Abstract
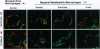
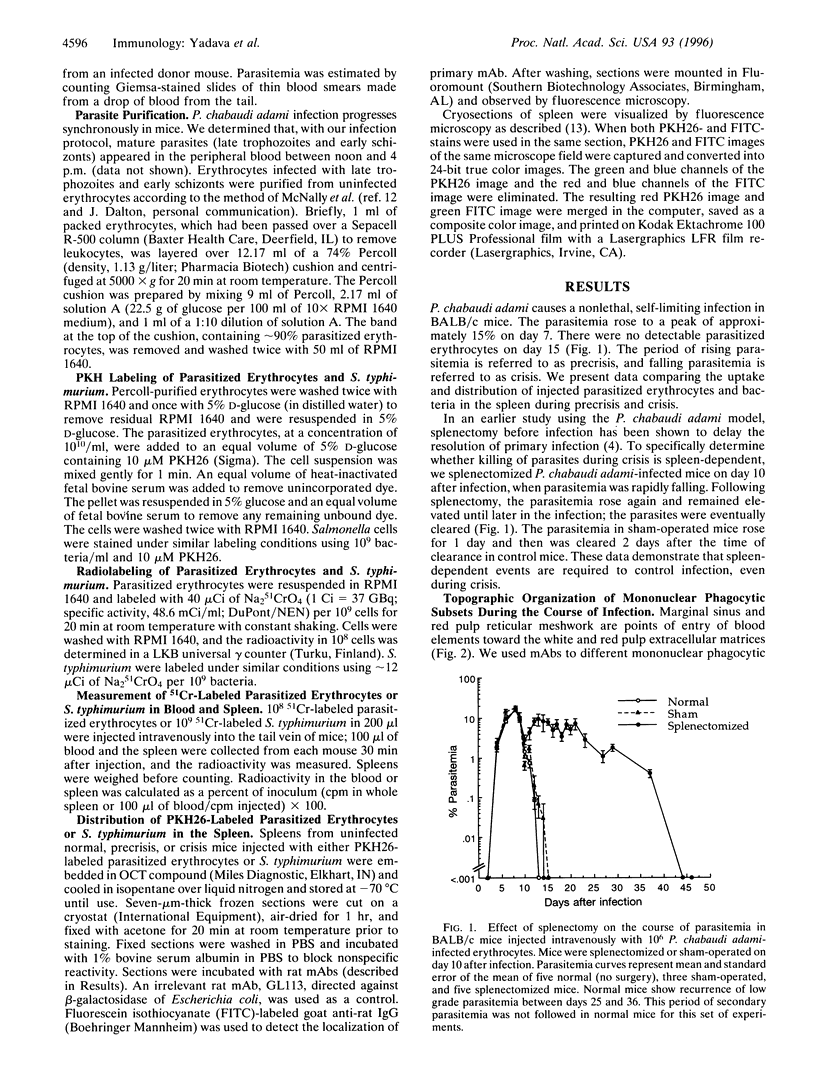
Plasmodium chabaudi adami causes a nonlethal infection in mice. We found that crisis, the time of rapidly dropping parasitemia, was abrogated by splenectomy, indicating the role of spleen in parasite killing. The factors that mediate spleen-dependent immunity are not known. An earlier study in Plasmodium berghei-infected rats showed an association between increased clearance of heat-treated erythrocytes and the onset of crisis [Wyler, D. J., Quinn, T. C. & Chen, L.-T. (1982) J. Clin. Invest. 67, 1400-1404]. To determine the potential effects of different vascular beds in parasite killing, we studied the distribution of parasitized erythrocytes and bacteria in the spleens of P. chabaudi adami-infected mice during precrisis (a period of rising parasitemia) and during crisis. After intravenous injection, bacteria were localized predominantly in the marginal zone. In contrast, parasitized erythrocytes were found in the red pulp. We also found that during precrisis, a time of no immunity, the uptake of radiolabeled infected erythrocytes by the spleen was increased, not decreased. These data imply that no change occurs in the flow of parasitized erythrocytes through the spleen during the transition to an immune state (crisis). Our observations suggest that immune effector mechanisms, not circulatory changes, account for spleen-dependent parasite killing during a P. chabaudi adami infection in mice.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Crocker P. R., Gordon S. Mouse macrophage hemagglutinin (sheep erythrocyte receptor) with specificity for sialylated glycoconjugates characterized by a monoclonal antibody. J Exp Med. 1989 Apr 1;169(4):1333–1346. doi: 10.1084/jem.169.4.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkstra C. D., Van Vliet E., Döpp E. A., van der Lelij A. A., Kraal G. Marginal zone macrophages identified by a monoclonal antibody: characterization of immuno- and enzyme-histochemical properties and functional capacities. Immunology. 1985 May;55(1):23–30. [PMC free article] [PubMed] [Google Scholar]
- Dvorak J. A., Kobayashi S., Alling D. W., Hallahan C. W. Elucidation of the DNA synthetic cycle of Entamoeba spp. using flow cytometry and mathematical modeling. J Eukaryot Microbiol. 1995 Sep-Oct;42(5):610–616. doi: 10.1111/j.1550-7408.1995.tb05915.x. [DOI] [PubMed] [Google Scholar]
- Grun J. L., Long C. A., Weidanz W. P. Effects of splenectomy on antibody-independent immunity to Plasmodium chabaudi adami malaria. Infect Immun. 1985 Jun;48(3):853–858. doi: 10.1128/iai.48.3.853-858.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraal G. Cells in the marginal zone of the spleen. Int Rev Cytol. 1992;132:31–74. doi: 10.1016/s0074-7696(08)62453-5. [DOI] [PubMed] [Google Scholar]
- Kumar S., Good M. F., Dontfraid F., Vinetz J. M., Miller L. H. Interdependence of CD4+ T cells and malarial spleen in immunity to Plasmodium vinckei vinckei. Relevance to vaccine development. J Immunol. 1989 Sep 15;143(6):2017–2023. [PubMed] [Google Scholar]
- Kumar S., Gorden J., Flynn J. L., Berzofsky J. A., Miller L. H. Immunization of mice against Plasmodium vinckei with a combination of attenuated Salmonella typhimurium and malarial antigen. Infect Immun. 1990 Oct;58(10):3425–3429. doi: 10.1128/iai.58.10.3425-3429.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally J., O'Donovan S. M., Dalton J. P. Plasmodium berghei and Plasmodium chabaudi chabaudi: development of simple in vitro erythrocyte invasion assays. Parasitology. 1992 Dec;105(Pt 3):355–362. doi: 10.1017/s0031182000074527. [DOI] [PubMed] [Google Scholar]
- Quinn T. C., Wyler D. J. Resolution of acute malaria (Plasmodium berghei in the rat): reversibility and spleen dependence. Am J Trop Med Hyg. 1980 Jan;29(1):1–4. doi: 10.4269/ajtmh.1980.29.1. [DOI] [PubMed] [Google Scholar]
- Schmidt E. E., MacDonald I. C., Groom A. C. Comparative aspects of splenic microcirculatory pathways in mammals: the region bordering the white pulp. Scanning Microsc. 1993 Jun;7(2):613–628. [PubMed] [Google Scholar]
- Smith M. J., Koch G. L. Differential expression of murine macrophage surface glycoprotein antigens in intracellular membranes. J Cell Sci. 1987 Feb;87(Pt 1):113–119. doi: 10.1242/jcs.87.1.113. [DOI] [PubMed] [Google Scholar]
- Springer T., Galfré G., Secher D. S., Milstein C. Mac-1: a macrophage differentiation antigen identified by monoclonal antibody. Eur J Immunol. 1979 Apr;9(4):301–306. doi: 10.1002/eji.1830090410. [DOI] [PubMed] [Google Scholar]
- Stevenson M. M., Kraal G. Histological changes in the spleen and liver of C57BL/6 and A/J mice during Plasmodium chabaudi AS infection. Exp Mol Pathol. 1989 Aug;51(1):80–95. doi: 10.1016/0014-4800(89)90009-9. [DOI] [PubMed] [Google Scholar]
- Van den Eertwegh A. J., Boersma W. J., Claassen E. Immunological functions and in vivo cell-cell interactions of T cells in the spleen. Crit Rev Immunol. 1992;11(6):337–380. [PubMed] [Google Scholar]
- Weiss L., Geduldig U., Weidanz W. Mechanisms of splenic control of murine malaria: reticular cell activation and the development of a blood-spleen barrier. Am J Anat. 1986 Jul;176(3):251–285. doi: 10.1002/aja.1001760303. [DOI] [PubMed] [Google Scholar]
- Weiss L. The spleen in malaria: the role of barrier cells. Immunol Lett. 1990 Aug;25(1-3):165–172. doi: 10.1016/0165-2478(90)90109-4. [DOI] [PubMed] [Google Scholar]
- Wyler D. J., Quinn T. C., Chen L. T. Relationship of alterations in splenic clearance function and microcirculation to host defense in acute rodent malaria. J Clin Invest. 1981 May;67(5):1400–1404. doi: 10.1172/JCI110168. [DOI] [PMC free article] [PubMed] [Google Scholar]