Abstract
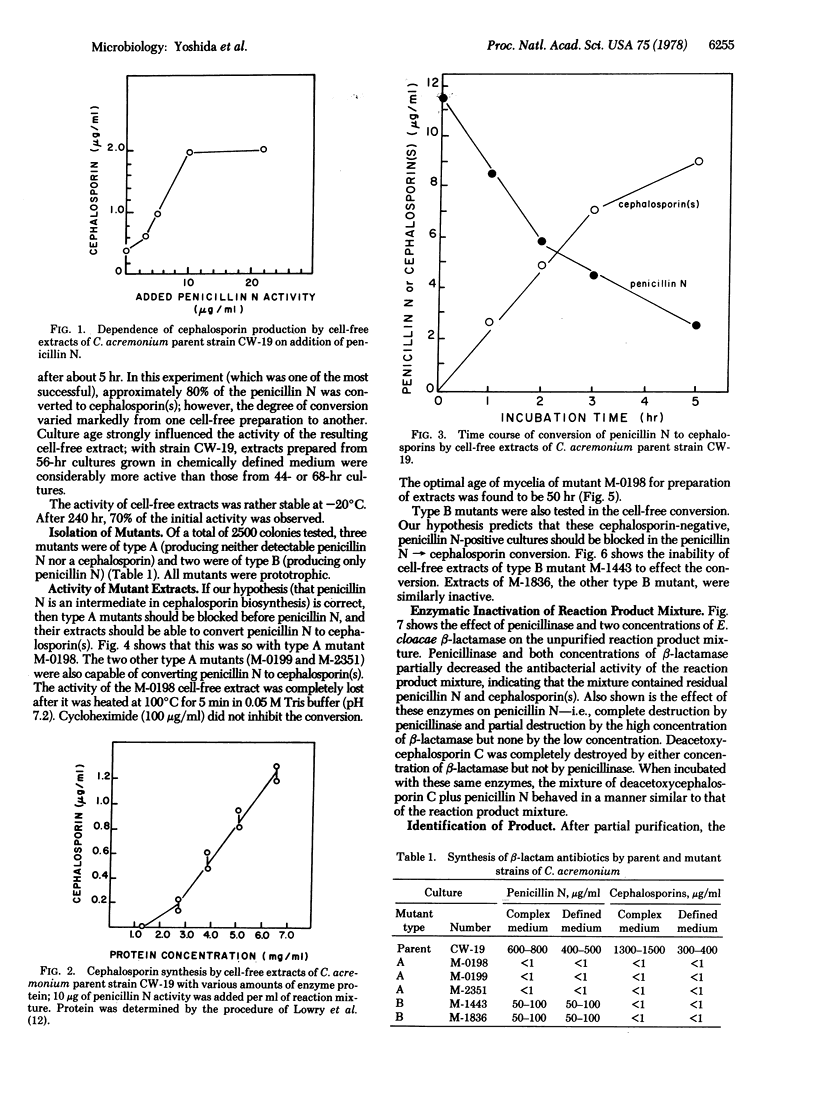
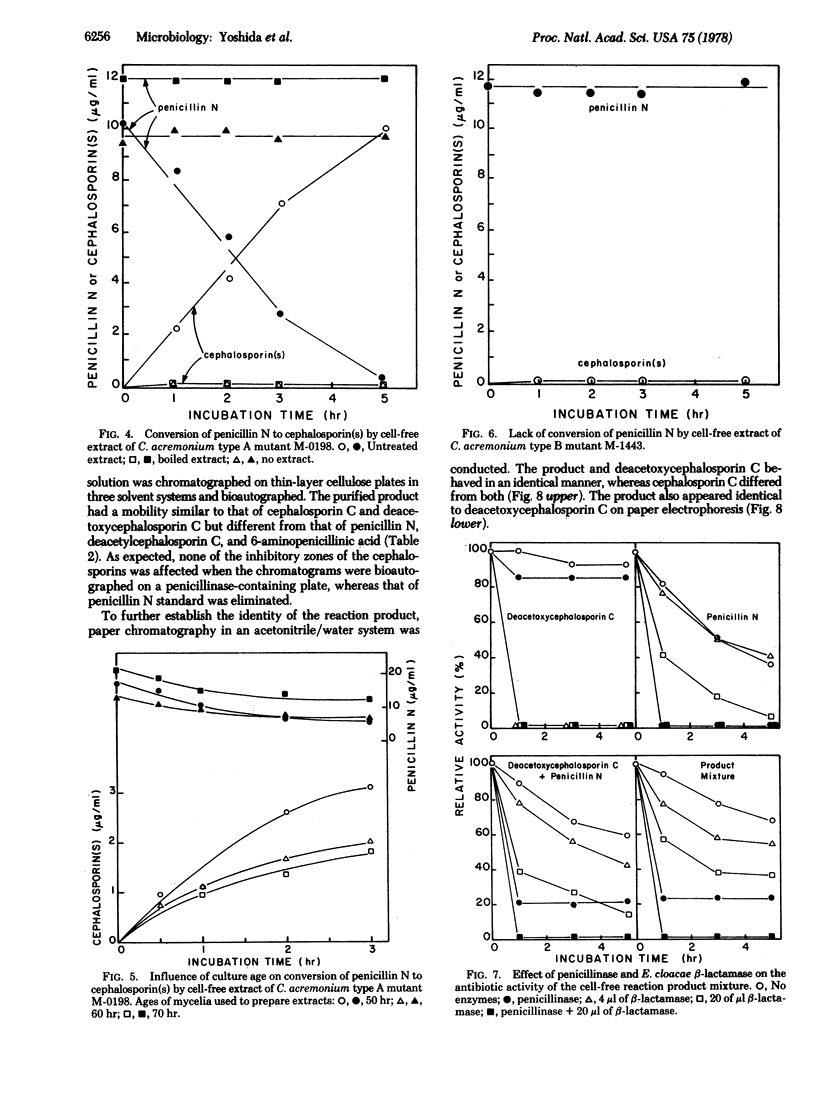
To examine microbiological ring expansion of penicillin N to a cephalosporin, we obtained five mutants of Cephalosporium acremonium blocked in beta-lactam antibiotic biosynthesis from 2500 survivors of mutagenesis. In submerged fermentation, mutants M-0198, M-0199, and M-2351 produced no beta-lactam antibiotic (type A), whereas mutants M-1443 and M-1836 formed penicillin N but not cephalosporin C (type B). Cell-free extracts of type A mutants converted penicillin N to a cephalosporin; those of type B mutants did not. The product of the cell-free reaction was identified as deacetoxycephalosporin C by thin-layer chromatography, paper chromatography, paper electrophoresis, and enzyme tests. These data strongly support our hypothesis that penicillin N is an intermediate of cephalosporin biosynthesis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bost P. E., Demain A. L. Studies on the cell-free biosynthesis of beta-lactam antibiotics. Biochem J. 1977 Mar 15;162(3):681–687. doi: 10.1042/bj1620681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caltrider P. G., Niss H. F. Role of methionine in cephalosporin synthesis. Appl Microbiol. 1966 Sep;14(5):746–753. doi: 10.1128/am.14.5.746-753.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett P. A., Usher J. J., Huddleston J. A., Bleaney R. C., Nisbet J. J., Abraham E. P. Synthesis of delta-(alpha-aminoadipyl)cysteinylvaline and its role in penicillin biosynthesis. Biochem J. 1976 Sep 1;157(3):651–660. doi: 10.1042/bj1570651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgens C. E., Hamill R. L., Sands T. H., Hoehn M. M., Davis N. E. Letter: The occurrence of deacetoxycephalosporin C in fungi and streptomycetes. J Antibiot (Tokyo) 1974 Apr;27(4):298–300. doi: 10.7164/antibiotics.27.298. [DOI] [PubMed] [Google Scholar]
- Kohsaka M., Demain A. L. Conversion of penicillin N to cephalosporin(s) by cell-free extracts of Cephalosporium acremonium. Biochem Biophys Res Commun. 1976 May 17;70(2):465–473. doi: 10.1016/0006-291x(76)91069-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lemke P. A., Nash C. H. Mutations that affect antibiotic synthesis by Cephalosporium acremonium. Can J Microbiol. 1972 Feb;18(2):255–259. doi: 10.1139/m72-038. [DOI] [PubMed] [Google Scholar]
- Loder P. B., Abraham E. P. Biosynthesis of peptides containing -aminoadipic acid and cysteine in extracts of a Cephalosporium sp. Biochem J. 1971 Jul;123(3):477–482. doi: 10.1042/bj1230477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loder P. B., Abraham E. P. Isolation and nature of intracellular peptides from a cephalosporin C-producing Cephalosporium sp. Biochem J. 1971 Jul;123(3):471–476. doi: 10.1042/bj1230471. [DOI] [PMC free article] [PubMed] [Google Scholar]


