longitudinal VL.
Acknowledgments
This work was funded by (i) the United States Agency for International Development (USAID) (to IAVI), (ii) United States National Institute of Allergy and Infectious Diseases (NIAID), through two R01 grants (AI071906 to R.A.K./J.T. and AI064060 to E.H.), (iii) Fogarty AIDS International Training and Research Program (AITRP) (grant FIC 2D43 TW001042 to S.L.). Although the submission of this study for publication required approval by representatives of the Kenya Medical Research Institute (KEMRI) and IAVI, the contents are the responsibility of the study authors and do not necessarily reflect the views of IAVI, NIAID, USAID or the United States government. We thank all members of the IAVI Africa HIV Prevention Partnership for their valuable contributions to cohort assembly and collection of prospective data. We are also grateful to several associates, especially Dongning He, Wei Song and Andrew Westfall, for assistance with genotyping and biostatistics. Part of this work was presented at the 19th Conference on Retroviruses and Opportunistic Infections (in Seattle, WA, March 5 to March 9, 2012).
Footnotes
Competing Interests
The authors declare no conflict of interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amornkul PN, Karita E, Kamali A, Rida WN, Sanders EJ, Lakhi S, Price MA, Kilembe W, Cormier E, Anzala O, Latka MH, Bekker LG, Allen SA, Gilmour J, Fast PE. Disease progression by infecting HIV-1 subtype in a seroconverter cohort in sub-Saharan Africa. AIDS. 2013 doi: 10.1097/QAD.0000000000000012. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apps R, Qi Y, Carlson JM, Chen H, Gao X, Thomas R, Yuki Y, Del Prete GQ, Goulder P, Brumme ZL, Brumme CJ, John M, Mallal S, Nelson G, Bosch R, Heckerman D, Stein JL, Soderberg KA, Moody MA, Denny TN, Zeng X, Fang J, Moffett A, Lifson JD, Goedert JJ, Buchbinder S, Kirk GD, Fellay J, McLaren P, Deeks SG, Pereyra F, Walker B, Michael NL, Weintrob A, Wolinsky S, Liao W, Carrington M. Influence of HLA-C expression level on HIV control. Science. 2013;340 (6128):87–91. doi: 10.1126/science.1232685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaout RA, Lloyd AL, O’Brien TR, Goedert JJ, Leonard JM, Nowak MA. A simple relationship between viral load and survival time in HIV-1 infection. Proc Natl Acad Sci USA. 1999;96 (20):11549–53. doi: 10.1073/pnas.96.20.11549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeten JM, Chohan B, Lavreys L, Chohan V, McClelland RS, Certain L, Mandaliya K, Jaoko W, Overbaugh J. HIV-1 subtype D infection is associated with faster disease progression than subtype A in spite of similar plasma HIV-1 loads. J Infect Dis. 2007;195 (8):1177–80. doi: 10.1086/512682. [DOI] [PubMed] [Google Scholar]
- Bansal A, Yue L, Conway J, Yusim K, Tang J, Kappes J, Kaslow RA, Wilson CM, Goepfert PA. Immunological control of chronic HIV-1 infection: HLA-mediated immune function and viral evolution in adolescents. AIDS. 2007;21 (18):2387–2397. doi: 10.1097/QAD.0b013e3282f13823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington M, Martin MP, van Bergen J. KIR-HLA intercourse in HIV disease. Trends Microbiol. 2008;16 (12):620–7. doi: 10.1016/j.tim.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, Hakim JG, Kumwenda J, Grinsztejn B, Pilotto JH, Godbole SV, Mehendale S, Chariyalertsak S, Santos BR, Mayer KH, Hoffman IF, Eshleman SH, Piwowar-Manning E, Wang L, Makhema J, Mills LA, de Bruyn G, Sanne I, Eron J, Gallant J, Havlir D, Swindells S, Ribaudo H, Elharrar V, Burns D, Taha TE, Nielsen-Saines K, Celentano D, Essex M, Fleming TR. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365 (6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SR, Napravnik S, Mugavero MJ, Lau B, Eron JJ, Jr, Saag MS. Copy-years viremia as a measure of cumulative human immunodeficiency virus viral burden. Am J Epidemiol. 2010;171 (2):198–205. doi: 10.1093/aje/kwp347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins MM, Konikoff J, Laeyendecker O, Celum C, Buchbinder SP, Seage GR, 3rd, Kirk GD, Moore RD, Mehta SH, Margolick JB, Brown J, Mayer KH, Koblin BA, Wheeler D, Justman JE, Hodder SL, Quinn TC, Brookmeyer R, Eshleman SH. HIV diversity as a biomarker for HIV incidence estimation: including a high resolution melting diversity assay in a multi-assay algorithm. J Clin Microbiol. 2013 doi: 10.1128/JCM.02040-13. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craney TA, Surles JG. Model-dependent variance inflation factor cutoff values. Qual Engin. 2002;14 (3):391–403. [Google Scholar]
- Crawford H, Lumm W, Leslie A, Schaefer M, Boeras D, Prado JG, Tang J, Farmer P, Ndung’u T, Lakhi S, Gilmour J, Goepfert P, Walker BD, Kaslow R, Mulenga J, Allen S, Goulder PJ, Hunter E. Evolution of HLA-B*5703 HIV-1 escape mutations in HLA-B*5703-positive individuals and their transmission recipients. J Exp Med. 2009;206 (4):909–21. doi: 10.1084/jem.20081984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkle KL, Stephenson R, Karita E, Chomba E, Kayitenkore K, Vwalika C, Greenberg L, Allen S. New heterosexually transmitted HIV infections in married or cohabiting couples in urban Zambia and Rwanda: an analysis of survey and clinical data. Lancet. 2008;371 (9631):2183–91. doi: 10.1016/S0140-6736(08)60953-8. [DOI] [PubMed] [Google Scholar]
- Farquhar C, Rowland-Jones S, Mbori-Ngacha D, Redman M, Lohman B, Slyker J, Otieno P, Obimbo E, Rostron T, Ochieng J, Oyugi J, Bosire R, John-Stewart G. Human leukocyte antigen (HLA) B*18 and protection against mother-to-child HIV type 1 transmission. AIDS Res Hum Retroviruses. 2004;20 (7):692–7. doi: 10.1089/0889222041524616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellay J, Ge D, Shianna KV, Colombo S, Ledergerber B, Cirulli ET, Urban TJ, Zhang K, Gumbs CE, Smith JP, Castagna A, Cozzi-Lepri A, De Luca A, Easterbrook P, Gunthard HF, Mallal S, Mussini C, Dalmau J, Martinez-Picado J, Miro JM, Obel N, Wolinsky SM, Martinson JJ, Detels R, Margolick JB, Jacobson LP, Descombes P, Antonarakis SE, Beckmann JS, O’Brien SJ, Letvin NL, McMichael AJ, Haynes BF, Carrington M, Feng S, Telenti A, Goldstein DB. Common genetic variation and the control of HIV-1 in humans. PLoS Genet. 2009;5 (12):e1000791. doi: 10.1371/journal.pgen.1000791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fideli US, Allen S, Musunda R, Trask S, Hahn B, Mulenga J, Kasolo FC, Vermund SH, Aldrovandi G. Virologic and immunologic determinants of heterosexual transmission of human immunodeficiency virus type 1 (HIV-1) in Africa. AIDS Res Hum Retroviruses. 2001;17 (10):901–910. doi: 10.1089/088922201750290023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiebig EW, Wright DJ, Rawal BD, Garrett PE, Schumacher RT, Peddada L, Heldebrant C, Smith R, Conrad A, Kleinman SH, Busch MP. Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. AIDS. 2003;17 (13):1871–9. doi: 10.1097/00002030-200309050-00005. [DOI] [PubMed] [Google Scholar]
- Gao X, O’Brien TR, Welzel TM, Marti D, Qi Y, Goedert JJ, Phair J, Pfeiffer R, Carrington M. HLA-B alleles associate consistently with HIV heterosexual transmission, viral load, and progression to AIDS, but not susceptibility to infection. AIDS. 2010;24 (12):1835–40. doi: 10.1097/QAD.0b013e32833c3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaleebu P, Ross A, Morgan D, Yirrell D, Oram J, Rutebemberwa A, Lyagoba F, Hamilton L, Biryahwaho B, Whitworth J. Relationship between HIV-1 Env subtypes A and D and disease progression in a rural Ugandan cohort. AIDS. 2001;15 (3):293–9. doi: 10.1097/00002030-200102160-00001. [DOI] [PubMed] [Google Scholar]
- Karita E, Price M, Hunter E, Chomba E, Allen S, Fei L, Kamali A, Sanders EJ, Anzala O, Katende M, Ketter N. Investigating the utility of the HIV-1 BED capture enzyme immunoassay using cross-sectional and longitudinal seroconverter specimens from Africa. AIDS. 2007;21 (4):403–8. doi: 10.1097/QAD.0b013e32801481b7. [DOI] [PubMed] [Google Scholar]
- Kawashima Y, Pfafferott K, Frater J, Matthews P, Payne R, Addo M, Gatanaga H, Fujiwara M, Hachiya A, Koizumi H, Kuse N, Oka S, Duda A, Prendergast A, Crawford H, Leslie A, Brumme Z, Brumme C, Allen T, Brander C, Kaslow R, Tang J, Hunter E, Allen S, Mulenga J, Branch S, Roach T, John M, Mallal S, Ogwu A, Shapiro R, Prado JG, Fidler S, Weber J, Pybus OG, Klenerman P, Ndung’u T, Phillips R, Heckerman D, Harrigan PR, Walker BD, Takiguchi M, Goulder P. Adaptation of HIV-1 to human leukocyte antigen class I. Nature. 2009;458 (7238):641–5. doi: 10.1038/nature07746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiwanuka N, Laeyendecker O, Robb M, Kigozi G, Arroyo M, McCutchan F, Eller LA, Eller M, Makumbi F, Birx D, Wabwire-Mangen F, Serwadda D, Sewankambo NK, Quinn TC, Wawer M, Gray R. Effect of human immunodeficiency virus Type 1 (HIV-1) subtype on disease progression in persons from Rakai, Uganda, with incident HIV-1 infection. J Infect Dis. 2008;197 (5):707–13. doi: 10.1086/527416. [DOI] [PubMed] [Google Scholar]
- Kozak M, Zinski A, Leeper C, Willig JH, Mugavero MJ. Late diagnosis, delayed presentation and late presentation in HIV: proposed definitions, methodological considerations and health implications. Antivir Ther. 2013;18 (1):17–23. doi: 10.3851/IMP2534. [DOI] [PubMed] [Google Scholar]
- Lazaryan A, Song W, Lobashevsky E, Tang J, Shrestha S, Zhang K, McNicholl JM, Gardner LI, Wilson CM, Klein RS, Rompalo A, Mayer K, Sobel J, Kaslow RA. The influence of human leukocyte antigen class I alleles and their population frequencies on human immunodeficiency virus type 1 control among African Americans. Hum Immunol. 2011;72 (4):312–8. doi: 10.1016/j.humimm.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie A, Matthews PC, Listgarten J, Carlson JM, Kadie C, Ndung’u T, Brander C, Coovadia H, Walker BD, Heckerman D, Goulder PJ. Additive contribution of HLA class I alleles in the immune control of HIV-1 infection. J Virol. 2010;84 (19):9879–88. doi: 10.1128/JVI.00320-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie AJ, Pfafferott KJ, Chetty P, Draenert R, Addo MM, Feeney M, Tang Y, Holmes EC, Allen T, Prado JG, Altfeld M, Brander C, Dixon C, Ramduth D, Jeena P, Thomas SA, St John A, Roach TA, Kupfer B, Luzzi G, Edwards A, Taylor G, Lyall H, Tudor-Williams G, Novelli V, Martinez-Picado J, Kiepiela P, Walker BD, Goulder PJ. HIV evolution: CTL escape mutation and reversion after transmission. Nat Med. 2004;10 (3):282–9. doi: 10.1038/nm992. [DOI] [PubMed] [Google Scholar]
- Lyles CM, Dorrucci M, Vlahov D, Pezzotti P, Angarano G, Sinicco A, Alberici F, Alcorn TM, Vella S, Rezza G. Longitudinal human immunodeficiency virus type 1 load in the italian seroconversion study: correlates and temporal trends of virus load. J Infect Dis. 1999;180 (4):1018–24. doi: 10.1086/314980. [DOI] [PubMed] [Google Scholar]
- McLaren PJ, Ripke S, Pelak K, Weintrob AC, Patsopoulos NA, Jia X, Erlich RL, Lennon NJ, Kadie CM, Heckerman D, Gupta N, Haas DW, Deeks SG, Pereyra F, Walker BD, de Bakker PI. Fine-mapping classical HLA variation associated with durable host control of HIV-1 infection in African Americans. Hum Mol Genet. 2012;21 (19):4334–4347. doi: 10.1093/hmg/dds226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellors JW, Kingsley LA, Rinaldo CR, Jr, Todd JA, Hoo BS, Kokka RP, Gupta P. Quantitation of HIV-1 RNA in plasma predicts outcome after seroconversion. Ann Intern Med. 1995;122 (8):573–9. doi: 10.7326/0003-4819-122-8-199504150-00003. [DOI] [PubMed] [Google Scholar]
- Mellors JW, Margolick JB, Phair JP, Rinaldo CR, Detels R, Jacobson LP, Munoz A. Prognostic value of HIV-1 RNA, CD4 cell count, and CD4 Cell count slope for progression to AIDS and death in untreated HIV-1 infection. JAMA. 2007;297(21):2349–50. doi: 10.1001/jama.297.21.2349. [DOI] [PubMed] [Google Scholar]
- Merino AM, Sabbaj S, Easlick J, Goepfert P, Kaslow RA, Tang J. Dimorphic HLA-B signal peptides differentially influence HLA-E- and natural killer cell-mediated cytolysis of HIV-1-infected target cells. Clin Exp Immunol. 2013;174 (3):414–23. doi: 10.1111/cei.12187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merino AM, Song W, He D, Mulenga J, Allen S, Hunter E, Tang J, Kaslow RA. HLA-B signal peptide polymorphism influences the rate of HIV-1 acquisition but not viral load. J Infect Dis. 2012;205 (12):1797–805. doi: 10.1093/infdis/jis275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaner JS, Lima VD, Barrios R, Yip B, Wood E, Kerr T, Shannon K, Harrigan PR, Hogg RS, Daly P, Kendall P. Association of highly active antiretroviral therapy coverage, population viral load, and yearly new HIV diagnoses in British Columbia, Canada: a population-based study. Lancet. 2010;376 (9740):532–9. doi: 10.1016/S0140-6736(10)60936-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugavero MJ, Amico KR, Westfall AO, Crane HM, Zinski A, Willig JH, Dombrowski JC, Norton WE, Raper JL, Kitahata MM, Saag MS. Early retention in HIV care and viral load suppression: Implications for a test and treat approach to HIV prevention. J Acquir Immune Defic Syndr. 2012;59 (1):86–93. doi: 10.1097/QAI.0b013e318236f7d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugavero MJ, Napravnik S, Cole SR, Eron JJ, Lau B, Crane HM, Kitahata MM, Willig JH, Moore RD, Deeks SG, Saag MS. Viremia copy-years predicts mortality among treatment-naive HIV-infected patients initiating antiretroviral therapy. Clin Infect Dis. 2011;53 (9):927–35. doi: 10.1093/cid/cir526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngongo PB, Priddy F, Park H, Becker J, Bender B, Fast P, Anzala O, Mutua G, Ruzagira E, Kamali A, Karita E, Mugo P, Chomba E, Bekker LG, Roux S, Nanvubya A, Mebrahtu T. Developing standards of care for HIV prevention research in developing countries -- a case study of 10 research centers in Eastern and Southern Africa. AIDS Care. 2012;24 (10):1277–89. doi: 10.1080/09540121.2012.656572. [DOI] [PubMed] [Google Scholar]
- Novitsky V, Wang R, Margolin L, Baca J, Moyo S, Musonda R, Essex M. Dynamics and timing of in vivo mutations at Gag residue 242 during primary HIV-1 subtype C infection. Virology. 2010;403 (1):37–46. doi: 10.1016/j.virol.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmanov S, Pattou C, Walker N, Schwardlander B, Esparza J. Estimated global distribution and regional spread of HIV-1 genetic subtypes in the year 2000. J Acquir Immune Defic Syndr. 2002;29 (2):184–90. doi: 10.1097/00042560-200202010-00013. [DOI] [PubMed] [Google Scholar]
- Prentice HA, Porter TR, Price MA, Cormier E, He D, Farmer PK, Kamali A, Karita E, Lakhi S, Sanders EJ, Anzala O, Amornkul PN, Allen S, Hunter E, Kaslow RA, Gilmour J, Tang J. HLA-B*57 versus HLA-B*81 in HIV-1 infection: slow and steady wins the race? J Virol. 2013;87 (7):4043–51. doi: 10.1128/JVI.03302-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentice HA, Tang J. HIV-1 dynamics: A reappraisal of host and viral factors, as well as methodological issues. Viruses. 2012;4 (10):2080–96. doi: 10.3390/v4102080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price MA, Wallis CL, Lakhi S, Karita E, Kamali A, Anzala O, Sanders EJ, Bekker LG, Twesigye R, Hunter E, Kaleebu P, Kayitenkore K, Allen S, Ruzagira E, Mwangome M, Mutua G, Amornkul PN, Stevens G, Pond SL, Schaefer M, Papathanasopoulos MA, Stevens W, Gilmour J. Transmitted HIV type 1 drug resistance among individuals with recent HIV infection in East and Southern Africa. AIDS Res Hum Retroviruses. 2011;27 (1):5–12. doi: 10.1089/aid.2010.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn TC, Wawer MJ, Sewankambo N, Serwadda D, Li C, Wabwire-Mangen F, Meehan MO, Lutalo T, Gray RH. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000;342 (13):921–929. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- Rodriguez B, Sethi AK, Cheruvu VK, Mackay W, Bosch RJ, Kitahata M, Boswell SL, Mathews WC, Bangsberg DR, Martin J, Whalen CC, Sieg S, Yadavalli S, Deeks SG, Lederman MM. Predictive value of plasma HIV RNA level on rate of CD4 T-cell decline in untreated HIV infection. JAMA. 2006;296 (12):1498–506. doi: 10.1001/jama.296.12.1498. [DOI] [PubMed] [Google Scholar]
- Shrestha S, Aissani B, Song W, Wilson CM, Kaslow RA, Tang J. Host genetics and HIV-1 viral load set-point in African-Americans. AIDS. 2009;23 (6):673–7. doi: 10.1097/QAD.0b013e328325d414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W, He D, Brill I, Malhotra R, Mulenga J, Allen S, Hunter E, Tang J, Kaslow RA. Disparate associations of HLA class I markers with HIV-1 acquisition and control of viremia in an African population. PLoS ONE. 2011;6 (8):e23469. doi: 10.1371/journal.pone.0023469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart CA, Laugier-Anfossi F, Vely F, Saulquin X, Riedmuller J, Tisserant A, Gauthier L, Romagne F, Ferracci G, Arosa FA, Moretta A, Sun PD, Ugolini S, Vivier E. Recognition of peptide-MHC class I complexes by activating killer immunoglobulin-like receptors. Proc Natl Acad Sci U S A. 2005;102 (37):13224–9. doi: 10.1073/pnas.0503594102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Cormier E, Gilmour J, Price MA, Prentice HA, Song W, Kamali A, Karita E, Lakhi S, Sanders EJ, Anzala O, Amornkul PN, Allen S, Hunter E, Kaslow RA. Human leukocyte antigen variants B*44 and B*57 are consistently favorable during two distinct phases of primary HIV-1 infection in sub-Saharan Africans with several viral subtypes. J Virol. 2011;85 (17):8894–8902. doi: 10.1128/JVI.00439-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Malhotra R, Song W, Brill I, Hu L, Farmer PK, Mulenga J, Allen S, Hunter E, Kaslow RA. Human leukocyte antigens and HIV type 1 viral load in early and chronic infection: Predominance of evolving relationships. PLoS ONE. 2010;5 (3):e9629. doi: 10.1371/journal.pone.0009629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Shao W, Yoo YJ, Brill I, Mulenga J, Allen S, Hunter E, Kaslow RA. Human leukocyte antigen class I genotypes in relation to heterosexual HIV type 1 transmission within discordant couples. J Immunol. 2008;181 (4):2626–35. doi: 10.4049/jimmunol.181.4.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Shelton B, Makhatadze NJ, Zhang Y, Schaen M, Louie LG, Goedert JJ, Seaberg EC, Margolick JB, Mellors J, Kaslow RA. Distribution of chemokine receptor CCR2 and CCR5 genotypes and their relative contribution to human immunodeficiency virus type 1 (HIV-1) seroconversion, early HIV-1 RNA concentration in plasma, and later disease progression. J Virol. 2002a;76 (2):662–72. doi: 10.1128/JVI.76.2.662-672.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Tang S, Lobashevsky E, Myracle AD, Fideli U, Aldrovandi G, Allen S, Musonda R, Kaslow RA. Favorable and unfavorable HLA class I alleles and haplotypes in Zambians predominantly infected with clade C human immunodeficiency virus type 1. J Virol. 2002b;76 (16):8276–8284. doi: 10.1128/JVI.76.16.8276-8284.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tebit DM, Arts EJ. Tracking a century of global expansion and evolution of HIV to drive understanding and to combat disease. Lancet Infect Dis. 2011;11(1):45–56. doi: 10.1016/S1473-3099(10)70186-9. [DOI] [PubMed] [Google Scholar]
- Vasan A, Renjifo B, Hertzmark E, Chaplin B, Msamanga G, Essex M, Fawzi W, Hunter D. Different rates of disease progression of HIV type 1 infection in Tanzania based on infecting subtype. Clin Infect Dis. 2006;42(6):843–52. doi: 10.1086/499952. [DOI] [PubMed] [Google Scholar]
- Wang YE, Li B, Carlson JM, Streeck H, Gladden AD, Goodman R, Schneidewind A, Power KA, Toth I, Frahm N, Alter G, Brander C, Carrington M, Walker BD, Altfeld M, Heckerman D, Allen TM. Protective HLA class I alleles that restrict acute-phase CD8+ T-cell responses are associated with viral escape mutations located in highly conserved regions of human immunodeficiency virus type 1. J Virol. 2009;83(4):1845–55. doi: 10.1128/JVI.01061-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue L, Prentice HA, Farmer P, Song W, He D, Lakhi S, Goepfert P, Gilmour J, Allen S, Tang J, Kaslow RA, Hunter E. Cumulative impact of host and viral factors on HIV-1 viral load control during early infection. J Virol. 2013;87(2):708–715. doi: 10.1128/JVI.02118-12. [DOI] [PMC free article] [PubMed] [Google Scholar]