Abstract
Objectives
To assess innate and humoral immune responses in middle ear effusion of obese pediatric patients with otitis media with effusion (OME).
Methods
We evaluated 219 children with OME, of whom 21 were obese and 198 were non-obese. We compared the expression in middle ear effusion of mRNAs encoding toll-like receptors (TLR) 2, 4, 5, and 9; nucleotide-binding oligomerization domains (NOD) 1 and 2; retinoic acid-inducible gene (RIG)-I; interleukins (IL)-6, -10, and -12; interferon (IFN)-γ; and tumor necrosis factor (TNF)-α mRNAs. We also compared the expression of immunoglobulins IgG, IgA, and IgM and the bacterial detection rate in the two groups.
Results
TLR2-mediated expression of IL-6 mRNA, TLR4-mediated expression of IL-6 and IL-10 mRNA, TLR5-mediated expression of IL-6, IL-10, and TNF-α mRNA, TLR9-mediated expression of IL-6 mRNA, and NOD2-mediated expression of IL-6, IL-12, and TNF-α mRNA were significantly lower in obese than in non-obese children (P<0.05). However, concentrations of IgG, IgA, and IgM in middle ear effusion were lower in obese than in non-obese children, but none of these differences was significant (P>0.05).
Conclusion
Mean body mass index was higher and pattern-recognition receptor-mediated cytokine mRNA expression was lower in obese than in non-obese children with OME.
Keywords: Otitis media, Obesity, Pattern-recognition receptors, Immunoglobulin, Immunity, Bacteria
INTRODUCTION
Otitis media with effusion (OME), a disease in which secreted fluid accumulates in the middle ear cavity, is a major cause of hearing loss in children [1]. OME may be caused by Eustachian tube dysfunction, bacterial or viral infection, adenoid hypertrophy, nasopharyngeal infection, sinusitis, allergic and/or immunological factors, exposure to second-hand smoke in the home, formula rather than breast-feeding, nutritional deficiency, attendance at day care, pollution, and family history [2]. These factors may be affected by time, environmental changes, weather, lifestyle, and changes in overall constitution. More recently, obesity has been linked to OME [3-5].
Obesity may cause many other diseases, in both adults and children. Obesity is characterized by low-grade systemic inflammation, with obese individuals showing greater expression of inflammatory markers than lean individuals. Several findings have suggested that obesity increase susceptibility to OME. The first is changes in cytokine levels. Increased expression of interleukin (IL)-6, tumor necrosis factor (TNF)-α, and plasminogen activator inhibitor (PAI)-1 have been observed in middle ear effusion of patients with active cellular responses, persistent inflammation in the middle ear cavity, hyper-secretion of middle ear effusion fluid in response to mucosal changes, and adipose tissue accumulation. Furthermore, host immunity changes in response to obesity, with altered T cell responses, production of interferon (IFN)-γ, and increased leptin concentrations, lead to increased rates of upper respiratory infection. Third, obesity has been found to increase intragastric pressure and transdiaphragmatic gastroesophageal pressure gradient; after rendering the lower esophageal sphincter inactive, this gastric reflux reaches the middle ear through the Eustachian tubes. Fourth, fat accumulation around the Eustachian tube and nasopharynx results in structural dysfunction, with weak structural closures of the Eustachian tube [4,6].
Although the immunologic etiology and mechanisms of OME have been investigated, little is known about how the immune system reacts in the middle ear cavity of obese and non-obese children. We therefore evaluated innate and humoral immunity in obese and non-obese children with OME.
MATERIALS AND METHODS
Study subjects
The experimental group consisted of children aged 1-11 years with OME who visited the Department of Ear, Nose and Throat at Kyung Hee University Hospital for unilateral or bilateral ventilation insertion.
Patients were included if they had (1) an amber-colored tympanic membrane for at least 2-3 months on otoscopic examination; (2) a B- or C-type tympanogram upon impedance audiometry; (3) progressive hearing loss as shown by an increase in the pure tone threshold; (4) progressive retraction of the tympanic membrane as revealed by otoscopy. If an air-fluid or air bubbles were seen in the tympanic membrane, we made it a rule to wait-and-see for 3 months at least. We then reevaluated each such patient as described above. A tympanostomy tube was inserted in all patients who met these inclusion criteria. Children with head-and-neck anomalies, systemic disease, chronic disease, or suspected of having acquired immune deficiency disease, were excluded.
Body mass index (BMI) for each child was calculated as the directly measured weight (kg)/square of height (m2). The standard measurements of physical growth of children and adolescents of Korea, proposed by the Korean Academy of Pediatrics and based on factors such as age, gender, and BMI, recommend that individuals with BMI at or above the 95th percentile value be defined as obese [7-9].
Children were enrolled after approval was obtained from the Medical Ethics Committee of Kyung Hee University Hospital. All parents or guardians provided written informed consent.
Middle ear effusion fluid
A tympanostomy tube was inserted after a radial shaped incision was made in the anterior inferior quadrant of the tympanic membrane. Prior to surgery, the external auditory canal was washed with potadine solution, and middle ear effusion fluid was collected using a collector (Xomed Treace Products, Jacksonville, FL, USA) by aseptic procedures, taking care to avoid bleeding. Each effusion fluid sample was collected using sterilized cotton swabs, added to Stuart transport medium, and used to inoculate blood agar medium and thioglycollate liquid medium. All cultures were incubated for at least 24 hours at 35℃, and the resultant bacteria were identified by Gram-staining and biochemical analyses. The rate of bacterial occurrence was compared in the two groups.
Amplification
Total RNA was extracted from effusion using the RNA-Bee solution kit (Tel-Test, Friendswood, TX, USA) following the manufacturer's protocol. First-strand cDNA synthesis was performed by reverse transcription in a total volume of 20 mL reaction mixture containing 1 mg of RNA, 1x reaction buffer, 1 mM dNTP, 5 µM random primers, 20 units RNase inhibitor, and 20 units AMV reverse transcriptase (Promega, Madison, WI, USA). The reaction mixture was incubated at 42℃ for 1 hour, terminated by heating at 95℃ for 5 minutes. Toll-like receptors (TLRs) 2, 4, 5, 9, and nucleotide-binding oligomerization domain-containing proteins (NODs) 1 and 2, retinoic acid-inducible gene (RIG)-I, cytokines (IL-6, -10, -12, IFN-γ, TNF-α), transcription factors (B-cell leukemia lymphoma-6 [Bcl-6], B-lymphocyte inducer of maturation program 1 [Blimp-1], paired box gene 5 [Pax-5], X-box binding protein 1 [XBP-1]), and nitric oxide primers are shown in Table 1. Real-time polymerase chain reaction (PCR) was performed using a Chromo4 Detector real-time system (Bio-Rad, Hercules, CA, USA) with the SsoFast EvaGreen supermix (Bio-Rad). PCR was performed with 2 µL of cDNA in a 20 µL reaction mixture of 10 mL SsoFast EvaGreen supermix, 2 mL primers and 6 mL PCR grade water. The reaction parameters were as follows: denaturation at 95℃ for 30 seconds followed by 45 cycles at 95℃ for 5 seconds, 55℃ to 64℃ for 12 seconds. The crossing point of TLR2, 4, 5, 9, and NOD1, 2, RIG-I, cytokines (IL-6, IL-10, IL-12, IFN-γ, TNF-α), transcription factors (Bcl-6, Blimp-1, Pax-5, XBP-1), and nitric oxide with β-actin was applied to the formula, 2-(target gene-β actin) and the relative amounts were quantitated. Between-group differences in pattern-recognition receptor (PRR)-mediated cytokine mRNA expression were assessed.
Table 1.
Primers for real-time PCR amplification

TLR, toll-like receptor; NOD, nucleotide-binding oligomerization domain-containing protein; RIG, retinoic acid-inducible gene; IL, interleukin; IFN, interferon; TNF, tumor necrosis factor; Bcl-6, B-cell leukemia lymphoma-6; Blimp-1, B-lymphocyte inducer of maturation program 1; Pax-5, paired box gene 5; XBP-1, X-box binding protein 1.
Enzyme-linked immunosorbent assay
All samples of middle ear retention fluid were stored at -80℃, and their IgG, IgA, and IgM concentrations were measured by enzyme-linked immunosorbent assay (ELISA). Briefly, 50 µL 1:100 goat anti-human IgG, IgA, IgM in coating buffer (1.59 g Na2CO3+2.93 g NaHCO3+5% NaN3, pH 9.6) was placed in each well of a 96 well plate and incubated overnight at 4℃. The wells were washed 6 times, blocking antibody was added and to each well was added 50 µL of each sample, and the plates were incubated at room temperature for 3 hours. The wells were washed 6 times, and purified goat anti-human IgG, IgA, and IgM conjugated to horseradish peroxidase in phosphate buffered saline/tween/bovine serum albumin solution was added, and the plates were incubated at room temperature. The plates were washed 6 times, substrate solution (2,2'-AZINO-Bis) was added, and the optical absorbance was measured at 450 nm (Bethyl, Montgomery, TX, USA).
Statistical analysis
Statistical analysis was performed using SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA), with the Mann-Whitney U-test employed for between-group analyses. A P<0.05 was considered to be statistically significant.
RESULTS
Patients
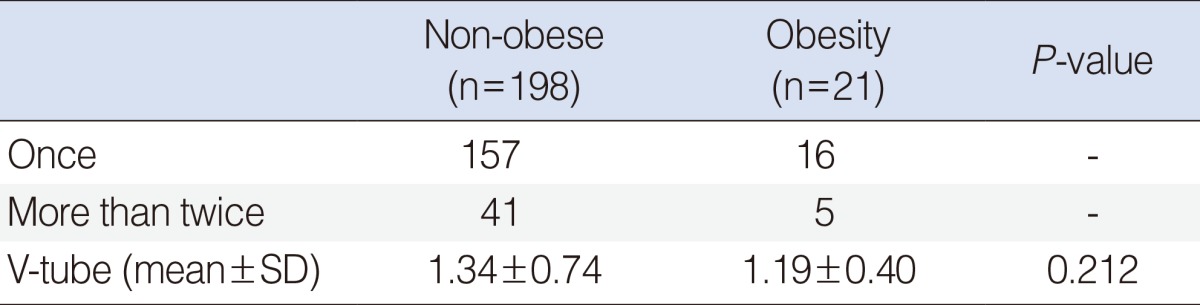
We evaluated 219 patients (152 boys, 67 girls) who underwent ventilation tube insertion for treatment of OME. Of these patients, 21 (12 boys, 9 girls; mean age, 4.13±2.36 years) were considered obese, 198 (126 boys, 72 girls; mean age, 4.76±2.17 years) were considered non-obese. Mean age and sex distribution were similar in the two groups (P>0.05 each). Mean BMI was significantly greater in the obese than in the non-obese group (21.31±2.66 kg/m2 vs. 15.89±1.66 kg/m2; P<0.05). Mean follow-up times were 15.3 months, ranged from 3 months to 3 years. The frequency of ventilation tube insertion did not differ significantly in the two groups (P>0.05) (Table 2).
Table 2.
Frequency of V-tube insertion
Culture results from middle ear effusion fluid
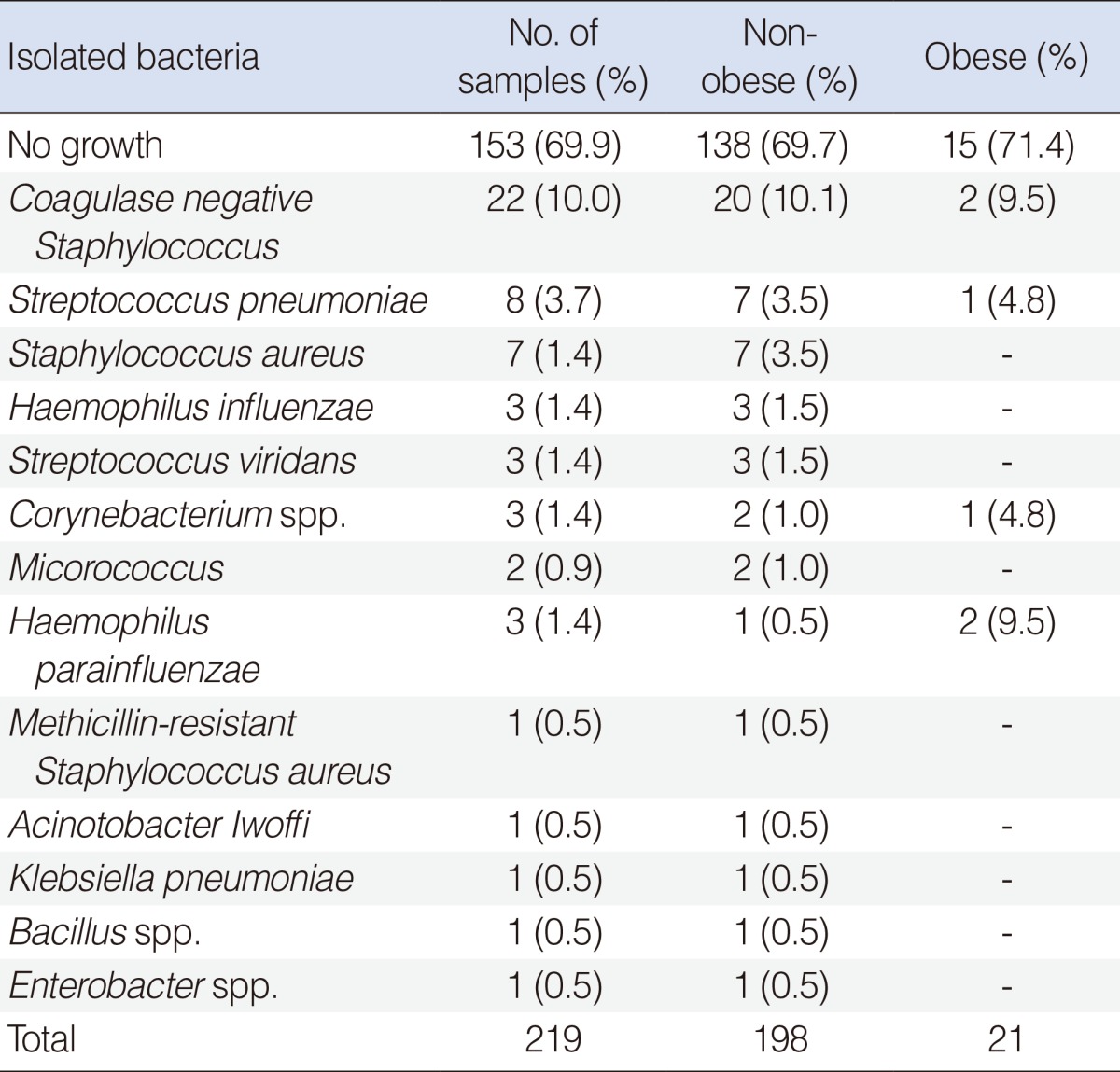
Bacteria were detected in 6 of 21 obese (28.6%) and 60 of 198 non-obese children (27.2%) by standard culture methods. Chi-squared analysis revealed that the detection rate in the two groups did not differ significantly (χ2=0.548, P=0.755) (Table 3).
Table 3.
Bacteriologic result
Pattern recognition receptor-mediated cytokine mRNA expression
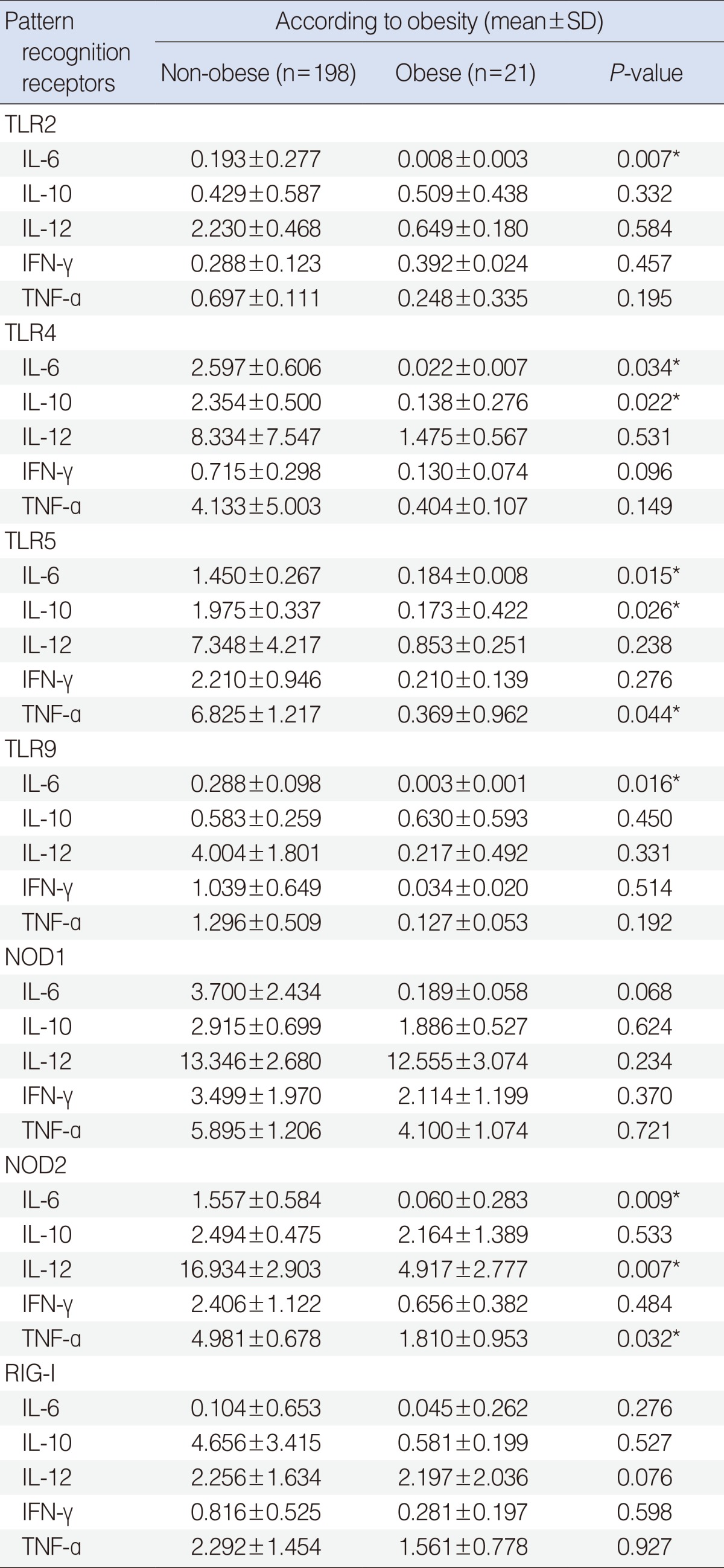
We found that all of these transcripts were expressed in both groups. TLR2-mediated expression of IL-6 mRNA, TLR4-mediated expression of IL-6 and IL-10 mRNA, TLR5-mediated expression of IL-6, IL-10, and TNF-α mRNA, TLR9-mediated expression of IL-6 mRNA, and NOD2-mediated expression of IL-6, IL-12, and TNF-α mRNA were significantly lower in obese than in non-obese children (P<0.05) (Table 4).
Table 4.
Pattern-recognition receptor-mediated cytokine mRNA expression
TLR, toll-like receptor; IL, interleukin; IFN, interferon; TNF, tumor necrosis facto; NOD, nucleotide-binding oligomerization domain-containing protein; RIG, retinoic acid-inducible gene.
*P<0.05.
IgG, IgA, IgM, and transcription factors
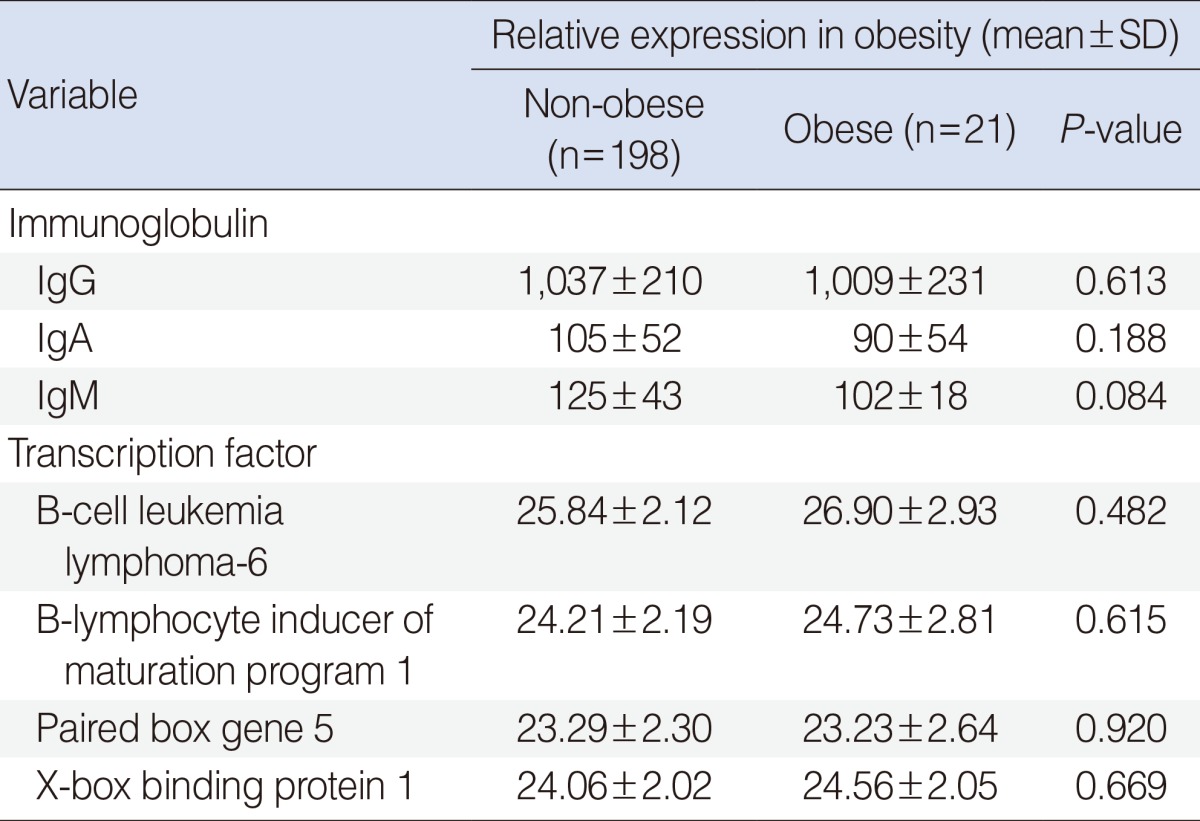
We found that the concentrations of IgG, IgA, and IgM in middle ear effusion were lower in the obese than in the non-obese group, but none of these differences was significant (P>0.05 each). We also found no between group differences in expression of the transcription factors Bcl-6, Blimp-1, Pax-5, and XBP-1 associated with immunoglobulin secretion (P>0.05) (Table 5).
Table 5.
Secretion of immunoglobulins and expression of transcription factors
DISCUSSION
Obesity, defined as the increased accumulation of body fat, is a chronic disease of multifactorial origin. Obesity is caused by interactions of social, behavioral, psychological, metabolic, cellular, and molecular factors. The worldwide prevalence of obesity has progressively increased over the past 30 years. The World Health Organization has estimated that about 700 million adults worldwide will be obese by 2015 [8,10]. Obesity has been associated with many diseases, including type 2 diabetes mellitus, systemic arterial hypertension, ischemic heart diseases, obstructive sleep apnea, asthma, peripheral vascular disease, fatty liver disease, liver failure, gout, arthritis, orthopedic disorders, psychosocial stress, and colorectal cancer [11,12]. Mean weight has been found to be markedly higher in children with than without OME, suggesting that childhood obesity may affect the occurrence of OME [3-5,13].
The rate of bacterial detection is very high in patients with acute otitis media, with the 3 most common bacteria being Streptococcus pneumoniae, Haemophilus influenza, and Moraxella catarrhalis. In contrast, about 60% of patients with chronic OME are culture negative, and staphylococci and anaerobes may play greater roles in chronic than in acute disease [14]. We also found that 71.4% of our patients with chronic OME were culture negative and that, among culture positive children, Staphylococci were the most commonly detected bacteria. In contrast to our expectation, that bacterial infection would be more frequent in obese than in non-obese patients, we found that the bacterial detection rate was similar in the two groups.
Obesity is considered a chronic low-grade inflammatory condition. Obesity results in the increased expression and release of proinflammatory cytokines, including TNF-α and IL-6. Moreover, obesity has been associated with the activation of inflammatory signaling pathways, due to the autonomous dysregulation of homeostatic pathways. In addition, the higher fatty acid content in obese individuals can activate proinflammatory pathways, resulting in the development of insulin resistance. All of these effects may be mediated by activation of TLRs in several tissues, which may contribute to the development of obesity-associated insulin resistance [10]. TLRs are among the well-known PRRs and play a crucial role in the innate immune system, which detects the presence and the nature of infectious agents and is the first line of host defense [15]. PRRs, which are expressed in cells of the innate immune system, include Toll-like receptors, NOD-like receptors, and RIG-I. Pathogen-associated molecular patterns (PAMPs), derived from bacteria, fungi, parasites and viruses, are recognized by PRRs, resulting in the release of inflammatory cytokines and type I interferons and the boosting of host defenses [16,17]. Activation of TLRs in fat cells induces cytokine secretion, which triggers further inflammation [18]. Cytokines are glycoproteins, produced by inflammatory and epithelial cells, which modulate immune responses. These cytokines are responsible for many of the inflammatory changes induced by pathogenic organisms during otitis media. Bacteria can trigger cytokine secretion of monocytes and macrophages as well as TLR expression. Gram-positive bacteria tend to induce expression of IL-12, IFN-γ and TNF, whereas Gram-negative bacteria tend to induce expression of IL-6, IL-8, and IL-10 [19]. Gram-positive bacteria present in obese children included coagulase negative Staphylococcus, S. pneumonia, S. aureus, and Corynebacterium, whereas the only Gram-negative bacterium detected was H. parainfluenzae. Since the number of subjects in this study was small, we could not compare differences in cytokine expression levels between subjects with Gram-positive and Gram-negative bacteria.
We found that TLR2, 4, 5, 9 and NOD1, 2 mRNAs, all of which are related to bacterial infection, were expressed in both groups. When we analyzed PRR-mediated cytokine expression, we found that TLR2-mediated expression of IL-6 mRNA, TLR4-mediated expression of IL-6 and IL-10 mRNA, TLR5-mediated expression of IL-6, IL-10, and TNF-α mRNA, TLR9-mediated expression of IL-6 mRNA, and NOD2-mediated expression of IL-6, IL-12, and TNF-α mRNA were significantly lower in obese than in non-obese children. These findings suggest that inflammatory responses linked with innate immunity may be affected by obesity, whereas effusion fluid may not fully reflect inflammatory reactions in the middle ear cavity.
As stated above, obesity is one of low-grade systemic inflammation. In this sense, the discordance between chronic systemic inflammation by obesity and decreased PRR-mediated cytokine expression in obese group should be discussed. We assumed the reason of discordance as follows: First, what we compare is not the simple mRNA expression of every PRRs and cytokines but PRR-mediated cytokine expression in both groups. That is, decreased PRRs-mediated cytokine expression in chronic systemic inflammation due to obesity may suggest that local immune reaction to protect middle ear is reduced in the obese group. Second, local immunity in middle ear may act in a different manner contrary to systemic immunity affected by obesity. Third, methodologically, transcription level used in this study does not always correlated with protein level and cytokine expression is regulated both at the transcriptional level and translational mechanisms [20]. However this assumption needs further verification.
Because effusion fluid contained acid phosphatase, alkaline phosphatase, oxaloacetic transaminase, creatine phosphokinase, mucin, cytokines, enzymes, immunoglobulin producing cells, immunoglobulin and secretory unit, this fluid in the middle ear was caused by a local immune reaction in the middle ear cavity, protecting against microbes [21,22]. The activity of antibodies involved in the defense of the middle-ear cavity is regulated by transcription factors that regulate the synthesis of antibodies. Among these transcription factors are Bcl-6, Bimp-1, Pax-5, and XBP-1. Bcl-6 and Pax-5 suppress, while Blimp-1 and XBP-1 enhance, immunoglobulin secretion [23]. Although immunoglobulin concentrations tended to be lower in the obese than in the non-obese group, these differences were not significant, nor were any differences in the concentrations of the transcriptional factors Bcl-6, Blimp-1, Pax-5, and XBP-1. We could not therefore evaluate the relationship between humoral immunity and obesity in children with OME.
This study had several limitations. We did not include any children with early stage OME. The exudates we examined were collected during surgery, 2-3 months after the initial onset of otitis media. Therefore we could not determine the levels of expression of PRRs, cytokines, and transcription factors mRNAs and of immunoglobulins at initial diagnosis. Moreover, we observed all patients until tubes were extracted. The follow-up times ranged from 3 months to 3 years, with a median of 7.0 months, which may have been too short to accurately determine the frequency of operation. This may have led to an overestimation of the frequency of operation.
Furthermore, for ethical reasons, we could not harvest middle ear mucosa from these patients. We therefore performed experiments on exudates, which contained only a few partially exfoliated mucosal epithelial cells, inflammatory cells, and immune cells; therefore, our findings would not fully reflect the immune response in the middle ear mucosa during otitis media.
Mean BMI was higher and pattern-recognition receptor-mediated cytokine mRNA expression was lower in obese than in non-obese children with OME. However, factors associated with humoral immune responses were similar in the middle ear cavities of obese and non-obese children with OME.
ACKNOWLEDGMENTS
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (No. 2011-0030072).
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Klein JO, Tos M, Hussl B, Naunton RF, Ohyama M, van Cauwenberge PB. Recent advances in otitis media: definition and classification. Ann Otol Rhinol Laryngol Suppl. 1989 Apr;139:10. [PubMed] [Google Scholar]
- 2.Bluestone CD, Klein JO. Clinical practice guideline on otitis media with effusion in young children: strengths and weaknesses. Otolaryngol Head Neck Surg. 1995 Apr;112(4):507–511. doi: 10.1177/019459989511200401. [DOI] [PubMed] [Google Scholar]
- 3.Kim JB, Park DC, Cha CI, Yeo SG. Relationship between pediatric obesity and otitis media with effusion. Arch Otolaryngol Head Neck Surg. 2007 Apr;133(4):379–382. doi: 10.1001/archotol.133.4.379. [DOI] [PubMed] [Google Scholar]
- 4.Lee SK, Yeo SG. Relationship between pediatric obesity and otitis media with effusion. Curr Allergy Asthma Rep. 2009 Nov;9(6):465–472. doi: 10.1007/s11882-009-0069-3. [DOI] [PubMed] [Google Scholar]
- 5.Kim SH, Park DC, Byun JY, Park MS, Cha CI, Yeo SG. The relationship between overweight and otitis media with effusion in children. Int J Obes (Lond) 2011 Feb;35(2):279–282. doi: 10.1038/ijo.2010.144. [DOI] [PubMed] [Google Scholar]
- 6.Fernández-Sánchez A, Madrigal-Santillan E, Bautista M, Esquivel-Soto J, Morales-Gonzalez A, Esquivel-Chirino C, et al. Inflammation, oxidative stress, and obesity. Int J Mol Sci. 2011;12(5):3117–3132. doi: 10.3390/ijms12053117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahn HS. Pediatrics. 8th ed. Seoul: Daehan Printing & Publishing; 2005. [Google Scholar]
- 8.Ebbeling CB, Pawlak DB, Ludwig DS. Childhood obesity: public-health crisis, common sense cure. Lancet. 2002 Aug;360(9331):473–482. doi: 10.1016/S0140-6736(02)09678-2. [DOI] [PubMed] [Google Scholar]
- 9.Barlow SE, Dietz WH. Obesity evaluation and treatment: expert Committee recommendations. The Maternal and Child Health Bureau, Health Resources and Services Administration and the Department of Health and Human Services. Pediatrics. 1998 Sep;102(3):E29. doi: 10.1542/peds.102.3.e29. [DOI] [PubMed] [Google Scholar]
- 10.Könner AC, Bruning JC. Toll-like receptors: linking inflammation to metabolism. Trends Endocrinol Metab. 2011 Jan;22(1):16–23. doi: 10.1016/j.tem.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 11.Gortmaker SL, Dietz WH, Jr, Sobol AM, Wehler CA. Increasing pediatric obesity in the United States. Am J Dis Child. 1987 May;141(5):535–540. doi: 10.1001/archpedi.1987.04460050077035. [DOI] [PubMed] [Google Scholar]
- 12.Chan RS, Woo J. Prevention of overweight and obesity: how effective is the current public health approach. Int J Environ Res Public Health. 2010 Mar;7(3):765–783. doi: 10.3390/ijerph7030765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kirk SF, Kuhle S, Ohinmaa A, Colman I, Veugelers PJ. Health care utilization from prevalent medical conditions in normal-weight, overweight, and obese children. J Pediatr. 2012 Feb;160(2):216–221.e1. doi: 10.1016/j.jpeds.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 14.Blustone CD, Casseobrant ML, Dohar JE. Targeted therapies otitis media and ititis externa. Hamilton, ON: BC Decker Inc; 2003. [Google Scholar]
- 15.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007 Oct;449(7164):819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 16.Kim MJ, Cho YK. Pattern recognition receptors in immune modulation. Biowave. 2006 Jun;8(12):1–22. [Google Scholar]
- 17.Kawai T, Akira S. The roles of TLRs, RLRs and NLRs in pathogen recognition. Int Immunol. 2009 Apr;21(4):317–337. doi: 10.1093/intimm/dxp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poulain-Godefroy O, Le Bacquer O, Plancq P, Lecoeur C, Pattou F, Frühbeck G, et al. Inflammatory role of Toll-like receptors in human and murine adipose tissue. Mediators Inflamm. 2010;2010:823486. doi: 10.1155/2010/823486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Skovbjerg S, Martner A, Hynsjo L, Hessle C, Olsen I, Dewhirst FE, et al. Gram-positive and gram-negative bacteria induce different patterns of cytokine production in human mononuclear cells irrespective of taxonomic relatedness. J Interferon Cytokine Res. 2010 Jan;30(1):23–32. doi: 10.1089/jir.2009.0033. [DOI] [PubMed] [Google Scholar]
- 20.Kuczkowski J, Sakowicz-Burkiewicz M, Izycka-Swieszewska E, Mikaszewski B, Pawełczyk T. Expression of tumor necrosis factor-α, interleukin-1α, interleukin-6 and interleukin-10 in chronic otitis media with bone osteolysis. ORL J Otorhinolaryngol Relat Spec. 2011;73(2):93–99. doi: 10.1159/000323831. [DOI] [PubMed] [Google Scholar]
- 21.Howie VM, Ploussard JH, Sloyer JL, Johnston RB., Jr Immunoglobulins of the middle ear fluid in acute otitis media: relationship to serum immunoglobulin concentrations and bacterial cultures. Infect Immun. 1973 Apr;7(4):589–593. doi: 10.1128/iai.7.4.589-593.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yeo SG, Park DC, Lee SK, Cha CI. Relationship between effusion bacteria and concentrations of immunoglobulin in serum and effusion fluid in otitis media with effusion patients. Int J Pediatr Otorhinolaryngol. 2008 Mar;72(3):337–342. doi: 10.1016/j.ijporl.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 23.Tumang JR, Frances R, Yeo SG, Rothstein TL. Spontaneously Ig-secreting B-1 cells violate the accepted paradigm for expression of differentiation-associated transcription factors. J Immunol. 2005 Mar;174(6):3173–3177. doi: 10.4049/jimmunol.174.6.3173. [DOI] [PubMed] [Google Scholar]


