Abstract
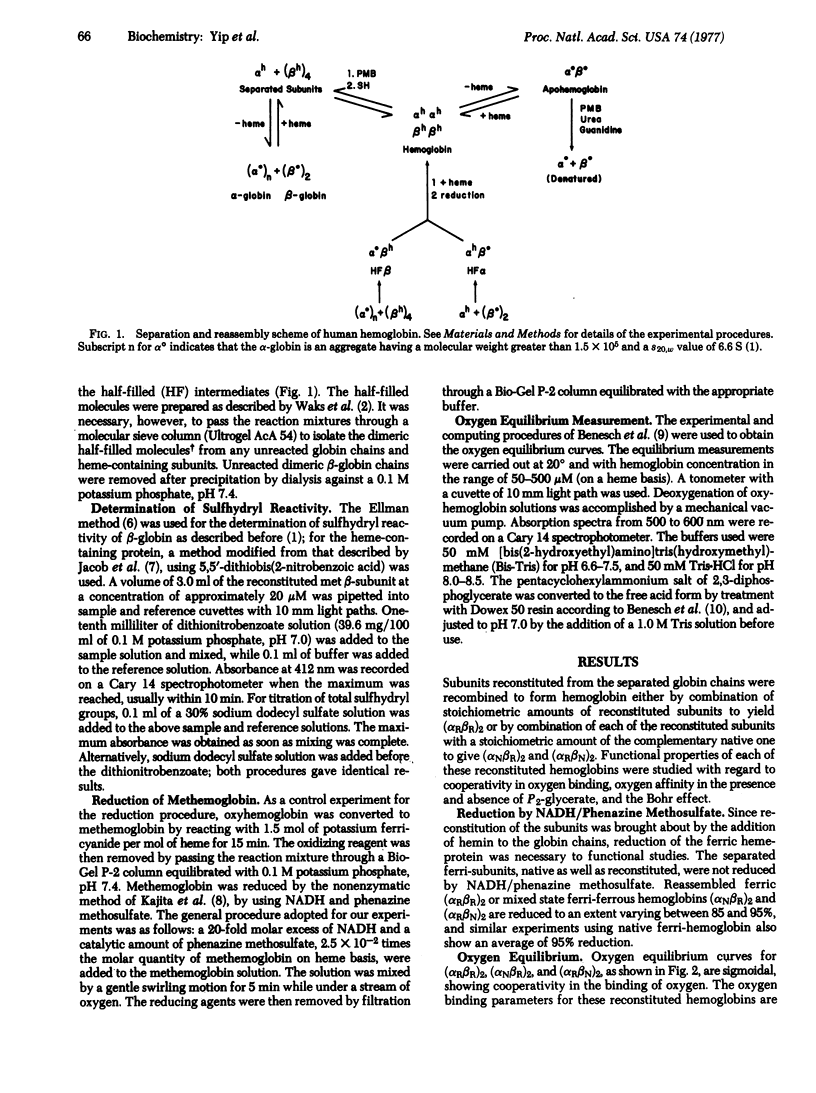
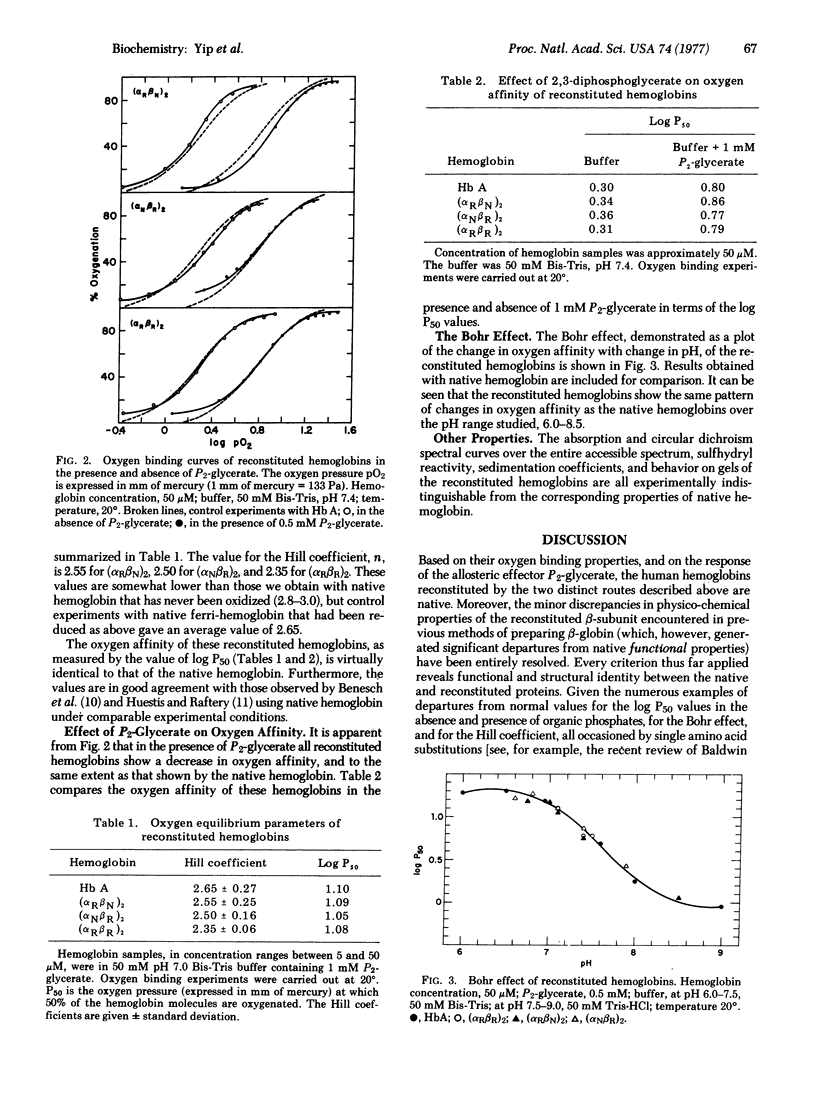
A complete experimental format is given for the reconstitution of human hemoglobin from the separated heme-free alpha- and beta-globin chains (alpha degrees, beta degrees) and hemin, by two alternative routes. Based on their oxygen binding properties, the reaction of the ferri-forms with reducing agent, and the response of the oxygen binding curves to pH variation and to the addition of the allosteric effector 2,3-diphosphoglycerate, the molecules are native. One reconstitution route uses direct addition of hemin to the separated globin chains with production of the separated subunits, which can then be recombined and reduced. This procedure occasions losses by precipitation in the heme-addition step except at high dilutions, and the yields are low. In the second pathway, either globin chain is mixed with the complementary untreated subunit to form the half-filled (with heme) intermediates, which combine stoichiometrically with hemin. No precipitation accompanies these reactions. For alpha-globin, the yield is about 50% because of incomplete combination with the heme-containing beta chain. For beta-globin, the yield is better than 70%. It is suggested that experiments intended to test either globin chain should use the second route in preparation for structural or functional comparisons with native hemoglobin.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENESCH R., MACDUFF G., BENESCH R. E. DETERMINATION OF OXYGEN EQUILIBRIA WITH A VERSATILE NEW TONOMETER. Anal Biochem. 1965 Apr;11:81–87. doi: 10.1016/0003-2697(65)90045-x. [DOI] [PubMed] [Google Scholar]
- Baldwin J. M. Structure and function of haemoglobin. Prog Biophys Mol Biol. 1975;29(3):225–320. doi: 10.1016/0079-6107(76)90024-9. [DOI] [PubMed] [Google Scholar]
- Banerjee R., Cassoly R. Preparation and properties of the isolated alpha and beta chains of human hemoglobin in the ferri state. Investigation of oxidation-reduction equilibria. J Mol Biol. 1969 Jun 14;42(2):337–349. doi: 10.1016/0022-2836(69)90047-3. [DOI] [PubMed] [Google Scholar]
- Benesch R. E., Benesch R., Renthal R. D., Maeda N. Affinity labeling of the polyphosphate binding site of hemoglobin. Biochemistry. 1972 Sep 12;11(19):3576–3582. doi: 10.1021/bi00769a013. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- GIBSON Q. H., ANTONINI E. Rates of reaction of native human globin with some hemes. J Biol Chem. 1963 Apr;238:1384–1388. [PubMed] [Google Scholar]
- Geraci G., Parkhurst L. J., Gibson Q. H. Preparation and properties of alpha- and beta-chains from human hemoglobin. J Biol Chem. 1969 Sep 10;244(17):4664–4667. [PubMed] [Google Scholar]
- Huestis W. H., Raftery M. A. A study of cooperative interactions in hemoglobin using fluorine nuclear magnetic resonance. Biochemistry. 1972 Apr 25;11(9):1648–1654. doi: 10.1021/bi00759a018. [DOI] [PubMed] [Google Scholar]
- Jacob H. S., Brain M. C., Dacie J. V., Carrell R. W., Lehmann H. Abnormal haem binding and globin SH group blockade in unstable haemoglobins. Nature. 1968 Jun 29;218(5148):1214–1217. doi: 10.1038/2181214a0. [DOI] [PubMed] [Google Scholar]
- Kajita A., Noguchi K., Shukuya R. A simple non-enzymatic method to regenerate oxyhemoglobin from methemoglobin. Biochem Biophys Res Commun. 1970;39(6):1199–1204. doi: 10.1016/0006-291x(70)90688-1. [DOI] [PubMed] [Google Scholar]
- Waks M., Yip Y. K., Beychok S. Influence of prosthetic groups on protein folding and subunit assembly. Recombination of separated human alpha-and beta-globin chains with heme and alloplex interactions of globin chains with heme-containing subunits. J Biol Chem. 1973 Sep 25;248(18):6462–6470. [PubMed] [Google Scholar]
- Yip Y. K., Waks M., Beychok S. Influence of prosthetic groups on protein folding and subunit assembly. I. Conformational differences between separated human alpha- and beta- globins. J Biol Chem. 1972 Nov 25;247(22):7237–7244. [PubMed] [Google Scholar]


