Abstract
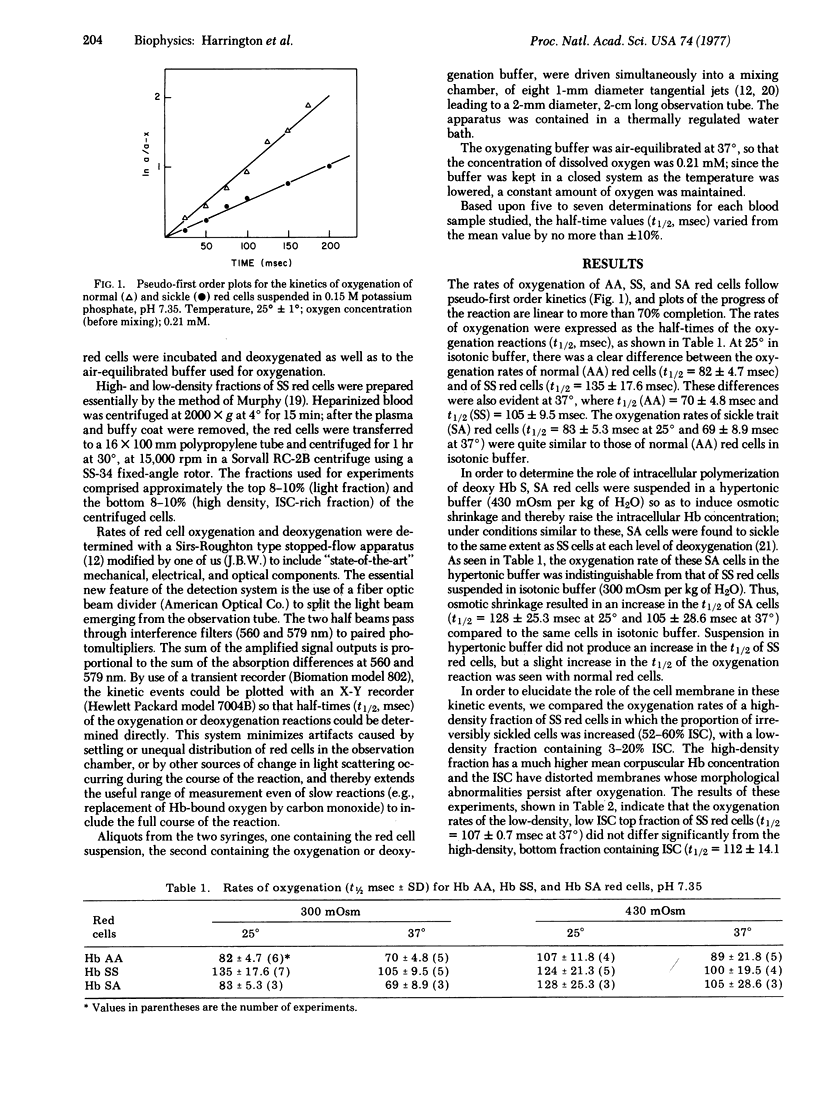
Oxygen uptake of fully deoxygenated sickle (SS) erythrocytes is slower than that of normal (AA) erythrocytes, as demonstrated by the half-times of the overall oxygenation reactions: at 25 degrees in an isotonic phosphate buffer the normal red cells have a t1/2 = 82 +/- 4.7 msec, as compared to sickle red cells where t1/2 = 135 +/- 17.6 msec. The effects of temperature, extracellular osmolality, and the presence of an antisickling agent (n-butylurea) on the rate of red cell oxygenation strongly suggest that the differences in oxygenation rates encountered with sickle red cells is directly related to the intracellular polymerization of deoxyhemoglobin S.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BECKLAKE M. R., GRIFFITHS S. B., McGREGOR M., GOLDMAN H. I., SCHREVE J. P. Oxygen dissociation curves in sickle cell anemia and in subjects with the sickle cell trait. J Clin Invest. 1955 May;34(5):751–755. doi: 10.1172/JCI103129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookchin R. M., Balazs T., Landau L. C. Determinants of red cell sickling. Effects of varying pH and of increasing intracellular hemoglobin concentration by osmotic shrinkage. J Lab Clin Med. 1976 Apr;87(4):597–616. [PubMed] [Google Scholar]
- Bromberg P. A., Jensen W. N. Blood oxygen dissociation curves in sickle cell disease. J Lab Clin Med. 1967 Sep;70(3):480–488. [PubMed] [Google Scholar]
- Elbaum D., Nagel R. L., Bookchin R. M., Herskovits T. T. Effect of alkylureas on the polymerization of hemoglobin S. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4718–4722. doi: 10.1073/pnas.71.12.4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FORSTER R. E., ROUGHTON F. J., KREUZER F., BRISCOE W. A. Photocolorimetric determination of rate of uptake of CO and O2 by reduced human red cell suspensions at 37 degrees C. J Appl Physiol. 1957 Sep;11(2):260–268. doi: 10.1152/jappl.1957.11.2.260. [DOI] [PubMed] [Google Scholar]
- GIBSON Q. H., KREUZER F., MEDA E., ROUGHTON F. J. The kinetics of human haemoglobin in solution and in the red cell at 37 degrees C. J Physiol. 1955 Jul 28;129(1):65–89. doi: 10.1113/jphysiol.1955.sp005339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENBERG M. S., KASS E. H., CASTLE W. B. Studies on the destruction of red blood cells. XII. Factors influencing the role of S hemoglobin in the pathologic physiology of sickle cell anemia and related disorders. J Clin Invest. 1957 Jun;36(6 Pt 1):833–843. doi: 10.1172/JCI103489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartridge H., Roughton F. J. The rate of distribution of dissolved gases between the red blood corpuscle and its fluid environment: Part I. Preliminary experiments on the rate of uptake of oxygen and carbon monoxide by sheep's corpuscles. J Physiol. 1927 Jan 12;62(3):232–242. doi: 10.1113/jphysiol.1927.sp002354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEGGE J. W., ROUGHTON F. J. W. Some observations on the kinetics of haemoglobin in solution and in the haemoglobin in solution and in the red blood corpuscle. Biochem J. 1950 Jun-Jul;47(1):43–52. doi: 10.1042/bj0470043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURAYAMA M. Titratable sulfhydryl groups of normal and sickle cell hemoglobins at O degrees and 38 degrees. J Biol Chem. 1957 Sep;228(1):231–240. [PubMed] [Google Scholar]
- May A., Huehns E. R. The mechanism of the low oxygen affinity of red cells in sickle cell disease. Hamatol Bluttransfus. 1972;10:279–283. [PubMed] [Google Scholar]
- Milner P. F. Oxygen transport in sickle cell anemia. Arch Intern Med. 1974 Apr;133(4):565–572. [PubMed] [Google Scholar]
- Murphy J. R. Influence of temperature and method of centrifugation on the separation of erythrocytes. J Lab Clin Med. 1973 Aug;82(2):334–341. [PubMed] [Google Scholar]
- ROUGHTON F. J. Kinetics of gas transport in the blood. Br Med Bull. 1963 Jan;19:80–89. doi: 10.1093/oxfordjournals.bmb.a070013. [DOI] [PubMed] [Google Scholar]
- SINGER K., CHERNOFF A. I., SINGER L. Studies on abnormal hemoglobins. I. Their demonstration in sickle cell anemia and other hematologic disorders by means of alkali denaturation. Blood. 1951 May;6(5):413–428. [PubMed] [Google Scholar]
- SIRS J. A., ROUGHTON F. J. Stopped-flow measurements of CO and O2 uptake by hemoglobin in sheep erythrocytes. J Appl Physiol. 1963 Jan;18:158–165. doi: 10.1152/jappl.1963.18.1.158. [DOI] [PubMed] [Google Scholar]
- Seakins M., Gibbs W. N., Milner P. F., Bertles J. F. Erythrocyte Hb-S concentration. An important factor in the low oxygen affinity of blood in sickle cell anemia. J Clin Invest. 1973 Feb;52(2):422–432. doi: 10.1172/JCI107199. [DOI] [PMC free article] [PubMed] [Google Scholar]


