Abstract
Objective
The aim of this study was to determine the role of Suppressor of Cytokine Signaling 1 (SOCS1) in graft arteriosclerosis (GA).
Background
GA, the major cause of late cardiac allograft failure, is initiated by immune-mediated endothelial activation resulting in vascular inflammation and consequent neointima formation. SOCS1, a negative regulator of cytokine signaling, is highly expressed in endothelial cells (ECs) and may prevent endothelial inflammatory responses and phenotypic activation.
Methods
Clinical specimens of coronary arteries with GA, atherosclerosis, or without disease were collected for histological analysis. SOCS1 knockout or vascular endothelial SOCS1 transgenic mice (VESOCS1) were used in an aorta transplant model of GA. Mouse aortic ECs were isolated for in vitro assays.
Results
Dramatic but specific reduction of endothelial SOCS1 was observed in human GA and atherosclerosis specimens which suggested the importance of SOCS1 in maintaining normal endothelial function. SOCS1 deletion in mice resulted in basal EC dysfunction. After transplantation, SOCS1-deficient aortic grafts augmented leukocyte recruitment and neointima formation, whereas endothelial overexpression of SOCS1 diminished arterial rejection. Induction of endothelial adhesion molecules in early stages of GA was suppressed by the VESOCS1 transgene and this effect was confirmed in cultured aortic ECs. Moreover, VESOCS1 maintained better vascular function during GA progression. Mechanistically, endothelial SOCS1, by modulating both basal and cytokine-induced expression of the adhesion molecules PECAM-1, ICAM-1 and VCAM-1, restrained leukocyte adhesion and trans-endothelial migration during inflammatory cell infiltration.
Conclusions
SOCS1 prevents GA progression by preserving endothelial function and attenuating cytokine-induced adhesion molecule expression in vascular endothelium.
Keywords: graft arteriosclerosis, SOCS1, endothelial activation, endothelial adhesion molecule
INTRODUCTION
Pathological vascular remodeling is the major cause of cardiovascular diseases. A prototypic example of pathological vascular remodeling is graft arteriosclerosis (GA), also referred to as cardiac allograft vasculopathy, which leads to clinical failure of organ allografts after the first year post-transplantation [1, 2]. The development of GA arises from the recruitment of acute inflammatory cells (e.g. macrophages) and subsequent alloantigen-responsive T cells on recognition of graft endothelial cells (ECs). The local production of pro-inflammatory cytokines, in turn, drives vascular smooth muscle cell migration and proliferation within the neointima, leading to luminal obstruction and allograft ischemia. A critical initiating event in this process is EC activation and the consequent induction of endothelial adhesion molecules, including E-selectin, P-selectin, intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), and platelet/endothelial cell adhesion molecule 1 (PECAM-1). The enhanced expression of endothelial adhesion molecules provides an environment for initial adhesion and subsequent migration of inflammatory cells into the vessel wall. Leukocyte adhesion to activated ECs is a sequential, multistep process consisting of tethering, rolling, firm adhesion, and transmigration [3]. Each adhesion molecule has specific functions in the adhesion cascade of inflammatory cell recruitment; selectins are associated with cell rolling, ICAM-1 and VCAM-1 correlate to firm adhesion, while PECAM-1 is responsible for paracellular transmigration. The major stimuli for induction of endothelial adhesion molecules are pro-inflammatory cytokines, which typically include interleukin (IL)-6 and interferon-γ (IFNγ) in GA.
Among IFNγ-induced genes, Suppressor of cytokine signaling 1 (SOCS1) is an attractive candidate as a putative regulator of GA pathogenesis. SOCS1, a member of the SOCS family of proteins, was first identified as a negative feedback inhibitor of cytokine signaling. So far, SOCS1 has been implicated in the regulation of dozens of cytokines, including IL-6 and IFNγ. It not only functions as a powerful attenuator of JAK-STAT signaling, but has also been shown to disrupt other inflammatory pathways by regulating NF-κB [4] and ASK1 degradation based on our previous work [5, 6]. Besides these in vitro studies, the physiological role of SOCS1 is extensively established in immune function and tumor progression. SOCS1 is abundantly expressed in the thymus and spleen and is induced in all immune cells by a large panel of cytokines and pathogens. SOCS1-deficient mice die around 2–3 weeks postnatally due to overactive inflammatory responses [7, 8]. On the other hand, SOCS1 overexpression in vivo results in a reduction of inflammatory cytokines produced by infiltrating immunocytes [9, 10]. The distinct role of SOCS1 in inflammation regulation is correlated with several immunological diseases, such as multiple sclerosis [11], inflammatory arthritis [12], and diabetes [13, 14]. Additionally, SOCS1 functions as a tumor suppressor in carcinogenesis. Decreased SOCS1 expression in cancer cells and increased expression in stromal cells have been observed in a variety of tumor samples. In clinical practice, SOCS1 has been set up as a diagnostic biomarker of tumors at both the transcriptional [15, 16] and post-translational levels [17, 18]. Although we have previously dissected part of the SOCS1 signal transduction pathway in cultured ECs [5, 6], little is known regarding the pathophysiological role of SOCS1 in the cardiovascular system. In the present study, we investigated the role of SOCS1 in vascular ECs within GA based on both analysis of clinical specimens and experimental research in mouse models of disease.
METHODS
Expanded materials and methods are provided in the online supplement, including the descriptions of clinical specimens, generation of SOCS1 transgenic mice, mouse aortic transplantation model, graft analyses, cell culture, immunoprecipitation/immunoblotting, leukocyte-endothelial adhesion and transmigration assays, and statistical analyses.
RESULTS
Loss of endothelial SOCS1 expression correlates with arterial disease in clinical samples
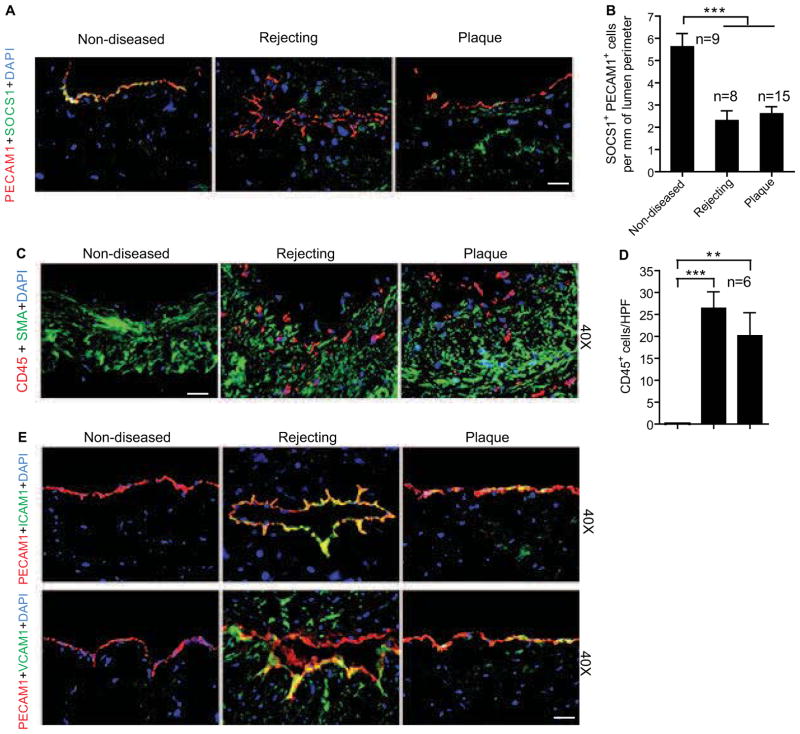
SOCS1 expression in human vascular cells has been described in several in vitro studies. To determine the expression pattern of SOCS1 in clinical specimens of vascular disease, human coronary arteries with GA from transplanted hearts and with atherosclerotic plaque or no disease from non-transplanted hearts were collected for histological examination. SOCS1 was abundantly expressed in non-diseased arteries, while it was dramatically reduced in diseased arteries especially within the luminal endothelial layer identified by PECAM-1 staining (Fig. 1A with quantification in 1B). However, no significant difference was detected at the mRNA level between healthy and diseased arteries (Supplemental Fig. S1), suggesting diminished SOCS1 protein synthesis. Histology also demonstrated obvious intimal expansion with plentiful leukocyte infiltration as evidenced by CD45 immunostaining of diseased arteries (Fig. 1C with quantifications in 1D). Therefore, to further determine a link between endothelial SOCS1 expression and vascular inflammation, the adhesion molecules and markers of EC activation, ICAM-1 and VCAM-1, were assessed by immunofluorescence. Opposite to the pattern for SOCS1, expression of ICAM-1 and VCAM-1 was markedly enhanced while PECAM1 was slightly increased in the endothelium of diseased arteries compared to non-diseased controls (Fig. 1E). These results suggest a protective role of SOCS1 in pathological vascular remodeling by preventing endothelial activation and vascular inflammation.
Fig. 1. Loss of SOCS1 expression correlates with clinical pathological vascular remodeling.
Human coronary artery specimens with GA from chronically rejecting heart allografts and with atherosclerotic plaques or no disease from non-transplanted hearts were collected. (A–B) Dramatically decreased endothelial SOCS1 expression in diseased vessel wall. Endothelial SOCS1 expression is demonstrated by immunofluorescence analysis of artery cross-sections stained for SOCS1 and the endothelial marker, PECAM-1 with DAPI labeling of nuclei. Representative images are shown in A with quantifications in B. Scale bar: 50 μm. (C–D) Inflammatory cell and vascular smooth muscle cell accumulation are demonstrated by immunofluorescence analysis of artery cross-sections stained for the leukocyte common marker, CD45 and the smooth muscle cell marker, smooth muscle α-actin (SMA) with DAPI labeling of nuclei. Representative images are shown with quantification in the histogram. Scale bar: 50 μm. (E) Induction of endothelial adhesion molecules has the opposite expression pattern as endothelial SOCS1. Representative histological analysis of artery cross-sections stained with ICAM-1 or VCAM-1 and PECAM-1 antibodies. Immunofluorescence sections were counterstained with DAPI. Scale bar: 50 μm. Data presented in B and D are mean±SEM from independent clinical specimens of varying number per group as indicated; **P<0.01, ***P<0.001, one-way ANOVA followed by Bonferroni’s post-hoc test.
SOCS1 deletion in mice exacerbates GA and EC dysfunction
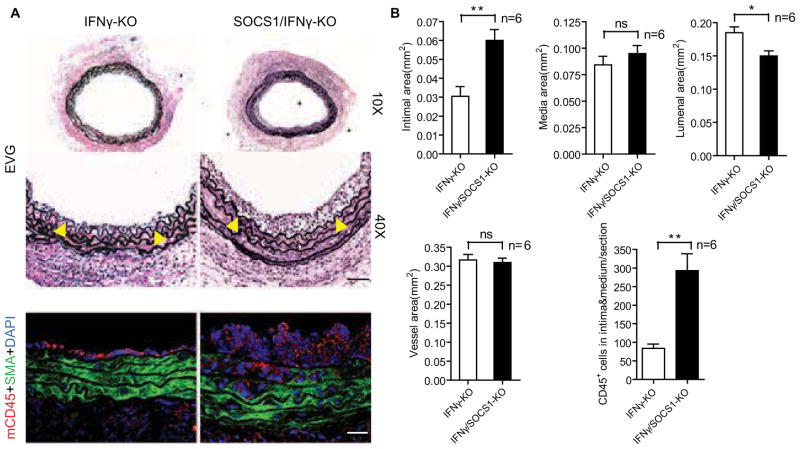
To further investigate the function of SOCS1 in vessel wall cells under a pathological setting, we used a mouse aorta transplantation model of GA which we established in a previous study [19, 20]. Briefly, a segment of male donor thoracic aorta is interposed into the abdominal aorta of a female recipient of the same background strain. The host then mounts an alloimmune response against the male-specific H–Y minor histocompatibility antigen expressed by the graft in which leukocyte–derived pro-inflammatory cytokines induce endothelial activation and drive graft neointima formation [19–22]. Since SOCS1 knockout mice (SOCS1-KO) die perinatally due to overproduction of inflammatory cytokines, we used SOCS1-KO mice on an IFNγ-deficient background in which the mice survive normally[23]. IFNγ-KO male C57BL/6 (B6) to female B6 aorta transplantation induced GA as characterized by graft infiltration with leukocytes and neointima formation (Fig. 2A, with quantification in Fig. 2B). Strikingly, SOCS1/IFNγ-KO male B6 donor grafts to female B6 recipients generated significantly larger neointima that contained more CD45-positive infiltrating leukocytes resulting in lumen loss compared to the IFNγ-KO donor group (Fig. 2A with quantification in Fig. 2B).
Fig. 2. SOCS1 deletion enhances GA in mice.
Aortas from IFNγ-KO or SOCS1/IFNγ-KO male B6 donors were transplanted to female B6 recipients and the allografts were harvested at 2 weeks post-operatively. (A) Histological analysis of artery grafts by EVG staining and immunostaining by anti-SMA and anti-CD45 antibodies with DAPI labeling of nuclei. Representative photomicrographs are shown. Arrowheads mark the internal elastic lamina to delineate the intima from media. Scale bar: 50 μm. (B) Morphometric assessment of artery graft intima, media, lumen, and vessel areas and quantification of CD45+ cells infiltrating the intima and media of each artery graft cross-section. Data are mean±SEM from 6 mice per group; *P<0.05, **P<0.001, unpaired t-test.
Since EC activation/dysfunction has been implicated as the early step for arteriosclerosis progression, we investigated if SOCS1 deficiency may affect endothelial function of the vessel wall. To this end, aortas from IFNγ-KO or SOCS1/IFNγ-KO mice were isolated for vessel function assays. Vascular reactivity of isolated aortic rings was determined by examining the responses to the vasoconstrictor phenylepherine (PE), endothelium-dependent vasodilator acetylcholine (Ach), and the NOS inhibitor L-NAME. Compared to IFNγ-KO, aortas from SOCS1/IFNγ-KO mice showed increased constriction in responsive to PE but reduced relaxation in response to Ach (Supplemental Fig. S2). These results are consistent with our above clinical observations and support a role for SOCS1 in preventing GA pathogenesis, in which SOCS1 regulated EC function may be a potential mechanism.
Vascular endothelial SOCS1 overexpression in mice inhibits GA
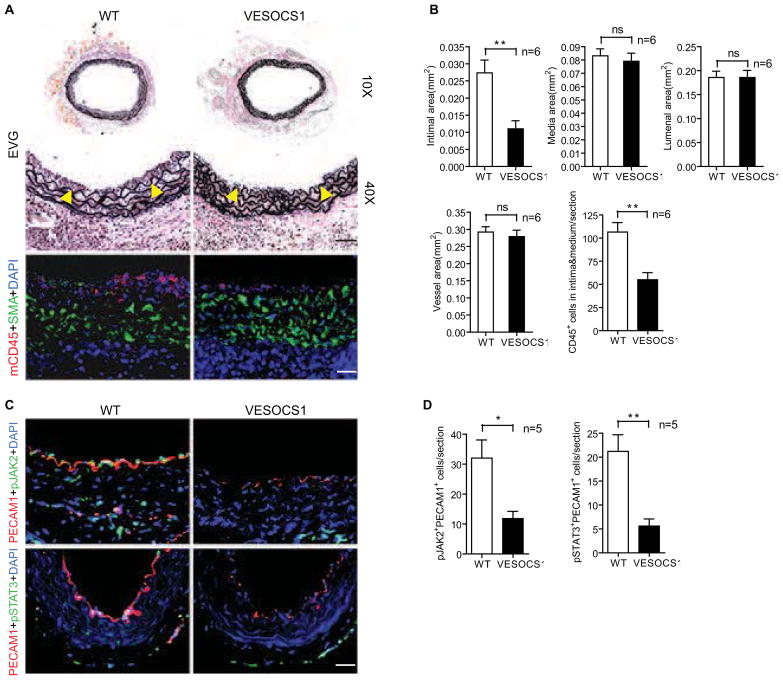
The conclusions drawn above led us to hypothesize that SOCS1 may function as a negative regulator of endothelial activation during GA. To clarify this issue, we generate a VE-cadherin promoter-driven SOCS1 transgenic mouse (VESOCS1) (Supplemental Fig. S3A) and backcrossed it onto the B6 background for more than 10 generations. EC-specific expression of SOCS1 was determined at both the mRNA and protein levels (Supplemental Fig. S3B–D). Non-transgenic littermates (WT) and VESOCS1 male mice were used as aorta donors in the GA model as described above. Donor grafts were harvested at 2 weeks after transplantation for histological analysis and morphometric assessment. Aorta grafts overexpressing SOCS1 generated significantly less neointima and contained fewer CD45+ inflammatory cells than did the WT vessels (Fig. 3A,B). To assess the effects on inflammation-induced EC signaling, IL-6 and IFNγ were selected for evaluation since they represent major pro-inflammatory cytokines produced in GA [24–28]. Real-time qPCR results showed no significant difference in the production of either cytokine between WT and VESOCS1 groups (Supplemental Fig. S4). In contrast, there was decreased cytokine signaling as assessed by immunostaining for phosphorylated JAK2 and phosphorylated STAT3 in aortas overexpressing SOCS1 at 3 days post-operatively (Fig. 3C,D). The expression of these activated signaling molecules co-localized with PECAM-1 demonstrating events within the graft ECs. Since JAK-STAT signaling is one of the major pathways in pro-inflammatory cytokine-mediated EC activation, these results provide clues to explore the effects of SOCS1 in GA progression.
Fig. 3. EC-specific overexpression of SOCS1 inhibits GA in mice.
Aortas from non-transgenic littermate WT and VESOCS1 male B6 mice were transplanted to female B6 recipients and the allografts were harvested at 2 weeks or 3 days post-operatively. (A) Histological analysis of 2-week artery grafts by EVG and immunostaining by anti-SMA and anti-CD45 antibodies with DAPI labeling of nuclei. Representative photomicrographs are shown. Arrowheads mark the internal elastic lamina to delineate the intima from media. Scale bar: 50 μm. (B) Morphometric assessment of artery graft intima, media, lumen, and vessel areas and quantification of CD45+ cells infiltrating the intima and media of each artery graft. (C) EC-specific expression of SOCS1 inhibited pro-inflammatory cytokine-induced JAK2 and STAT3 activation in vessel grafts. Cross sections of WT or VESOCS1 grafts at day 3 were co-immunostained with antibodies to phosphorylated (p)-JAK2 or p-STAT3 and the EC marker PECAM-1 with DAPI labeling of nuclei. Representative images are shown. Scale bar: 50 μm. (D) Quantification of the endothelial signaling data in C. Data are mean±SEM from 5–6 mice per group; *P<0.05, **P<0.001, unpaired t-test.
SOCS1 is a negative regulator of endothelial activation in GA
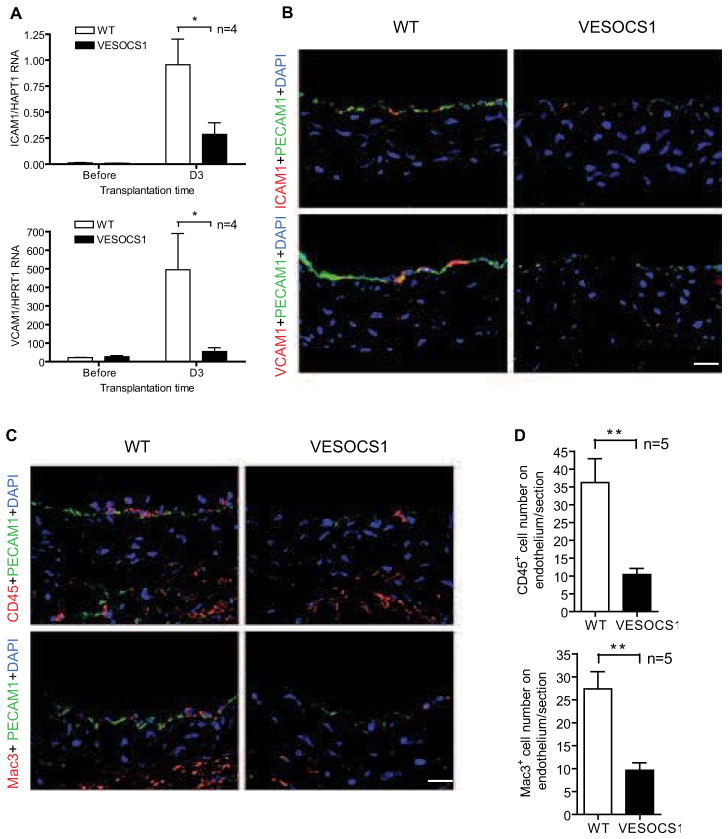
To further determine the function of SOCS1 in GA, we examined for markers of EC activation early after transplantation of WT and VESOCS1 grafts prior to discernable neointima formation. At 3 days post-operatively, real-time qPCR revealed decreased mRNA expression of the inducible endothelial adhesion molecules ICAM-1 and VCAM-1 (but not PECAM-1) in VESOCS1 aortas compared to the WT group (Fig. 4A). Immunostaining confirmed an inhibitory effect of SOCS1 on the expression of ICAM-1 and VCAM-1 and also of PECAM-1 in the graft endothelium (Fig. 4B). Correspondingly, decreased numbers of infiltrating leukocytes were observed in VESOCS1 grafts compared to the WT group (Fig. 4C,D). On the other hand, vessel function assays revealed reduced constriction of VESOCS1 graft in response to PE but enhanced relaxation in response to Ach, compared to B6 controls (Supplemental Fig. S5). Taken together, these results demonstrate a direct role of SOCS1 in preventing endothelial activation/dysfunction during GA formation.
Fig. 4. EC-specific expression of SOCS1 inhibits endothelial adhesion molecule induction and corresponding leukocytic infiltration in GA.
Aortas from non-transgenic littermate WT and VESOCS1 male B6 mice were transplanted to female B6 recipients and the allografts were harvested at 3 days post-operatively. (A) Transcripts for the endothelial adhesion molecules, ICAM-1 and VCAM-1 from WT and VESOCS1 grafts were quantified by qPCR and normalized to HPRT. (B) Induction of endothelial adhesion molecules was also determined by immunostaining with antibodies to ICAM-1 or VCAM-1 and PECAM-1 with DAPI labeling of nuclei. Representative images are shown. Scale bar: 50 μm. (C) Leukocyte infiltration was determined by immunostaining with antibodies to the pan-leukocyte marker, CD45 or the macrophage marker, Mac3 and endothelium marker PECAM-1. Representative images are shown. Scale bar: 50 μm. (D) Quantification of the number of leukocytes in C. Data are mean±SEM from 4–5 mice per group; *P<0.05, **P<0.001, unpaired t-test.
SOCS1 overexpression restrains expression of adhesion molecules and leukocyte-endothelial adhesion and transmigration
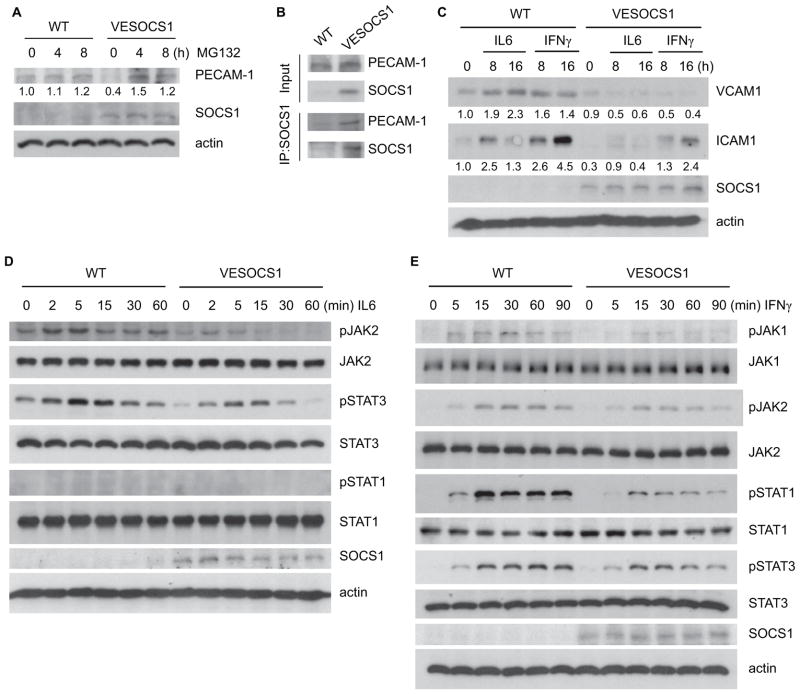
To define how SOCS1 mediates gene expression of PECAM-1, ICAM-1 and VCAM-1 in vascular EC, we further examined the effects of the SOCS1 transgene in isolated primary mouse aortic EC. We observed that the basal level of PECAM-1, a major scaffold protein but also an adhesion molecule of ECs, is clearly suppressed in the presence of the SOCS1 transgene (Fig. 5A). Consistent with the in vivo findings, PECAM-1 mRNA was not altered by SOCS1 transgene expression (data not shown), suggesting that SOCS1 may regulate PECAM-1 expression at a post-translational level. We investigated if SOCS1 mediates PECAM-1 proteasomal degradation as reported previously [29]. In aortic ECs from VESOCS1 animals, the low expression level of PECAM-1 was strongly reversed by the presence of MG132, a pan-proteasome inhibitor (Fig. 5A). Moreover, associations between SOCS1 and PECAM-1 could be detected by a co-immunoprecipitation assay with anti-PECAM-1 followed by Western blot for SOCS1 in the same primary cells (Fig. 5B). Thereby, these results demonstrate that SOCS1 binds PECAM-1 and mediates its proteasomal degradation.
Fig. 5. EC-specific expression of SOCS1 weakens pro-inflammatory cytokine responses in primary cultured aortic EC.
(A) SOCS1 promotes proteasomal degradation of PECAM-1. Primary cultured WT and VESOCS1 mouse aortic ECs were treated with the pan-proteasome inhibitor MG132 for the indicated times. Expression of PECAM-1 and SOCS1 was determined by Western blot with the respective antibodies. PECAM-1 expression was quantified with untreated WT normalized as a value of 1.0. (B) SOCS1 associates with PECAM-1 in mouse aortic EC. Primary cultured WT and VESOCS1 mouse aortic ECs were lysed in RIPA buffer. Association of SOCS1 with PECAM-1 was determined by a co-immunoprecipitation assay with anti-SOCS1 followed by Western blot with anti-PECAM-1 or anti-SOCS1. (C) Isolated WT and VESOCS1 mouse aortic ECs were cultured in serum-free medium for 24 hours, followed by treatment with mouse IFNγ (10 ng/mL) or IL-6 (10 ng/mL) for the indicated times. Induction of endothelial adhesion molecules was determined. VCAM-1 and ICAM-1 expression was quantified with untreated WT normalized as a value of 1.0. (D–E) Isolated WT and VESOCS1 mouse aortic ECs were cultured in serum-free medium for 24 hours, followed by treatment with mouse IFNγ (10 ng/mL) or IL-6 (10 ng/mL) for the indicated times. Phosphorylation of JAK1, JAK2, STAT1, and STAT3 and total proteins were determined by Western blot with the respective antibodies. SOCS1 and β-actin were also determined. All experiments in A–E were repeated 3 times.
In contrast to PECAM-1, we observed that both the mRNA (not shown) and protein (Fig. 5C) levels of ICAM-1 and VCAM-1 were upregulated by IL-6 and IFN-γ, and this regulation was attenuated by the SOCS1 transgene (Fig. 5C). Since both IL-6 and IFN-γ utilize JAK-STATs signaling pathways to induce gene expression of proinflammatory molecules, we examined the signaling responses in aortic ECs. Primary cultured aortic ECs were treated with mouse IL-6 or IFNγ and activation of their downstream signaling mediators was determined by Western blot with phospho-specific antibodies. IL-6 induced activation of JAK2 and STAT3, and the SOCS1 transgene reduced IL-6-activated JAK2-STAT3 signaling (Fig. 5D). IFN-γ induced activation of JAK1/2 and STAT1/3 in aortic ECs, and IFN-γ-activated signaling was attenuated by the SOCS1 transgene in EC (Fig. 5C). These effects of SOCS1 on JAK-STAT activation are consistent with the in vivo observations (Fig. 3). In summary, these results demonstrate that SOCS1 specifically inhibits pro-inflammatory cytokine responses in vascular EC.
To correlate the role of SOCS1 in pro-inflammatory cytokine signaling to its effect on vascular inflammation, we determined the effects of SOCS1 overexpression on in vitro assays of leukocyte-endothelial adhesion and transmigration, two critical steps involved in inflammatory cell recruitment [30, 31]. In an adhesion assay using fluorescently pre-labeled mouse monocytes seeded onto confluent primary cultured aortic EC, SOCS1 overexpression had no effect on monocyte attachment on resting ECs, but prevented pro-inflammatory cytokine-mediated monocyte attachment as compared to the WT group (Supplemental Fig. S6A,B). Similarly, an inhibitory effect of the SOCS1 transgene on monocyte transmigration across cytokine-activated ECs was observed in a transendothelial migration assay [30] (Supplemental Fig. S6C,D). These data suggest that SOCS1 specifically regulates pro-inflammatory cytokine–dependent functions in vascular ECs that are relevant for leukocyte trafficking and the development of GA.
DISCUSSION
In the present study, we investigated the role of SOCS1 in GA by assessing clinical specimens and employing a mouse aorta transplantation model, across the H–Y–dependent minor histocompatibility antigen barrier, which closely mimics GA progression as we have previously described [20]. Here we demonstrate that SOCS1 is highly expressed in the luminal endothelium of non-diseased human coronary arteries, while its expression is decreased in ECs of chronically rejecting heart grafts and of atherosclerotic plaques. Such dramatic loss of expression accompanied with abundant immune cell infiltration in the pathologically remodeled vessel wall suggests a regulatory role for SOCS1 in GA pathogenesis. The clinical observation is supported by enhanced intimal expansion and increased leukocyte infiltration as well as EC dysfunction in SOCS1-deficient murine allografts. Since endothelial activation initiates leukocytic infiltration and neointima formation, a critical role for SOCS1 specifically in the endothelium of the transplanted aortas is implied. To this end, transgenic mice overexpressing SOCS1 in vascular endothelial cells were generated. The grafts from VESOCS1 mice exhibit dramatically decreased endothelial activation, leukocyte infiltration, and neointima formation. ECs from VESOCS1 grafts also blunt pro-inflammatory cytokine signaling and induction of adhesion molecules. This mirrors the finding of decreased endogenous SOCS1 and enhanced adhesion molecule expression in the endothelium of clinical GA specimens. Mechanistically, we show that SOCS1 overexpression in ECs significantly reduces IL-6- and IFNγ-induced JAK2-STAT1/3 signaling and leukocyte-endothelial adhesion and transmigration. Furthermore, we show that SOCS1 directly binds to PECAM-1 and interrupts PECAM-1 stability. We conclude that endothelial SOSC1 prevents GA by downregulating pro-inflammatory cytokine-induced EC activation and subsequent leukocyte infiltration.
Negative role of SOCS1 in endothelial inflammation
GA progression depends closely on inflammation, the important amplification mechanism of innate and adaptive immunity. Infiltration of immune cells into the vessel wall, including neutrophils, macrophages, and T cells, requires vascular endothelial activation. Characterized by the induction of adhesion molecules, endothelial activation results in adherence of immune cells from the circulation onto endothelium followed by trans-endothelial migration into the artery wall. In the present study, the clinical analysis suggests a special inhibitory role of SOCS1 on endothelial sensing and responses during vascular rejection. This observation supports our further investigations into the role of SOCS1 in maintaining EC homeostasis. Besides preserving EC vessel function, we also find that SOCS1 overexpression in vascular EC inhibits the induction of endothelial adhesion molecules by pro-inflammatory cytokines. To this extent, our results reveal an important role of SOCS1 as a negative regulator of endothelial activation and subsequent arterial inflammation.
Dual regulatory mechanisms of endothelial adhesion molecule expression by SOCS1
Most studies of the SOCS family have focused on the immune system, including the regulation of cytokine production by immune cells in vascular diseases. To determine the effect of endothelial SOCS1 overexpression on inflammatory cytokine generation, we assessed the levels of IL-6 and IFNγ transcripts typically expressed in GA lesions. No significant differences were detected between WT and VESOCS1 groups. This suggests that the responses of SOCS1-expressing ECs are critical to understanding GA progression and need to be elucidated. Upregulation of adhesion molecule expression generally involves transcriptional mechanisms. Besides NF-κB, STAT family members are major transcriptional factors controlling the expression of inducible adhesion molecules, such as ICAM-1 and VCAM-1, and are canonical signaling mediators for many inflammatory stimuli [32, 33]. Upon the binding of different pro-inflammatory cytokines to their receptors, intracellular activation of the Janus kinases, JAK1 and JAK2 is initiated which leads to auto-phosphorylation and downstream STAT recruitment and phosphorylation. Activated STATs subsequently form homodimers and translocate to the nucleus to launch target gene expression. In our present study, inhibition of pro-inflammatory cytokine-induced JAK-STAT signaling by endothelial SOCS1 is identified as its molecular function on endothelial activation. Of note, IL-6 activates STAT1 while IFNγ induces broader STAT activation in vascular ECs. More interestingly, our findings illustrate a novel mechanism of SOCS1 on endothelial adhesion molecule expression in addition to transcription. We identify that SOCS1 directly interacts with PECAM-1 to enhance its degradation. The association of PECAM-1 downregulation and its phosphorylation in activated EC has been previously reported [29], but little is known regarding the mechanisms. Here we demonstrate that SOCS1 is a potent negative mediator in this process.
Pathophysiological function of SOCS1 in pathological vascular remodeling
Being a major member of the SOCS family, SOCS1 has received the most attention as a critical negative regulator of immune cells. In pathological vascular remodeling, infiltrating immune cells are the major source of pro-inflammatory cytokines which activate vascular ECs and vascular smooth muscle cells as well as other vessel wall cells. In response to pro-inflammatory cytokines, SOCS1 is induced in these inflammatory infiltrating cells and negatively regulate the generation of these cytokines [34]. Based on both clinical and experimental data, the present study reveals a critical function of SOCS1 in vascular EC activation, dysfunction and homeostasis. Our results shed light on the role of SOCS1 in vessel wall cells, which expands the understanding of SOCS family beyond their traditional role in the immune system. To our surprise, endothelial SOCS1 expression is inhibited and not induced by the occurrence of both clinical GA and atherosclerosis. Since SOCS1 mRNA was not reduced in these specimens, it suggests that protein degradation may represent a novel regulation mechanism for SOCS1 expression. However, we cannot exclude the expression of endogenous SOCS1 transcripts in vessel wall cell types other than ECs, such as infiltrating leukocytes. Recent work from other lab reports that SOCS1 expressed in smooth muscle cells is a key regulator of vascular cell responses in atherosclerosis [35]. Our observation is also consistent for SOCS1 induction in the underlying neointima and shoulder regions of plaques beyond endothelium (Fig. 1A). Herewith, SOCS1 is an important regulator in maintaining normal function of the vasculature in pathological vascular remodeling. Modulation of endothelial SOCS1 expression and activity may represent a novel strategy for the treatment of cardiovascular diseases, such as GA and atherosclerosis.
Supplementary Material
Acknowledgments
Dr. Wang Min is supported by NIH grants R01 HL085789 and R01 HL109420. Dr. Luyang Yu is supported by Natural Science Foundation of China (81270357), the Fundamental Research Funds for the Central Universities and American Heart Association Scientist Development Grant (12SDG9320033).
Abbreviations list
- EC
endothelial cell
- GA
graft arteriosclerosis
- ICAM1
intercellular adhesion molecule 1
- JAK
Janus kinase
- PECAM-1
platelet/endothelial cell adhesion molecule 1
- STAT
Signal transducers and activators of transcription
- SOCS1
Suppressor of cytokine signaling 1
- VCAM-1
vascular cell adhesion molecule 1
Footnotes
Conflict of Interest: The authors declare that they have no conflict
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Libby P, Pober JS. Chronic rejection. Immunity. 2001;14:387–97. doi: 10.1016/s1074-7613(01)00119-4. [DOI] [PubMed] [Google Scholar]
- 2.Lietz K, Miller LW. Current understanding and management of allograft vasculopathy. Seminars in thoracic and cardiovascular surgery. 2004;16:386–94. doi: 10.1053/j.semtcvs.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–14. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 4.Ryo A, Suizu F, Yoshida Y, Perrem K, Liou YC, Wulf G, et al. Regulation of NF-kappaB signaling by Pin1-dependent prolyl isomerization and ubiquitin-mediated proteolysis of p65/RelA. Mol Cell. 2003;12:1413–26. doi: 10.1016/s1097-2765(03)00490-8. [DOI] [PubMed] [Google Scholar]
- 5.He Y, Zhang W, Zhang R, Zhang H, Min W. SOCS1 inhibits tumor necrosis factor-induced activation of ASK1-JNK inflammatory signaling by mediating ASK1 degradation. J Biol Chem. 2006;281:5559–66. doi: 10.1074/jbc.M512338200. [DOI] [PubMed] [Google Scholar]
- 6.Yu L, Min W, He Y, Qin L, Zhang H, Bennett AM, et al. JAK2 and SHP2 reciprocally regulate tyrosine phosphorylation and stability of proapoptotic protein ASK1. J Biol Chem. 2009;284:13481–8. doi: 10.1074/jbc.M809740200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naka T, Matsumoto T, Narazaki M, Fujimoto M, Morita Y, Ohsawa Y, et al. Accelerated apoptosis of lymphocytes by augmented induction of Bax in SSI-1 (STAT-induced STAT inhibitor-1) deficient mice. Proc Natl Acad Sci U S A. 1998;95:15577–82. doi: 10.1073/pnas.95.26.15577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Starr R, Metcalf D, Elefanty AG, Brysha M, Willson TA, Nicola NA, et al. Liver degeneration and lymphoid deficiencies in mice lacking suppressor of cytokine signaling-1. Proc Natl Acad Sci U S A. 1998;95:14395–9. doi: 10.1073/pnas.95.24.14395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu CR, Mahdi RR, Oh HM, Amadi-Obi A, Levy-Clarke G, Burton J, et al. Suppressor of cytokine signaling-1 (SOCS1) inhibits lymphocyte recruitment into the retina and protects SOCS1 transgenic rats and mice from ocular inflammation. Invest Ophthalmol Vis Sci. 2011;52:6978–86. doi: 10.1167/iovs.11-7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Solomon M, Flodstrom-Tullberg M, Sarvetnick N. Differences in suppressor of cytokine signaling-1 (SOCS-1) expressing islet allograft destruction in normal BALB/c and spontaneously-diabetic NOD recipient mice. Transplantation. 2005;79:1104–9. doi: 10.1097/01.tp.0000162979.66954.53. [DOI] [PubMed] [Google Scholar]
- 11.Balabanov R, Strand K, Kemper A, Lee JY, Popko B. Suppressor of cytokine signaling 1 expression protects oligodendrocytes from the deleterious effects of interferon-gamma. J Neurosci. 2006;26:5143–52. doi: 10.1523/JNEUROSCI.0737-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Egan PJ, Lawlor KE, Alexander WS, Wicks IP. Suppressor of cytokine signaling-1 regulates acute inflammatory arthritis and T cell activation. J Clin Invest. 2003;111:915–24. doi: 10.1172/JCI16156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chong MM, Chen Y, Darwiche R, Dudek NL, Irawaty W, Santamaria P, et al. Suppressor of cytokine signaling-1 overexpression protects pancreatic beta cells from CD8+ T cell-mediated autoimmune destruction. J Immunol. 2004;172:5714–21. doi: 10.4049/jimmunol.172.9.5714. [DOI] [PubMed] [Google Scholar]
- 14.Flodstrom-Tullberg M, Yadav D, Hagerkvist R, Tsai D, Secrest P, Stotland A, et al. Target cell expression of suppressor of cytokine signaling-1 prevents diabetes in the NOD mouse. Diabetes. 2003;52:2696–700. doi: 10.2337/diabetes.52.11.2696. [DOI] [PubMed] [Google Scholar]
- 15.Roman-Gomez J, Jimenez-Velasco A, Castillejo JA, Cervantes F, Barrios M, Colomer D, et al. The suppressor of cytokine signaling-1 is constitutively expressed in chronic myeloid leukemia and correlates with poor cytogenetic response to interferon-alpha. Haematologica. 2004;89:42–8. [PubMed] [Google Scholar]
- 16.Sasi W, Jiang WG, Sharma A, Mokbel K. Higher expression levels of SOCS 1,3,4,7 are associated with earlier tumour stage and better clinical outcome in human breast cancer. BMC cancer. 2010;10:178. doi: 10.1186/1471-2407-10-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Melnikov AA, Scholtens D, Talamonti MS, Bentrem DJ, Levenson VV. Methylation profile of circulating plasma DNA in patients with pancreatic cancer. Journal of surgical oncology. 2009;99:119–22. doi: 10.1002/jso.21208. [DOI] [PubMed] [Google Scholar]
- 18.Galm O, Yoshikawa H, Esteller M, Osieka R, Herman JG. SOCS-1, a negative regulator of cytokine signaling, is frequently silenced by methylation in multiple myeloma. Blood. 2003;101:2784–8. doi: 10.1182/blood-2002-06-1735. [DOI] [PubMed] [Google Scholar]
- 19.Min W, Pober JS. AIP1 in graft arteriosclerosis. Trends Cardiovasc Med. 2011;21:229–33. doi: 10.1016/j.tcm.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu L, Qin L, Zhang H, He Y, Chen H, Pober J, et al. AIP1 prevents graft arteriosclerosis by inhibiting IFN-γ-dependent smooth muscle cell proliferation and intimal expansion. Cir Res. 2011;109:418–27. doi: 10.1161/CIRCRESAHA.111.248245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitchell RN. Allograft arteriopathy: pathogenesis update. Cardiovasc Pathol. 2004;13:33–40. doi: 10.1016/S1054-8807(03)00108-X. [DOI] [PubMed] [Google Scholar]
- 22.Koulack J, McAlister VC, MacAulay MA, Bitter-Suermann H, MacDonald AS, Lee TD. Importance of minor histocompatibility antigens in the development of allograft arteriosclerosis. Clin Immunol Immunopathol. 1996;80:273–7. doi: 10.1006/clin.1996.0123. [DOI] [PubMed] [Google Scholar]
- 23.Marine JC, Topham DJ, McKay C, Wang D, Parganas E, Stravopodis D, et al. SOCS1 deficiency causes a lymphocyte-dependent perinatal lethality. Cell. 1999;98:609–16. doi: 10.1016/s0092-8674(00)80048-3. [DOI] [PubMed] [Google Scholar]
- 24.Boratynska M, Klinger M, Szyber P, Patrzalek D, Polak K. Interleukin-6 in chronic renal allograft rejection: influence of nonimmunologic risk factors. Transplant Proc. 2001;33:1215–7. doi: 10.1016/s0041-1345(00)02393-9. [DOI] [PubMed] [Google Scholar]
- 25.Deng MC, Plenz G, Labarrere C, Marboe C, Baba HA, Erre M, et al. The role of IL6 cytokines in acute cardiac allograft rejection. Transplant immunology. 2002;9:115–20. doi: 10.1016/s0966-3274(02)00004-7. [DOI] [PubMed] [Google Scholar]
- 26.Plenz G, Eschert H, Erren M, Wichter T, Bohm M, Flesch M, et al. The interleukin-6/interleukin-6-receptor system is activated in donor hearts. J Am Coll Cardiol. 2002;39:1508–12. doi: 10.1016/s0735-1097(02)01791-6. [DOI] [PubMed] [Google Scholar]
- 27.Tellides G, Pober JS. Interferon-gamma axis in graft arteriosclerosis. Circ Res. 2007;100:622–32. doi: 10.1161/01.RES.0000258861.72279.29. [DOI] [PubMed] [Google Scholar]
- 28.Tellides G, Tereb DA, Kirkiles-Smith NC, Kim RW, Wilson JH, Schechner JS, et al. Interferon-gamma elicits arteriosclerosis in the absence of leukocytes. Nature. 2000;403:207–11. doi: 10.1038/35003221. [DOI] [PubMed] [Google Scholar]
- 29.Zehnder JL, Hirai K, Shatsky M, McGregor JL, Levitt LJ, Leung LL. The cell adhesion molecule CD31 is phosphorylated after cell activation. Down-regulation of CD31 in activated T lymphocytes. J Biol Chem. 1992;267:5243–9. [PubMed] [Google Scholar]
- 30.Muller WA, Luscinskas FW. Assays of transendothelial migration in vitro. Methods Enzymol. 2008;443:155–76. doi: 10.1016/S0076-6879(08)02009-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Langer HF, Chavakis T. Leukocyte-endothelial interactions in inflammation. Journal of cellular and molecular medicine. 2009;13:1211–20. doi: 10.1111/j.1582-4934.2009.00811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roebuck KA, Finnegan A. Regulation of intercellular adhesion molecule-1 (CD54) gene expression. J Leukoc Biol. 1999;66:876–88. doi: 10.1002/jlb.66.6.876. [DOI] [PubMed] [Google Scholar]
- 33.Saha B, Jyothi Prasanna S, Chandrasekar B, Nandi D. Gene modulation and immunoregulatory roles of interferon gamma. Cytokine. 2010;50:1–14. doi: 10.1016/j.cyto.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 34.Davey GM, Heath WR, Starr R. SOCS1: a potent and multifaceted regulator of cytokines and cell-mediated inflammation. Tissue antigens. 2006;67:1–9. doi: 10.1111/j.1399-0039.2005.00532.x. [DOI] [PubMed] [Google Scholar]
- 35.Ortiz-Munoz G, Martin-Ventura JL, Hernandez-Vargas P, Mallavia B, Lopez-Parra V, Lopez-Franco O, et al. Suppressors of cytokine signaling modulate JAK/STAT-mediated cell responses during atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:525–31. doi: 10.1161/ATVBAHA.108.173781. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.