Abstract
Over the past few decades, food fortification and infant formula supplementation with high levels of vitamins have led to a sharp increase in vitamin intake among infants, children and adults. This is followed by a sharp increase in the prevalence of obesity and related diseases, with significant disparities among countries and different groups within a country. It has long been known that B vitamins at doses below their toxicity threshold strongly promote body fat gain. Studies have demonstrated that formulas, which have very high levels of vitamins, significantly promote infant weight gain, especially fat mass gain, a known risk factor for children developing obesity. Furthermore, ecological studies have shown that increased B vitamin consumption is strongly correlated with the prevalence of obesity and diabetes. We therefore hypothesize that excess vitamins may play a causal role in the increased prevalence of obesity. This review will discuss: (1) the causes of increased vitamin intake; (2) the non-monotonic effect of excess vitamin intake on weight and fat gain; and (3) the role of vitamin fortification in obesity disparities among countries and different groups within a country.
Keywords: Vitamin fortification, Refined grain, Infant formula, Obesity, Diabetes, Insulin resistance, Oxidative stress, Glycemic index, Formula feeding, Epigenetic
Core tip: B vitamins are a known fat gain promoting factor. Food fortification-induced high vitamin consumption is followed by a rapid increase in obesity prevalence. Why is the fat gain effect of B vitamins neglected in obesity studies? Why does obesity prevalence vary from country to country? Why are the poor in developed countries but the rich in developing countries at high risk of obesity? Why is obesity prevalence higher in blacks than whites in the United States? Why does formula feeding (which is associated with high energy expenditure) increase the risk for obesity? Why is physical inactivity associated with increased obesity risk? This paper reviews the role of excess vitamins in obesity and proposes a unified answer to these questions.
INTRODUCTION
Obesity, a state of excessive accumulation of fat in the body, is a major risk factor for many diseases, such as type 2 diabetes and cardiovascular disease[1,2]. In the 1970s and 1980s, a rapid increase in the prevalence of obesity occurred almost simultaneously in many developed countries. Since then, developing countries have also experienced a rapid increase in obesity rates[3,4]. Nowadays, obesity has become a global epidemic[5]. It is worth noting that the prevalence of obesity differs greatly among countries[3,4,6,7] as well as groups within a country[8-12]. It is more prevalent among those with low socioeconomic status (SES) in developed countries[6,8-10] but with high SES in developing countries, especially at their early stage of development[10-12]. Interestingly, compared with breast-fed infants, formula-fed infants have higher rather than lower levels of energy expenditure[13,14] and are more at risk for obesity in later life[15-17]. Therefore, the rapidly increased prevalence of obesity cannot be simply explained by genetic factors or decreased energy expenditure.
Recently, it has been suggested that changes in the global food system may play a role in the increased prevalence of obesity[4]. If this is the case, the global food system must have sharply changed in the 1970s-1980s. Notably, in the 1970s and 1980s, the contents of vitamins (organic chemicals affecting the body’s functioning) in the food system of many developed countries were sharply increased due to modifications or changes in their rules, laws and regulations regarding food fortification[18-20]. This led to a nationwide increase in the consumption of many vitamins, especially fat synthesis-promoting B vitamins[21-24], including B1 (thiamin), B2 (riboflavin), B3 (niacin) and B6, in many countries[18-20]. Thus, there is a possibility that the food fortification-induced high vitamin intake may be related to the sudden increase in the prevalence of obesity in the 1970s-1980s. Indeed, emerging evidence suggests that this food fortification-induced excess vitamin intake might play a major role in the increased prevalence of obesity[25,26]. In this review, we will discuss the cause of increased vitamin intake and its possible role in obesity, as well as the obesity disparities among countries and groups within countries.
CAUSES OF EXCESS VITAMIN INTAKE
Until the mid 1930s when the first commercial yeast extract vitamin B complex and semi-synthetic vitamin C supplement tablets were sold, vitamins were obtained solely through natural foods and seasonal changes in diet usually greatly altered the types and amounts of vitamins ingested. For example, the intake of fresh vegetable-derived vitamins might be high in summer but low in winter. However, through evolution, humans have adapted to this seasonal variations in vitamin intake by developing mechanisms to maintain the vitamin homeostasis. While the intake of vitamins is higher in summer, their elimination through sweat and sebum[27-30] may also increase because the secretion of sweat and sebum is higher in summer than in winter[28,31,32]. Moreover, the body can store a certain amount of vitamins when the supply is adequate, which can be used for some time when the intake is inadequate. For example, it will take several months before the first symptoms of vitamin C deficiency appear in a vitamin C deprivation condition[33]. From this point of view, it seems unnecessary to take vitamins everyday, although estimated daily average requirements (EARs) and the recommended dietary allowances are given (Table 1). Yet over the past several decades, the actual intake of vitamins has been significantly higher than the EARs due to the following causes.
Table 1.
The estimated daily average requirements and recommended dietary allowances for selected vitamins (mg/d)1
| Vitamin |
Adult man |
Adult woman |
Pregnancy |
|||
| EAR | RDA | EAR | RDA | EAR | RDA | |
| Thiamin | 1.0 | 1.2 | 0.9 | 1.1 | 1.2 | 1.4 |
| Riboflavin | 1.1 | 1.3 | 0.9 | 1.1 | 1.2 | 1.4 |
| Niacin | 12 | 16 | 11 | 14 | 14 | 18 |
| Vitamin B6 | 1.1 | 1.3 | 1.1 | 1.3 | 1.6 | 1.9 |
| Vitamin C | 75 | 90 | 60 | 75 | 70 | 90 |
| Vitamin E | 12 | 15 | 12 | 15 | 12 | 15 |
1Data are from the United States Food and Nutrition Board. EAR: Estimated daily average requirement, available from: URL: http://iom.edu/Activities/Nutrition/SummaryDRIs/~/media/Files/Activity%20Files/Nutrition/DRIs/EAR%20Table.pdf. RDA: Recommended dietary allowance, available from: URL: http://iom.edu/Activities/Nutrition/SummaryDRIs/~/media/Files/Activity%20Files/Nutrition/DRIs/RDA%20and%20AIs_Vitamin%20and%20Elements.pdf.
Increased vitamin intake from vegetable/fruit sources
Over the past several decades, many fresh vegetables and fruits with better quality can be obtained year round due to widespread out-of-season cultivation. This has not only led to an increase in the intake of vegetable/fruit-derived vitamins (e.g., vitamin C), but also abolished the seasonal vitamin intake variations. Taking the United States as an example, the per capita consumption of vegetables and fruits showed an increasing trend in the 1970s through the 1990s (Figure 1A), leading to an increase in vitamin C intake since the mid-1960s[34].
Figure 1.
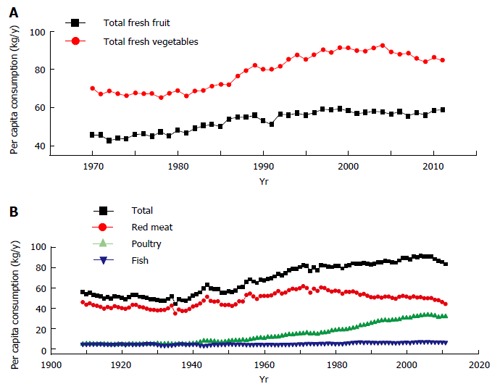
Trends in United States per capita consumption of vegetables, fruits (A) and meats (B). Data are from the Economic Research Service of the United States Department of Agriculture. Available from: URL: http://www.ers.usda.gov/data-products/food-availability-(per-capita)-data-system/.aspx.
Increased vitamin intake from animal sources
The consumption of animal-based foods significantly increased in developed countries in the second half of the last century. Dietary patterns in developing countries have been shifting to a more meat-centric diet over the past few decades[5,35]. Such a nutrition transition has increased the intake of vitamins (especially nicotinamide, a form of niacin) from animal-based foods. For example, United States per capita consumption of total meat showed an increasing trend between the 1930s and 2000 (Figure 1B), which increased the daily intake of meat-derived nicotinamide from 6.8 mg in the 1930s to 11.4 mg in 2000, according to the data on meat contribution to daily niacin intake[34].
Increased vitamin intake from artificial sources
Besides increased natural vitamin sources, vitamins may be obtained from artificial sources, which involves food fortification, infant formula fortification, and vitamin-enriched drinks. Fortification is the process of adding synthetic vitamins to foods and infant milk (including breast milk or formula) to increase its overall vitamin content[34]. Some staple foods (such as flour and maize) are used as a vehicle for fortification. Wheat flour fortification with synthetic vitamins (B1, B2 and niacin) was started first in the United States in the late 1930s, which was soon adopted by many developed countries and then introduced to developing countries[19,20]. Notably, ready-to-eat cereals are a major vehicle of fortification of B vitamins (B1, B2, B6 and niacin). Especially since 1974 when the food fortification standards for cereals were updated, ready-to-eat cereals have become the top food source of many vitamins[20,34]. The levels of vitamins in fortified ready-to-eat cereals are so high (Table 2) that consumption of less than a quarter pound of them (because foods per se also contain some amount of vitamins) meets the daily need for these vitamins in an adult. Many sugar-sweetened beverages are also supplemented with vitamins[36,37], which is also an important cause of increased vitamin intake. Since the 1950s, synthetic vitamins have been added to infant formulas[38]. In the 1980s, the governments of most countries established minimum nutrient requirements for commercial infant formulas[39], resulting in a significant increase in the content of vitamins in formulas. The levels of vitamins in some formulas for premature infants are more than 20 times higher than that of human milk (i.e., about the minimum limit for nutrients) (Table 3). This leads to a high vitamin intake in infancy.
Table 2.
Fortification recommendations for ready-to-eat cereals
| Vitamin | U.S. RDA (mg/d) | 1974-1992 amount | 1974-2000 amount |
| (mg/per pound)1 | (mg/per pound)1 | ||
| Thiamin | 1.5 | 6 | 5.7 |
| Niacin | 20 | 80 | 76 |
| Riboflavin | 1.7 | 6.8 | 6.4 |
| Vitamin C | 60 | 240 | 227 |
| Vitamin B6 | 2 | 8 | 7.6 |
Data are from Reference 34. RDA: Recommended dietary allowance.
Table 3.
The minimum limit for infant formulas in the United States and commercially labeled values of nutrients (per 100 kcal)
| Nutrient | ML1 | TF2 | TF/ML | PF2 | PF/ML |
| Macronutrients | |||||
| Protein (g) | 1.8 | 2.71 | 1.5 | 3 | 1.7 |
| Fat (g) | 3.3 | 5.27 | 1.6 | 5.43 | 1.7 |
| Vitamins | |||||
| Vitamin B1 (μg) | 40 | 100 | 2.5 | 250 | 6.3 |
| Vitamin B2 (μg) | 60 | 150 | 2.5 | 620 | 10.3 |
| Niacin (nicotinamide, μg) | 250 | 1050 | 4.2 | 5000 | 20.0 |
| Vitamin B6 (μg) | 35 | 60 | 1.7 | 250 | 7.1 |
| Vitamin B12 (μg) | 0.15 | 0.25 | 1.7 | 0.55 | 3.7 |
| Vitamin C (mg) | 8 | 9 | 1.1 | 37 | 4.6 |
| Biotin (μg) | 1.5 | 4.4 | 2.9 | 37 | 24.7 |
| Pantothenic acid (mg) | 300 | 450 | 1.5 | 1900 | 6.3 |
| Folic acid (μg) | 4 | 15 | 3.8 | 37 | 9.3 |
| Vitamin A (IU) | 250 | 300 | 1.2 | 1250 | 5.0 |
| Vitamin D (IU) | 40 | 60 | 1.5 | 150 | 3.8 |
| Vitamin E (IU) | 0.7 | 1.5 | 2.1 | 4 | 5.7 |
| Vitamin K (μg) | 4 | 8 | 2.0 | 12 | 3.0 |
The minimum limit for nutrients set by the United States Infant Formula Act of 1980[40];
Similac formulas (http://abbottnutrition.com/brands/similac). ML: Minimum limit; TF: A similac formula for term infants (Similac Expert Care® 24 Cal With Iron); PF: A Similac formula for low-birth-weight infants and premature infants (Similac® Special Care® 20 With Iron).
As a result of the combination of the above factors, the intake of vitamins has been significantly increased over the past few decades. As shown in Figure 2, United States per capita daily consumption of vitamin B1, B2 and niacin has doubled from the 1930s to 2000, which is significantly higher than the EARs.
Figure 2.
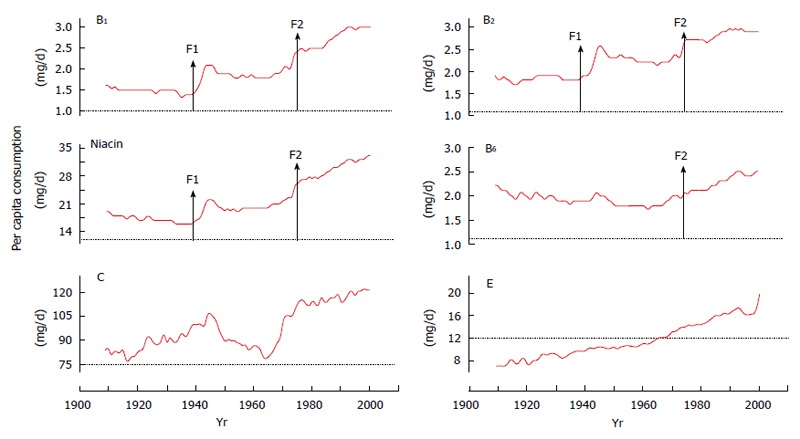
United States per capita daily vitamin consumption in 1909-2000. Data are from the Economic Research Service of the United States Department of Agriculture (http://search.ers.usda.gov/searchaffiliate=ers&query=nutrients.xls). Red line indicates per capita consumption. Dot line indicates EAR. F1: Initiation of flour fortification; F2: Update of nutrient fortification standards for breakfast cereals in 1974[34]; EAR: The estimated daily average requirement.
FOOD FORTIFICATION-RELATED DISPARITIES
Food fortification may lead to differential exposure to synthetic vitamins. The major differences include: (1) Different vitamin exposure among countries. Food fortification has caused significant differences in daily synthetic-vitamin consumption among countries due to different fortification policies and fortification standards[19], as shown in Table 4. Nationwide exposure to fortified foods in developing countries occurs much later than in developed countries[19], e.g., it was not until 1994 that China began mandatory fortification[41]; (2) Different vitamin exposure among groups within countries. Wheat flour is fortified with B vitamins. Thus, those who use wheat flour products as staple foods possibly consume a higher amount of synthetic B vitamins. Vitamin-fortified foods are cheaper than fresh and natural foods in developed countries[34,47], which may lead to a higher intake of synthetic vitamins in low SES groups than in high SES groups in these countries[47,48]. In contrast, in developing countries, those who live in urban areas may consume more fortified foods than those who live in rural areas[49,50]. Infant formula milk (Table 3) and children foods (e.g., ready-to-eat cereals[34]) are highly fortified with vitamins. Thus, infants fed formula milk and children are likely to have excess vitamin intake, as reported in the literature[51-54]; and (3) Different tolerance to fortified foods among population groups. Water-soluble vitamins can be eliminated through sweat[27,28]. Thus, under the same conditions of high vitamin intake, people who often sweat (e.g., doing physical work and/or living in hot regions) may have a lower risk of excess accumulation of water-soluble vitamins in the body than those who rarely sweat (e.g., living a sedentary life and/or in cold regions).
Table 4.
Obesity rate in selected countries with different wheat flour fortification policies
| Country | Food policy |
Standard (mg/kg flour, min) |
Obesity rate | ||
| Niacin | Vitamin B1 | Vitamin B2 | in children | ||
| Canada | Mandatory1 | 52.9 | 6.4 | 4 | 9-104 |
| United States | Mandatory1 | 52.9 | 6.4 | 4 | 6.85 |
| Kuwait | Mandatory1 | 52.9 | 6.4 | 4 | 14.66 |
| Saudi Arabia | Mandatory1 | 52.9 | 6.4 | 4 | 6-6.77 |
| United Kingdom | Mandatory1 | 16 | 2.4 | 0 | 5.15 |
| Finland | Prohibited2 | 0 | 0 | 0 | 2.55 |
| Norway | Prohibited2 | 0 | 0 | 0 | 2.25 |
| France | Prohibited3 | 0 | 0 | 0 | 1.65 |
Reference 36;
Reference 19;
Reference 42;
Reference 43, children (7-13 years) in 1996;
Reference 44, children (10 to 16 years) in 2001-2002;
Reference 45, children (10 to 14 years) in 2005-2006;
Reference 46 Children (1 to 18 years).
VITAMIN FORTIFICATION AND OBESITY PREVALENCE
Although there are few studies linking the increased prevalence of obesity to vitamin fortification, existing evidence suggests that high-risk populations are those who are most likely to have an increased intake of synthetic vitamins and decreased vitamin elimination, e.g., populations in fortified countries[6], individuals with low SES in developed countries[6-10] or with high SES in developing countries[11,12,55], formula-fed infants[15-17], and those who live in fortified countries with less rigorous physical activity[56-59].
The prevalence of obesity varies from country to country. It seems that this variation may be related to different food fortification policies and standards among countries. As shown in Table 4, the ranking of countries according to their prevalence of child obesity is similar to the ranking by the fortification standards of B vitamins. Evidently, flour fortification prohibited countries have a low prevalence of obesity, while countries with high flour fortification standard have high rates of obesity. Over the past few decades, food fortification has spread from developed countries to developing countries[19]. Therefore, it is possible that the spread of obesity from developed countries to developing countries may reflect the time sequence of implementing food fortification with vitamins.
Implementation of a vitamin fortification policy in a country will surely cause a sudden nationwide increase in vitamin intake in a short period. The initiation of food fortification with B vitamins in the late 1930s-1940s and the update of fortification standards in the 1970s in developed countries led to three phases in the consumption of vitamin B1, B2 and niacin: a rapid increase in the 1940s, followed by a plateau period between the 1950s and the 1960s and a steep increase thereafter, as shown in Figure 2. Available evidence has suggested an association between these food events and the prevalence of obesity. Two birth cohort studies conducted in Switzerland[60] and Denmark[61] showed that there was a significant increase in the prevalence of being overweight and obesity which occurred mainly in the cohorts born in the 1930s and the 1940s and in the cohorts born in the late 1960s to the 1970s. A Fels longitudinal study also showed that the child obesity epidemic in the United States is a sudden event that started in the 1970s and the 1980s[62]. A similar phenomenon is also seen in Saudi Arabia. Saudi Arabia started wheat flour fortification in the 1970s[63]. Following its food system change, Saudi Arabia experienced a rapid increase in obesity rates in the 1980s and the 1990s, and its obesity rate in schoolboys sharply increased from 3.4% in 1988 to 24.5% in 2005[64]. Our ecological studies clearly showed that there are strong lagged correlations between United States per capita consumption of B vitamins (B1, B2 and niacin) and the prevalence of obesity and diabetes[25,26]. Figure 3 clearly shows that both the initiation of food fortification in the 1940s and the update of fortification standards in 1974 are followed by a sharp increase in diabetes prevalence. The update of fortification standards followed a sharp rise in obesity prevalence.
Figure 3.
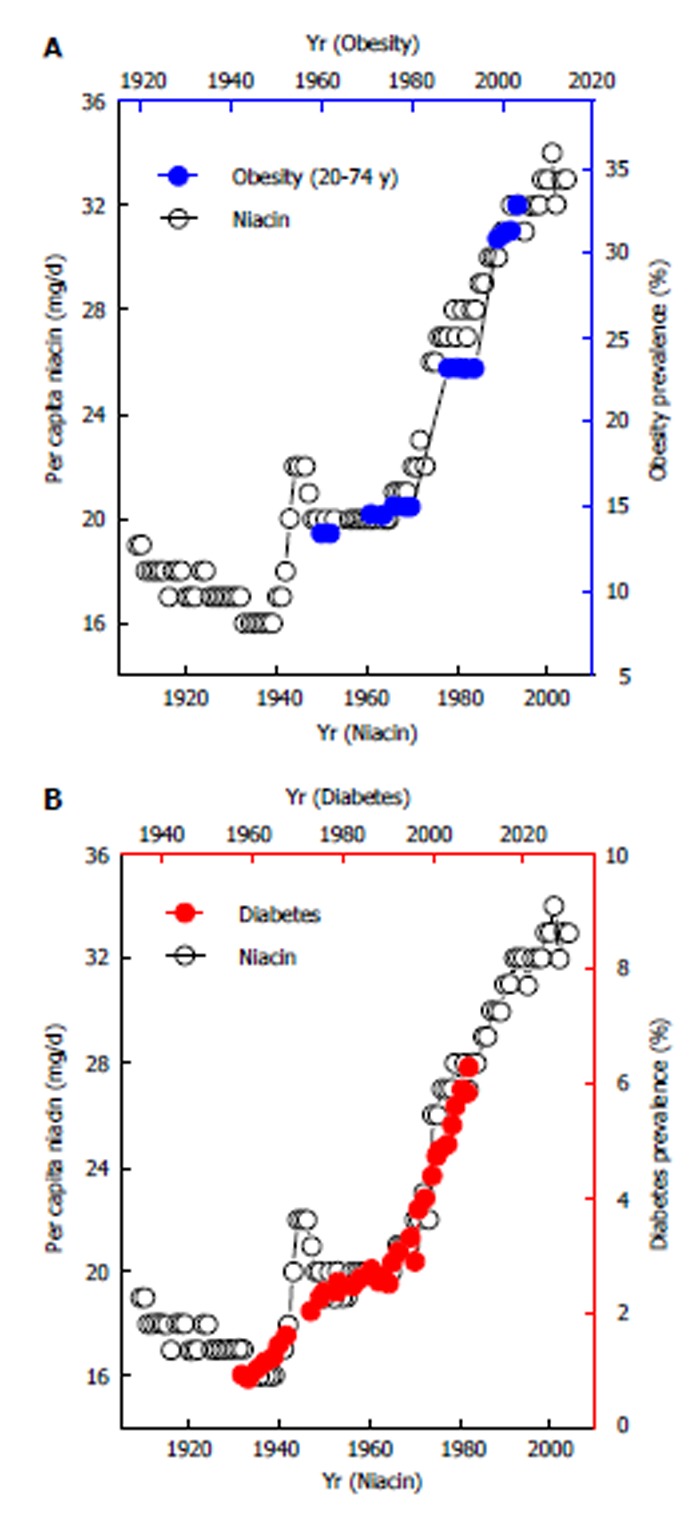
Lagged correlations between United States per capita niacin consumption and the prevalence of obesity and diabetes. The lag time between per capita niacin consumption and the prevalence of obesity and diabetes is 10 (A) and 26 years (B), respectively[25,26].
As mentioned above, low SES groups in developed countries but high SES groups in developing counties may have a high synthetic vitamin intake from fortified foods. This may explain the findings that obesity is more prevalent in low SES groups in developed countries[6-10] but in high SES groups in developing countries[10-12,55]. Formula-fed infants have a high vitamin intake. Studies have demonstrated that formula-fed infants have a higher plasma level of vitamins compared with human milk-fed infants[51-53]. It is known that formula feeding[65-67] and micronutrient-fortified human milk feeding[68,69] can lead to rapid infant weight gain, a known major risk factor for children developing obesity[70-72]. Therefore, excess vitamin intake may mediate the link between formula feeding and childhood obesity.
In most developed countries, the energy expenditure needed for daily life has decreased since the beginning of the 20th century because of increasing mechanization, urbanization, motorization and computerization[4]. However, it is only since the 1970s, when food fortification standards were dramatically increased, that obesity prevalence has risen substantially. Moreover, although formula feeding is associated with an increased risk for obesity[15-17], there is no evidence indicating that there is a decrease in energy expenditure in formula-fed infants compared with breast-fed infants[73,74]. Instead, evidence shows that formula-fed infants may have higher total daily energy expenditure[13,14]. These data suggest that increased B vitamin intake rather than decreased energy expenditure may play a major role in the development of obesity. On the other hand, many studies, especially those conducted in highly B vitamin fortified countries, such as the United States[56], Canada[57], Saudi Arabia[58] and Kuwait[59], found that moderate to vigorous physical activity is associated with a reduced risk of obesity. It is proposed that this association may involve increased elimination of vitamins through sweat because moderate to vigorous physical activity can increase the sweat rate[28]. We have demonstrated that excess nicotinamide can be rapidly removed through sweating[75]. Sweat-mediated elimination of nicotinamide may be a crucial factor in preventing nicotinamide toxicity because human kidneys hardly excrete nicotinamide due to the reabsorption of renal tubules[76]. Therefore, it is conceivable that under the same conditions of high vitamin intake, those individuals who live a life that inhibits the activity of sweat glands (e.g., physical inactivity) may be at greater risk of obesity. From this point of view, black people should be more sensitive to excess vitamins than whites, because the activity of sweat glands of blacks is lower than that of whites in the same temperature environment[77]. There is evidence showing that black women may have lower levels of physical activity than black men[78]. This may explain why obesity prevalence is greater in blacks, especially black women, than in whites in the United States[79,80]. Taken together, it may be concluded that food fortification-induced high intake of vitamins, especially B vitamins, may be responsible for the increased global prevalence of obesity.
MECHANISM OF EXCESS VITAMINS-INDUCED OBESITY
Many vitamins are known to act as coenzymes or as parts of enzymes responsible for essential chemical reactions, e.g., the synthesis of fat and neurotransmitters. Excess vitamins may also affect the degradation of neurotransmitters and one-carbon metabolism. Therefore, excess vitamins may trigger obesity through multiple ways, including increasing fat synthesis, causing insulin resistance, disturbing neurotransmitter metabolism and inducing epigenetic changes.
B vitamins enhance fat synthesis
Obesity involves an accumulation of excess body fat. Early studies have already demonstrated that B vitamins play a crucial role in fat synthesis and there is a synergistic effect of B vitamins on fat synthesis. Vitamin B1 and B6 are required for the synthesis of fat from carbohydrate and protein[21-23] and their effects on fat synthesis are enhanced by the presence of other B vitamins. Vitamin B6 administered together with B1, B2 and B5 (pantothenic acid) resulted in a significant increase in body fat in rats[22]. Niacin has been found to increase daily feed intake, weight gain and percentage of abdominal fat in chicken when increasing supplementation from 0 to 60 mg nicotinic acid per kilogram diet[24]. It has been found that formula feeding leads to more fat gain, which may account for increased risk of later obesity[81,82]. Considering that formulas contain high levels of B vitamins (Table 3) that are a known factor increasing fat synthesis, we therefore propose that formula feeding-induced fat gain may be due to excess vitamins. Taken together, existing evidence suggests that excess vitamins, especially B vitamins, may play a role in the development of obesity.
Excess vitamins cause insulin resistance
Insulin resistance, a characteristic of obesity and type 2 diabetes[83], is a condition in which the tissues of the body do not respond appropriately to normal levels of insulin. It is known that glycemic and insulin responses are related to food. Foods can be classified by their glycemic index (GI, a relative measure of the incremental glucose response per gram of carbohydrate)[84]. Figure 4 shows the different glycemic and insulin responses to low GI food and high GI food. The typical glycemic response to high GI foods is a biphasic response, with an initial significantly higher blood glucose and insulin level (hyperglycemic phase) followed by significantly lower blood glucose level (postprandial reactive hypoglycemic phase)[85-87]. Postprandial reactive hypoglycemia stimulates appetite and may lead to increased caloric intake[86,88,89]. Therefore, it may be particularly important to understand how high GI foods induce a biphasic glycemic response.
Figure 4.
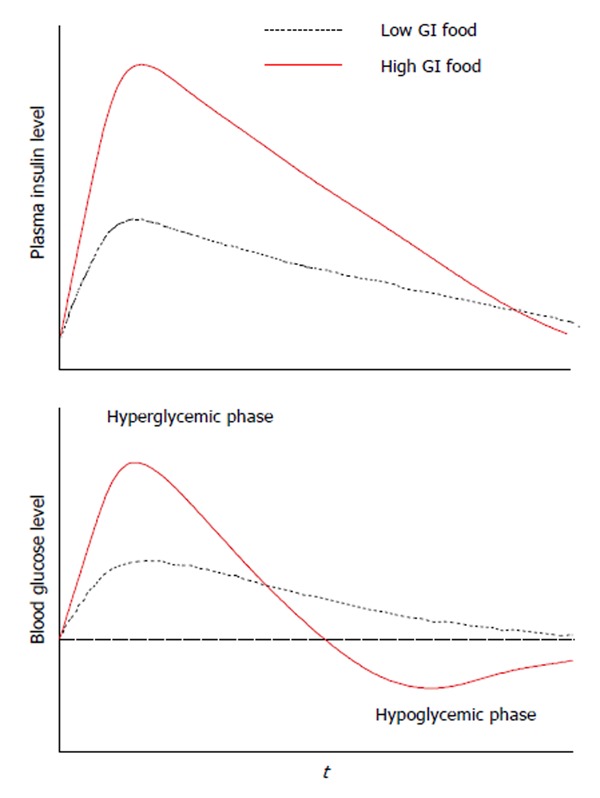
Typical glycemic responses to ingestion of a high glycemic index food and a low glycemic index I food. This figure is based on literature data[85,87]. GI: Glycemic index.
Grain foods are a major source of carbohydrates. Historically, high grain intake was associated with a low incidence of obesity. However, over the past few decades, refined (processed) grains became high GI foods[86,90,91]. Many processed grains (e.g., white bread) produce even higher glycemic responses than simple sugars[86]. It seems that the effect of refined grains is not merely a matter of increased rate of digestion and absorption of carbohydrate, but a matter of increased insulin resistance. Grain foods are used as a vehicle of B vitamin fortification. Therefore, it is possible that the increased GI of processed grains may be due to their increased levels of B vitamins. Among the B vitamins fortified in foods, niacin is known to induce insulin resistance and glucose intolerance[92-95]. Nicotinamide is the most common form of niacin used in food fortification and infant formula supplementation (e.g., Table 3). A study compared the glycemic and insulin responses of healthy subjects to glucose alone and glucose plus nicotinamide. The result showed that glucose plus nicotinamide significantly increased the levels of plasma insulin and hydrogen peroxide [a major component of reactive oxygen species (ROS)], followed by reactive hypoglycemia and hunger[26]. This study suggested for the first time that drinking nicotinamide-containing sugar-sweetened beverages may induce insulin resistance and nicotinamide fortification may contribute to the increased GI of refined grains.
It is known that increased ROS levels (i.e., oxidative stress) may play a causal role in insulin resistance[96,97]. We therefore hypothesize that oxidative stress may mediate the effect of nicotinamide. The mechanism may be as follows. After glucose and nicotinamide are absorbed into the circulation, increased blood glucose level stimulates insulin secretion, while increased nicotinamide level may induce oxidative stress due to increased ROS generation (as found in Ref 26), leading to a decrease in cell functions, including insulin signaling (i.e., insulin resistance). This results in a sharp increase in the level of blood glucose, which stimulates more insulin release (hyperglycemic phase). The clearance of ROS is more rapid than that of insulin. With the rapid clearance of ROS, cell response to insulin recovers quickly and as a result, the uptake of glucose by tissues (including adipose tissue) increases rapidly in response to relatively high insulin, which thus leads to a rapid fall in the level of blood glucose (hypoglycemic phase). Hypoglycemia initiates the feeling of hunger and subsequent feeding behavior. As mentioned above, B vitamins promote fat synthesis from carbohydrates. Thus, the cooperation of increased glucose uptake in the hypoglycemic phase and increased fat synthesis by high levels of B vitamins may induce excess fat storage and subsequent obesity (Figure 5). Unfortunately, the insulin resistance-inducing and obesity-promoting effects of B vitamins might have long been underestimated because traditional laboratory tests (e.g., glucose tolerance test) are usually performed under fasting conditions, in which most, if not all, of increased ROS produced in the degradation of excess vitamins must have been cleared up after overnight fasting. For example, we found that oral nicotinamide (300 mg) induced increase in circulating hydrogen peroxide had returned to normal at 3 h[26].
Figure 5.
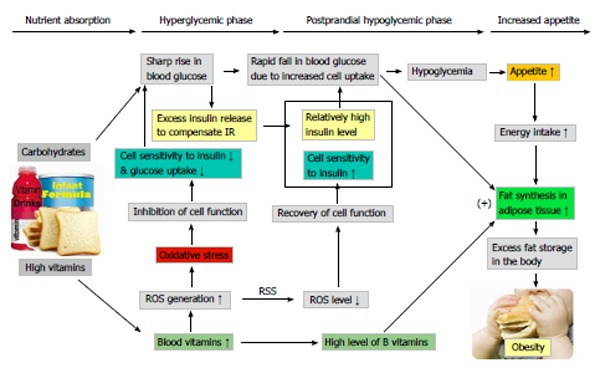
Proposed mechanism of excess vitamins-induced obesity. The absorption of sugar stimulates the release of insulin, while absorbed excess vitamins (from vitamin-fortified foods and drinks) generate ROS, leading to a decrease in the sensitivity of peripheral tissues to insulin (i.e., insulin resistance). To compensate the insulin resistance, additional insulin has to be secreted, resulting in a high blood insulin level. Then, the sensitivity of peripheral tissues recovered with the rapid clearance of ROS, consequently, relatively high insulin level causes a rapid decrease in blood glucose due to increased glucose uptake, which may trigger excess energy intake. The conversion of glucose to fat in adipose tissue is promoted by high levels of B vitamins. Therefore, long-term consumption of vitamin fortified foods (including formulas) and drinks may cause fat accumulation in the body and subsequent obesity. ROS: Reactive oxygen species; RSS: ROS scavenging system.
It has been demonstrated in rats that the weight/fat gain-promoting effect of B vitamins is more efficient when given in successive doses (added to the diet, like human food fortification) than in periodic doses[98]. This may explain why obesity prevalence significantly increased after the implementation of grain fortification with B vitamins. Because consumption of B vitamins-fortified foods may increase the burden of pancreatic islet B-cells, it is conceivable that obesity is closely associated with type 2 diabetes. In addition, other vitamins, even those that have antioxidant function (e.g., vitamin C and E[99]), when used in large doses can increase ROS generation. Thus, high consumption of other vitamins may also contribute to the development of obesity. The relationship between dietary carbohydrates, excess vitamins, oxidative stress, insulin resistance, postprandial hypoglycemia, increased appetite and the development of obesity is proposed in Figure 5.
From the excess vitamin point of view, it may be easy to understand why the price of fast food, which determines the consumption of synthetic vitamins from fast food, may affect the body mass index of teens with low SES[100] and why vitamin-rich formulas[15-17] and sugar-sweetened beverages may increase the risk for obesity and type 2 diabetes[17,37,101,102]. It is interesting that some overweight children become overweight adults, while others do not[103]. One possible explanation for this may be a changing vitamin intake during the lifetime. Whether obese infants become obese children and then obese adults may to a large degree depends on the intake of vitamins after weaning. In theory, infants, even with normal body weight, may become obese adults if they always consume high vitamin-fortified foods (e.g., refined grains) after weaning. We therefore recommend that the role of vitamin intake be taken into consideration in the study of the relationship between infant obesity and later obesity.
Excess vitamins may disturb neurotransmitter metabolism
Food intake is regulated by many neurotransmitters, including monoamine neurotransmitters (e.g., dopamine and serotonin[104,105]) in the central nervous system. Therefore, factors that affect monoamine neurotransmitters may affect feeding behavior. Some vitamins are known to play an important role in the synthesis of monoamine neurotransmitters (serotonin and catecholamines). For example, vitamin B6 is a cofactor for aromatic L-amino acid decarboxylase that catalyzes the formation of serotonin and dopamine[106]. Vitamin C enhances norepinephrine synthesis from dopamine by neuronal cells[107]. L-methylfolate, a derivate of the vitamin folate, also regulates the synthesis of the monoamine neurotransmitters serotonin, dopamine and norepinephrine[108].
Although small amount of vitamins can be directly eliminated through the urine, sweat[27,28,75] and sebum (such as vitamin E[29,30]), most of them usually undergo a series of phase I (oxidation, reduction and hydrolysis) and phase II (conjugation, including glutathione conjugation, sulfation, methylation and glucuronidation) biotransformation before elimination from the body. As a result, vitamin degradation produces many metabolites. For example, at least 18 metabolites of vitamin B1 are identified in the urine, of which six are major[109]. Niacin is degraded mainly to a number of methylated metabolites[110]. Vitamin C is degraded through sulfation[111] and glutathione conjugation[112]. Vitamin E also undergoes extensive metabolism and its conjugated metabolites (including sulfated) are also identified[113]. Because vitamins and neurotransmitters share the same biotransformation and detoxification system in the body[106,114], excess vitamins may affect the degradation of neurotransmitters by competing for the detoxification resources. For example, vitamin C has been known to inhibit the sulfation of other chemicals by competing for limited sulfate[111]. Although there are no systematic studies on the effect of vitamin fortification on the degradation of neurotransmitters, evidence has shown that excess vitamin C[115,116] and nicotinamide[117] can inhibit the degradation of catecholamines by depletion of sulfate and methyl groups, respectively. Thus, in theory, the effect of vitamins on the metabolism of monoamine neurotransmitters may affect the function of the nervous system. It is known that niacin can stimulate appetite. Niacin deficiency (i.e., pellagra) is associated with a loss of appetite[118], which might involve changes in neurotransmitter metabolism in the brain.
Excess vitamins-induced obesity may involve epigenetic changes
Epigenetic changes are biochemical modifications that affect gene expression without changing the sequence of DNA. Emerging evidence suggests that epigenetic mechanisms may play a role in the development of obesity[119]. Epigenetic mechanisms involve an environment-gene interaction[120,121]. Nutrition is a crucial environmental factor which affects health and disease. Both maternal undernutrition and overnutrition can induce persistent changes in gene expression and metabolism[120]. Over the past few decades, one of the biggest changes in our food system has been the extensive use of synthetic vitamins. Therefore, it is possible that excess vitamin intake may contribute to epigenetic changes.
DNA methylation, which occurs at cytosine residues in CpG dinucleotides in gene promoters, is one of several epigenetic modifications[122]. The primary function of DNA methylation is to suppress gene expression. Global DNA hypomethylation increases genomic instability[122]. Although the mechanism of global DNA hypomethylation is not well understood, a lack of methyl groups may play a role in abnormal DNA methylation, because an adequate supply of methyl groups is a prerequisite for DNA methylation[123]. The biotransformation of some vitamins, especially niacin[117], may increase the demand for labile methyl groups and therefore, an excess intake of these vitamins may disturb DNA methylation by competing methyl groups. Recently, we tested this possibility by investigating the effect of nicotinamide supplementation on DNA methylation in rats and found that long-term high nicotinamide exposure led to a decrease in the methyl pool and in the levels of hepatic DNA methylation associated with alteration of gene expression[123]. Moreover, maternal nicotinamide supplementation is also found to disturb fetal one-carbon metabolism in rats, including decreased global DNA methylation and decreased DNA uracil content in the brain and liver[124]. These data indicate that excess vitamins may be an important factor leading to epigenetic changes. The role of vitamin fortification in the development of methylation-related diseases is an open question.
NON-MONOTONIC EFFECT OF VITAMINS ON WEIGHT GAIN
Although it is known that B vitamins promote fat synthesis and vitamin-fortified foods and formulas increase the risk for obesity, why is there so little attention to the relationship between excess vitamin intake and obesity prevalence A possible reason may be due to ignorance of the fact that the effect of vitamins on weight gain is non-monotonic. While vitamins are an important weight gain-promoting factor, at toxic levels they are no longer associated with weight gain or even cause weight loss.
It has long been known that many micronutrients (vitamins and minerals) are essential for life at low concentrations but become toxic at high concentrations. This phenomenon is termed Bertrand’s rule[125]. The effect of vitamins on weight gain also follows this Bertrand’s rule. We may take the weight-gain effect of niacin as an example. Jiang and colleagues[24] investigated the effects of dietary supplemental nicotinic acid at different doses (0, 30, 60 and 120 mg/kg diet) on the growth performance of chicken. They found that increasing supplementation from 0 to 60 mg nicotinic acid/kg tended to increase the average daily feed intake, weight gain and fat gain, i.e., the maximum weight and fat gain was achieved at 60 mg/kg diet. Ivers and Veum found that among the doses used (6, 10, 14, 18, 22 and 44 mg/kg diet with adequate Trp), 14 mg of niacin/kg produced maximum weight gain in growing pigs[126]. Shibata et al[127] studied the effect of nicotinamide at doses of 0, 60, 1000 and 5000 mg/kg diet on rat weight gain. Their result showed that nicotinamide increased the food intake of rats, especially in the groups fed diet containing 60 and 1000 mg/kg of nicotinamide. The highest weight gain was observed at 60 mg/kg, while high-dose nicotinamide (5000 mg/kg diet) led to an inhibition of weight gain at the early stage of exposure due to its toxicity. These animal studies suggest that the supplemental dose for niacin to achieve maximum weight-gain effect may be around or less than 60 mg/kg diet. This dose is similar to that used in wheat flour fortification in some countries, e.g., the United States, Canada, Saudi Arabia and Kuwait (Table 4). Thus, food fortification with niacin in these countries might have induced a maximum weight gain effect. In this case, further supplementation with niacin or niacin-containing multivitamin may offset the weight gain effect due to increased toxic effects, such as hepatotoxicity[128-131] and oxidative tissue damage[123]. This may account for the observations that further multivitamin supplementation in the United States[132] and Canada[133] or large-dose niacin treatment for dyslipidemia (1-3 g/d)[134,135] does not show weight gain.
Some other vitamins at high doses may also have toxic effects, including death. Davis et al[136] found that sudden infant death syndrome (a sudden and unexplained infant death) was association with high serum thiamin levels. A randomized controlled trial on vitamin C supplementation in very preterm infants showed that the infants who died in the trial were those who had significantly higher level of plasma vitamin C before randomization than surviving infants[137]. A systematic review and meta-analysis showed that long-term supplementation with beta carotene, vitamin A and vitamin E may increase mortality[138]. Therefore, it is not surprising that multivitamin supplementation in those who live in high-dose vitamin-fortified countries, e.g., the United States[132] and Canada[133], may be associated with a slight weight loss. A similar phenomenon has been also observed in formula-fed infants. It has been found that formula feeding can lead to a more rapid weight gain, especially fat gain[81,82], compared to human milk feeding[17,65,67]. However, when formulas were further enriched with vitamins, their weight-gain effect was decreased rather than increased, compared with the standard formulas[139]. It seems clear that the weight-gain effect of vitamins has already been saturated at fortification doses used in infant formulas, children and adult foods, while further increasing the doses (i.e., fortification plus additional supplementation) may induce a weight-loss effect due to the toxic effect. Considering that high vitamin intake which may cause hepatotoxicity (e.g., niacin, as mentioned above) is very popular nowadays, we suggest that high vitamin intake may contribute to non-alcoholic fatty liver disease, the most frequent chronic liver disease in developed countries[140].
CONCLUSION
Since the late 1930s, when synthetic vitamins were first used, the human being has experienced the largest growth in vitamin intake in human history. It is possible that excess vitamins, especially B vitamins, may contribute to the development of obesity. Vitamin-rich formulas and food fortification with vitamins may, to a large extent, be responsible for the increased prevalence of obesity over the past several decades. Different fortification policies and standards may account for the differences in the prevalence between countries, while disparities in the consumption of fortified foods may contribute to the disparities in obesity between population groups within a country. Staple food fortification may be of great harm because it leads to a sustained high vitamin intake. Therefore, given that there has been a significant increase in vitamin supply from natural sources, it is necessary and urgent to review and modify the standards of vitamin fortification.
Footnotes
P- Reviewers: Paraskevas KI, Resmini E, Tomkin GH S- Editor: Cui XM L- Editor: Roemmele A E- Editor: Liu SQ
References
- 1.Hossain P, Kawar B, El Nahas M. Obesity and diabetes in the developing world--a growing challenge. N Engl J Med. 2007;356:213–215. doi: 10.1056/NEJMp068177. [DOI] [PubMed] [Google Scholar]
- 2.Cote AT, Harris KC, Panagiotopoulos C, Sandor GG, Devlin AM. Childhood obesity and cardiovascular dysfunction. J Am Coll Cardiol. 2013;62:1309–1319. doi: 10.1016/j.jacc.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 3.Sassi F, Devaux M, Cecchini M, Rusticelli E. The obesity epidemic: analysis of past and projected future trends in selected OECD countries. Paris: Organisation for Economic Co-operation and Development (OECD), Directorate for Employment, Labour And Social Affairs, Health Committee; 2009. [Google Scholar]
- 4.Swinburn BA, Sacks G, Hall KD, McPherson K, Finegood DT, Moodie ML, Gortmaker SL. The global obesity pandemic: shaped by global drivers and local environments. Lancet. 2011;378:804–814. doi: 10.1016/S0140-6736(11)60813-1. [DOI] [PubMed] [Google Scholar]
- 5.Malik VS, Willett WC, Hu FB. Global obesity: trends, risk factors and policy implications. Nat Rev Endocrinol. 2013;9:13–27. doi: 10.1038/nrendo.2012.199. [DOI] [PubMed] [Google Scholar]
- 6.Phipps SA, Burton PS, Osberg LS, Lethbridge LN. Poverty and the extent of child obesity in Canada, Norway and the United States. Obes Rev. 2006;7:5–12. doi: 10.1111/j.1467-789X.2006.00217.x. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Lim H. The global childhood obesity epidemic and the association between socio-economic status and childhood obesity. Int Rev Psychiatry. 2012;24:176–188. doi: 10.3109/09540261.2012.688195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ogden CL, Lamb MM, Carroll MD, Flegal KM. Obesity and socioeconomic status in children and adolescents: United States, 2005-2008. NCHS Data Brief. 2010;(51):1–8. [PubMed] [Google Scholar]
- 9.Singh GK, Siahpush M, Kogan MD. Rising social inequalities in US childhood obesity, 2003-2007. Ann Epidemiol. 2010;20:40–52. doi: 10.1016/j.annepidem.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 10.McLaren L. Socioeconomic status and obesity. Epidemiol Rev. 2007;29:29–48. doi: 10.1093/epirev/mxm001. [DOI] [PubMed] [Google Scholar]
- 11.Monteiro CA, Moura EC, Conde WL, Popkin BM. Socioeconomic status and obesity in adult populations of developing countries: a review. Bull World Health Organ. 2004;82:940–946. [PMC free article] [PubMed] [Google Scholar]
- 12.Dinsa GD, Goryakin Y, Fumagalli E, Suhrcke M. Obesity and socioeconomic status in developing countries: a systematic review. Obes Rev. 2012;13:1067–1079. doi: 10.1111/j.1467-789X.2012.01017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lubetzky R, Vaisman N, Mimouni FB, Dollberg S. Energy expenditure in human milk- versus formula-fed preterm infants. J Pediatr. 2003;143:750–753. doi: 10.1067/S0022-3476(03)00532-8. [DOI] [PubMed] [Google Scholar]
- 14.Guilfoy VM, Wright-Coltart S, Leitch CA, Denne SC. Energy expenditure in extremely low birth weight infants near time of hospital discharge. J Pediatr. 2008;153:612–615. doi: 10.1016/j.jpeds.2008.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Owen CG, Martin RM, Whincup PH, Smith GD, Cook DG. Effect of infant feeding on the risk of obesity across the life course: a quantitative review of published evidence. Pediatrics. 2005;115:1367–1377. doi: 10.1542/peds.2004-1176. [DOI] [PubMed] [Google Scholar]
- 16.Gibbs BG, Forste R. Socioeconomic status, infant feeding practices and early childhood obesity. Pediatr Obes. 2013:Apr 2; Epub ahead of print. doi: 10.1111/j.2047-6310.2013.00155.x. [DOI] [PubMed] [Google Scholar]
- 17.Davis JN, Koleilat M, Shearrer GE, Whaley SE. Association of infant feeding and dietary intake on obesity prevalence in low-income toddlers. Obesity (Silver Spring) 2013:Oct 12; Epub ahead of print. doi: 10.1002/oby.20644. [DOI] [PubMed] [Google Scholar]
- 18.Richardson DP. Food fortification. Proc Nutr Soc. 1990;49:39–50. doi: 10.1079/pns19900007. [DOI] [PubMed] [Google Scholar]
- 19.Food and Agriculture Organization of the United Nations. Food Fortification: Technology and Quality Control. FAO Food And Nutrition Paper-60, 1996 Available from: http://www.fao.org/docrep/w2840e/w2840e03.htm; [PubMed] [Google Scholar]
- 20.Backstrand JR. The history and future of food fortification in the United States: a public health perspective. Nutr Rev. 2002;60:15–26. doi: 10.1301/002966402760240390. [DOI] [PubMed] [Google Scholar]
- 21.McHenry EW, Gavin G. The B vitamins and fat metabolism: I. Effects of thiamin, riboflavin, and rice polish concentrate upon body fat. J Biol Chem. 1938;125:653–660. [Google Scholar]
- 22.McHenry EW, Gavin G. The B vitamins and fat metabolism: IV. The synthesis of fat from protein. J Biol Chem. 1941;138:471–475. [Google Scholar]
- 23.CARTER CW, PHIZACKERLEY PJ. The influence of pyridoxine on fat metabolism in the rat. Biochem J. 1951;49:227–232. doi: 10.1042/bj0490227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang RR, Zhao GP, Chen JL, Zheng MQ, Zhao JP, Li P, Hu J, Wen J. Effect of dietary supplemental nicotinic acid on growth performance, carcass characteristics and meat quality in three genotypes of chicken. J Anim Physiol Anim Nutr (Berl) 2011;95:137–145. doi: 10.1111/j.1439-0396.2010.01031.x. [DOI] [PubMed] [Google Scholar]
- 25.Zhou SS, Li D, Zhou YM, Sun WP, Liu QG. B-vitamin consumption and the prevalence of diabetes and obesity among the US adults: population based ecological study. BMC Public Health. 2010;10:746. doi: 10.1186/1471-2458-10-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li D, Sun WP, Zhou YM, Liu QG, Zhou SS, Luo N, Bian FN, Zhao ZG, Guo M. Chronic niacin overload may be involved in the increased prevalence of obesity in US children. World J Gastroenterol. 2010;16:2378–2387. doi: 10.3748/wjg.v16.i19.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.LUGG JW, ELLIS FP. Some water-soluble vitamins in the sweat of tropically acclimatized European men. Br J Nutr. 1954;8:71–77. doi: 10.1079/bjn19540011. [DOI] [PubMed] [Google Scholar]
- 28.Zhou SS, Li D, Zhou YM, Cao JM. The skin function: a factor of anti-metabolic syndrome. Diabetol Metab Syndr. 2012;4:15. doi: 10.1186/1758-5996-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Passi S, De Pità O, Grandinetti M, Simotti C, Littarru GP. The combined use of oral and topical lipophilic antioxidants increases their levels both in sebum and stratum corneum. Biofactors. 2003;18:289–297. doi: 10.1002/biof.5520180233. [DOI] [PubMed] [Google Scholar]
- 30.Ekanayake-Mudiyanselage S, Kraemer K, Thiele JJ. Oral supplementation with all-Rac- and RRR-alpha-tocopherol increases vitamin E levels in human sebum after a latency period of 14-21 days. Ann N Y Acad Sci. 2004;1031:184–194. doi: 10.1196/annals.1331.017. [DOI] [PubMed] [Google Scholar]
- 31.Sato K. The physiology, pharmacology, and biochemistry of the eccrine sweat gland. Rev Physiol Biochem Pharmacol. 1977;79:51–131. doi: 10.1007/BFb0037089. [DOI] [PubMed] [Google Scholar]
- 32.Piérard-Franchimont C, Piérard GE, Kligman A. Seasonal modulation of sebum excretion. Dermatologica. 1990;181:21–22. doi: 10.1159/000247853. [DOI] [PubMed] [Google Scholar]
- 33.Pemberton J. Medical experiments carried out in Sheffield on conscientious objectors to military service during the 1939-45 war. Int J Epidemiol. 2006;35:556–558. doi: 10.1093/ije/dyl020. [DOI] [PubMed] [Google Scholar]
- 34.Gerrior S, Bente L, Hiza H. Nutrient Content of the U.S. Food Supply: 1909-2000 (Home Economics Research Report No. 56), 2004 Available from: http: //www.cnpp.usda.gov/publications/foodsupply/foodsupply1909-2000.pdf; [Google Scholar]
- 35.Popkin BM. Nutrition in transition: the changing global nutrition challenge. Asia Pac J Clin Nutr. 2001;10 Suppl:S13–S18. [PubMed] [Google Scholar]
- 36.Wesley A, Ranum P. FORTIFICATION HANDBOOK: Vitamin and mineral fortification of wheat flour and maize meal. 2004. pp. 35–36. Available from: http: //www.micronutrient.org/resources/publications/Fort_handbook.pdf. [Google Scholar]
- 37.Malik VS, Hu FB. Sweeteners and risk of obesity and type 2 diabetes: the role of sugar-sweetened beverages. Curr Diab Rep. 2012;12:195–203. doi: 10.1007/s11892-012-0259-6. [DOI] [PubMed] [Google Scholar]
- 38.Fomon S. Infant feeding in the 20th century: formula and beikost. J Nutr. 2001;131:409S–420S. doi: 10.1093/jn/131.2.409S. [DOI] [PubMed] [Google Scholar]
- 39.Koletzko B, Baker S, Cleghorn G, Neto UF, Gopalan S, Hernell O, Hock QS, Jirapinyo P, Lonnerdal B, Pencharz P, et al. Global standard for the composition of infant formula: recommendations of an ESPGHAN coordinated international expert group. J Pediatr Gastroenterol Nutr. 2005;41:584–599. doi: 10.1097/01.mpg.0000187817.38836.42. [DOI] [PubMed] [Google Scholar]
- 40.PUBLIC LAW 96-359-SEPT. 26, 1980. Available from: http: //www.foodcomm.org.uk/pdfs/fortification.PDF; [Google Scholar]
- 41.Pray CE, Huang J. Biofortification for China: political responses to food fortification and GM technology, interest groups, and possible strategies. AgBioForum. 2007;10:161–169. [Google Scholar]
- 42.Bonner G, Warwick H, Bmardo M, Lobstein T. Fortification examined: How added nutrients can undermine good nutrition. The Food Commission (UK) Ltd: London, 1999 Available from: http: //www.foodcomm.org.uk/pdfs/fortification.PDF; [Google Scholar]
- 43.Tremblay MS, Katzmarzyk PT, Willms JD. Temporal trends in overweight and obesity in Canada, 1981-1996. Int J Obes Relat Metab Disord. 2002;26:538–543. [PubMed] [Google Scholar]
- 44.Janssen I, Katzmarzyk PT, Boyce WF, Vereecken C, Mulvihill C, Roberts C, Currie C, Pickett W. Comparison of overweight and obesity prevalence in school-aged youth from 34 countries and their relationships with physical activity and dietary patterns. Obes Rev. 2005;6:123–132. doi: 10.1111/j.1467-789X.2005.00176.x. [DOI] [PubMed] [Google Scholar]
- 45.El-Bayoumy I, Shady I, Lotfy H. Prevalence of obesity among adolescents (10 to 14 years) in Kuwait. Asia Pac J Public Health. 2009;21:153–159. doi: 10.1177/1010539509331786. [DOI] [PubMed] [Google Scholar]
- 46.El-Hazmi MA, Warsy AS. A comparative study of prevalence of overweight and obesity in children in different provinces of Saudi Arabia. J Trop Pediatr. 2002;48:172–177. doi: 10.1093/tropej/48.3.172. [DOI] [PubMed] [Google Scholar]
- 47.Drewnowski A, Darmon N. The economics of obesity: dietary energy density and energy cost. Am J Clin Nutr. 2005;82(1 Suppl):265S–273S. doi: 10.1093/ajcn/82.1.265S. [DOI] [PubMed] [Google Scholar]
- 48.Thornton LE, Bentley RJ, Kavanagh AM. Individual and area-level socioeconomic associations with fast food purchasing. J Epidemiol Community Health. 2011;65:873–880. doi: 10.1136/jech.2009.099614. [DOI] [PubMed] [Google Scholar]
- 49.Imhoff-Kunsch B, Flores R, Dary O, Martorell R. Wheat flour fortification is unlikely to benefit the neediest in Guatemala. J Nutr. 2007;137:1017–1022. doi: 10.1093/jn/137.4.1017. [DOI] [PubMed] [Google Scholar]
- 50.Steyn NP, Wolmarans P, Nel JH, Bourne LT. National fortification of staple foods can make a significant contribution to micronutrient intake of South African adults. Public Health Nutr. 2008;11:307–313. doi: 10.1017/S136898000700033X. [DOI] [PubMed] [Google Scholar]
- 51.Smith AM, Picciano MF, Deering RH. Folate intake and blood concentrations of term infants. Am J Clin Nutr. 1985;41:590–598. doi: 10.1093/ajcn/41.3.590. [DOI] [PubMed] [Google Scholar]
- 52.Davis RE, Icke GC, Hilton JM, Orr E. Serum thiamin, pyridoxal, cobalamin and folate concentrations in young infants. Acta Paediatr Scand. 1986;75:402–407. doi: 10.1111/j.1651-2227.1986.tb10221.x. [DOI] [PubMed] [Google Scholar]
- 53.Heiskanen K, Salmenperä L, Perheentupa J, Siimes MA. Infant vitamin B-6 status changes with age and with formula feeding. Am J Clin Nutr. 1994;60:907–910. doi: 10.1093/ajcn/60.6.907. [DOI] [PubMed] [Google Scholar]
- 54.Hennessy Á, Walton J, Flynn A. The impact of voluntary food fortification on micronutrient intakes and status in European countries: a review. Proc Nutr Soc. 2013;72:433–440. doi: 10.1017/S002966511300339X. [DOI] [PubMed] [Google Scholar]
- 55.Zhang YX, Wang SR. Rural-urban comparison in prevalence of overweight and obesity among adolescents in Shandong, China. Ann Hum Biol. 2013;40:294–297. doi: 10.3109/03014460.2013.772654. [DOI] [PubMed] [Google Scholar]
- 56.Mitchell JA, Mattocks C, Ness AR, Leary SD, Pate RR, Dowda M, Blair SN, Riddoch C. Sedentary behavior and obesity in a large cohort of children. Obesity (Silver Spring) 2009;17:1596–1602. doi: 10.1038/oby.2009.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chaput JP, Lambert M, Mathieu ME, Tremblay MS, O’Loughlin J, Tremblay A. Physical activity vs. sedentary time: independent associations with adiposity in children. Pediatr Obes. 2012;7:251–258. doi: 10.1111/j.2047-6310.2011.00028.x. [DOI] [PubMed] [Google Scholar]
- 58.Al-Hazzaa HM, Abahussain NA, Al-Sobayel HI, Qahwaji DM, Musaiger AO. Physical activity, sedentary behaviors and dietary habits among Saudi adolescents relative to age, gender and region. Int J Behav Nutr Phys Act. 2011;8:140. doi: 10.1186/1479-5868-8-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Al-Haifi AR, Al-Fayez MA, Al-Athari BI, Al-Ajmi FA, Allafi AR, Al-Hazzaa HM, Musaiger AO. Relative contribution of physical activity, sedentary behaviors, and dietary habits to the prevalence of obesity among Kuwaiti adolescents. Food Nutr Bull. 2013;34:6–13. doi: 10.1177/156482651303400102. [DOI] [PubMed] [Google Scholar]
- 60.Faeh D, Bopp M. Increase in the prevalence of obesity in Switzerland 1982-2007: birth cohort analysis puts recent slowdown into perspective. Obesity (Silver Spring) 2010;18:644–646. doi: 10.1038/oby.2009.310. [DOI] [PubMed] [Google Scholar]
- 61.Olsen LW, Baker JL, Holst C, Sørensen TI. Birth cohort effect on the obesity epidemic in Denmark. Epidemiology. 2006;17:292–295. doi: 10.1097/01.ede.0000208349.16893.e0. [DOI] [PubMed] [Google Scholar]
- 62.von Hippel PT, Nahhas RW. Extending the history of child obesity in the United States: The Fels Longitudinal Study, birth years 1930-1993. Obesity (Silver Spring) 2013;21:2153–2156. doi: 10.1002/oby.20395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.WHO-EM/NUT/259/E. Report on the Joint WHO/Flour Fortification Initiative harmonization workshop for wheat and maize flour fortification. Amman: Jordan; 2012. [February]. p. 4 Available from: http://applications.emro.who.int/docs/IC_Meet_Rep_2012_EN_14767.pdf. [Google Scholar]
- 64.Al-Hazzaa HM. Prevalence and trends in obesity among school boys in Central Saudi Arabia between 1988 and 2005. Saudi Med J. 2007;28:1569–1574. [PubMed] [Google Scholar]
- 65.Dewey KG, Heinig MJ, Nommsen LA, Peerson JM, Lönnerdal B. Growth of breast-fed and formula-fed infants from 0 to 18 months: the DARLING Study. Pediatrics. 1992;89:1035–1041. [PubMed] [Google Scholar]
- 66.Mihrshahi S, Battistutta D, Magarey A, Daniels LA. Determinants of rapid weight gain during infancy: baseline results from the NOURISH randomised controlled trial. BMC Pediatr. 2011;11:99. doi: 10.1186/1471-2431-11-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Oddy WH. Infant feeding and obesity risk in the child. Breastfeed Rev. 2012;20:7–12. [PubMed] [Google Scholar]
- 68.Kuschel CA, Harding JE. Multicomponent fortified human milk for promoting growth in preterm infants. Cochrane Database Syst Rev. 2004;(1):CD000343. doi: 10.1002/14651858.CD000343.pub2. [DOI] [PubMed] [Google Scholar]
- 69.Gathwala G, Chawla M, Gehlaut VS. Fortified human milk in the small for gestational age neonate. Indian J Pediatr. 2007;74:815–818. doi: 10.1007/s12098-007-0144-5. [DOI] [PubMed] [Google Scholar]
- 70.Dennison BA, Edmunds LS, Stratton HH, Pruzek RM. Rapid infant weight gain predicts childhood overweight. Obesity (Silver Spring) 2006;14:491–499. doi: 10.1038/oby.2006.64. [DOI] [PubMed] [Google Scholar]
- 71.Ong KK, Loos RJ. Rapid infancy weight gain and subsequent obesity: systematic reviews and hopeful suggestions. Acta Paediatr. 2006;95:904–908. doi: 10.1080/08035250600719754. [DOI] [PubMed] [Google Scholar]
- 72.Leunissen RW, Kerkhof GF, Stijnen T, Hokken-Koelega A. Timing and tempo of first-year rapid growth in relation to cardiovascular and metabolic risk profile in early adulthood. JAMA. 2009;301:2234–2242. doi: 10.1001/jama.2009.761. [DOI] [PubMed] [Google Scholar]
- 73.Butte NF, Wong WW, Ferlic L, Smith EO, Klein PD, Garza C. Energy expenditure and deposition of breast-fed and formula-fed infants during early infancy. Pediatr Res. 1990;28:631–640. doi: 10.1203/00006450-199012000-00019. [DOI] [PubMed] [Google Scholar]
- 74.Wells JC, Davies PS. Diet and behavioural activity in 12-week-old infants. Ann Hum Biol. 1995;22:207–215. doi: 10.1080/03014469500003872. [DOI] [PubMed] [Google Scholar]
- 75.Zhou SS, Li D, Sun WP, Guo M, Lun YZ, Zhou YM, Xiao FC, Jing LX, Sun SX, Zhang LB, et al. Nicotinamide overload may play a role in the development of type 2 diabetes. World J Gastroenterol. 2009;15:5674–5684. doi: 10.3748/wjg.15.5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Knip M, Douek IF, Moore WP, Gillmor HA, McLean AE, Bingley PJ, Gale EA. Safety of high-dose nicotinamide: a review. Diabetologia. 2000;43:1337–1345. doi: 10.1007/s001250051536. [DOI] [PubMed] [Google Scholar]
- 77.THOMSON ML. A comparison between the number and distribution of functioning eccrine sweat glands in Europeans and Africans. J Physiol. 1954;123:225–233. doi: 10.1113/jphysiol.1954.sp005045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dubbert PM, Robinson JC, Sung JH, Ainsworth BE, Wyatt SB, Carithers T, Newton R, Rhudy JL, Barbour K, Sternfeld B, et al. Physical activity and obesity in African Americans: the Jackson Heart Study. Ethn Dis. 2010;20:383–389. [PMC free article] [PubMed] [Google Scholar]
- 79.Ogden CL. Disparities in obesity prevalence in the United States: black women at risk. Am J Clin Nutr. 2009;89:1001–1002. doi: 10.3945/ajcn.2009.27592. [DOI] [PubMed] [Google Scholar]
- 80.Jackson CL, Szklo M, Yeh HC, Wang NY, Dray-Spira R, Thorpe R, Brancati FL. Black-white disparities in overweight and obesity trends by educational attainment in the United States, 1997-2008. J Obes. 2013;2013:140743. doi: 10.1155/2013/140743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gale C, Logan KM, Santhakumaran S, Parkinson JR, Hyde MJ, Modi N. Effect of breastfeeding compared with formula feeding on infant body composition: a systematic review and meta-analysis. Am J Clin Nutr. 2012;95:656–669. doi: 10.3945/ajcn.111.027284. [DOI] [PubMed] [Google Scholar]
- 82.de Zegher F, Sebastiani G, Diaz M, Gómez-Roig MD, López-Bermejo A, Ibáñez L. Breast-feeding vs formula-feeding for infants born small-for-gestational-age: divergent effects on fat mass and on circulating IGF-I and high-molecular-weight adiponectin in late infancy. J Clin Endocrinol Metab. 2013;98:1242–1247. doi: 10.1210/jc.2012-3480. [DOI] [PubMed] [Google Scholar]
- 83.Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000;106:473–481. doi: 10.1172/JCI10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Esfahani A, Wong JM, Mirrahimi A, Srichaikul K, Jenkins DJ, Kendall CW. The glycemic index: physiological significance. J Am Coll Nutr. 2009;28 Suppl:439S–445S. doi: 10.1080/07315724.2009.10718109. [DOI] [PubMed] [Google Scholar]
- 85.Last AR, Wilson SA. Low-carbohydrate diets. Am Fam Physician. 2006;73:1942–1948. [PubMed] [Google Scholar]
- 86.Ludwig DS. The glycemic index: physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA. 2002;287:2414–2423. doi: 10.1001/jama.287.18.2414. [DOI] [PubMed] [Google Scholar]
- 87.Brand-Miller JC, Liu V, Petocz P, Baxter RC. The glycemic index of foods influences postprandial insulin-like growth factor-binding protein responses in lean young subjects. Am J Clin Nutr. 2005;82:350–354. doi: 10.1093/ajcn.82.2.350. [DOI] [PubMed] [Google Scholar]
- 88.Melanson KJ, Westerterp-Plantenga MS, Saris WH, Smith FJ, Campfield LA. Blood glucose patterns and appetite in time-blinded humans: carbohydrate versus fat. Am J Physiol. 1999;277:R337–R345. doi: 10.1152/ajpregu.1999.277.2.R337. [DOI] [PubMed] [Google Scholar]
- 89.Chaput JP, Tremblay A. The glucostatic theory of appetite control and the risk of obesity and diabetes. Int J Obes (Lond) 2009;33:46–53. doi: 10.1038/ijo.2008.221. [DOI] [PubMed] [Google Scholar]
- 90.Brand JC, Nicholson PL, Thorburn AW, Truswell AS. Food processing and the glycemic index. Am J Clin Nutr. 1985;42:1192–1196. doi: 10.1093/ajcn/42.6.1192. [DOI] [PubMed] [Google Scholar]
- 91.Holt SH, Miller JC, Petocz P. An insulin index of foods: the insulin demand generated by 1000-kJ portions of common foods. Am J Clin Nutr. 1997;66:1264–1276. doi: 10.1093/ajcn/66.5.1264. [DOI] [PubMed] [Google Scholar]
- 92.Greenbaum CJ, Kahn SE, Palmer JP. Nicotinamide’s effects on glucose metabolism in subjects at risk for IDDM. Diabetes. 1996;45:1631–1634. doi: 10.2337/diab.45.11.1631. [DOI] [PubMed] [Google Scholar]
- 93.Kelly JJ, Lawson JA, Campbell LV, Storlien LH, Jenkins AB, Whitworth JA, O’Sullivan AJ. Effects of nicotinic acid on insulin sensitivity and blood pressure in healthy subjects. J Hum Hypertens. 2000;14:567–572. doi: 10.1038/sj.jhh.1001099. [DOI] [PubMed] [Google Scholar]
- 94.Chang AM, Smith MJ, Galecki AT, Bloem CJ, Halter JB. Impaired beta-cell function in human aging: response to nicotinic acid-induced insulin resistance. J Clin Endocrinol Metab. 2006;91:3303–3309. doi: 10.1210/jc.2006-0913. [DOI] [PubMed] [Google Scholar]
- 95.Libby A, Meier J, Lopez J, Swislocki AL, Siegel D. The effect of body mass index on fasting blood glucose and development of diabetes mellitus after initiation of extended-release niacin. Metab Syndr Relat Disord. 2010;8:79–84. doi: 10.1089/met.2009.0074. [DOI] [PubMed] [Google Scholar]
- 96.Henriksen EJ, Diamond-Stanic MK, Marchionne EM. Oxidative stress and the etiology of insulin resistance and type 2 diabetes. Free Radic Biol Med. 2011;51:993–999. doi: 10.1016/j.freeradbiomed.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhou SS, Zhou YM, Li D, Lun YZ. Dietary methyl-consuming compounds and metabolic syndrome. Hypertens Res. 2011;34:1239–1245. doi: 10.1038/hr.2011.133. [DOI] [PubMed] [Google Scholar]
- 98.Stielau WJ, Meyer JH. Periodic B-vitamin supplementation of growing rats fed a vitamin-free diet. J Nutr. 1963;81:330–334. doi: 10.1093/jn/81.4.330. [DOI] [PubMed] [Google Scholar]
- 99.Rietjens IM, Boersma MG, Haan Ld, Spenkelink B, Awad HM, Cnubben NH, van Zanden JJ, Woude Hv, Alink GM, Koeman JH. The pro-oxidant chemistry of the natural antioxidants vitamin C, vitamin E, carotenoids and flavonoids. Environ Toxicol Pharmacol. 2002;11:321–333. doi: 10.1016/s1382-6689(02)00003-0. [DOI] [PubMed] [Google Scholar]
- 100.Powell LM. Fast food costs and adolescent body mass index: evidence from panel data. J Health Econ. 2009;28:963–970. doi: 10.1016/j.jhealeco.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 101.Moreno LA, Rodríguez G. Dietary risk factors for development of childhood obesity. Curr Opin Clin Nutr Metab Care. 2007;10:336–341. doi: 10.1097/MCO.0b013e3280a94f59. [DOI] [PubMed] [Google Scholar]
- 102.Richelsen B. Sugar-sweetened beverages and cardio-metabolic disease risks. Curr Opin Clin Nutr Metab Care. 2013;16:478–484. doi: 10.1097/MCO.0b013e328361c53e. [DOI] [PubMed] [Google Scholar]
- 103.Serdula MK, Ivery D, Coates RJ, Freedman DS, Williamson DF, Byers T. Do obese children become obese? adults A review of the literature. Prev Med. 1993;22:167–177. doi: 10.1006/pmed.1993.1014. [DOI] [PubMed] [Google Scholar]
- 104.Meguid MM, Fetissov SO, Varma M, Sato T, Zhang L, Laviano A, Rossi-Fanelli F. Hypothalamic dopamine and serotonin in the regulation of food intake. Nutrition. 2000;16:843–857. doi: 10.1016/s0899-9007(00)00449-4. [DOI] [PubMed] [Google Scholar]
- 105.Vucetic Z, Reyes TM. Central dopaminergic circuitry controlling food intake and reward: implications for the regulation of obesity. Wiley Interdiscip Rev Syst Biol Med. 2010;2:577–593. doi: 10.1002/wsbm.77. [DOI] [PubMed] [Google Scholar]
- 106.Zhou SS, Zhou YM, Li D, Ma Q. Early infant exposure to excess multivitamin: a risk factor for autism. Autism Res Treat. 2013;2013:963697. doi: 10.1155/2013/963697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.May JM, Qu ZC, Nazarewicz R, Dikalov S. Ascorbic acid efficiently enhances neuronal synthesis of norepinephrine from dopamine. Brain Res Bull. 2013;90:35–42. doi: 10.1016/j.brainresbull.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Stahl SM. L-methylfolate: a vitamin for your monoamines. J Clin Psychiatry. 2008;69:1352–1353. doi: 10.4088/jcp.v69n0901. [DOI] [PubMed] [Google Scholar]
- 109.Ariaey-Nejad MR, Balaghi M, Baker EM, Sauberlich HE. Thiamin metabolism in man. Am J Clin Nutr. 1970;23:764–778. doi: 10.1093/ajcn/23.6.764. [DOI] [PubMed] [Google Scholar]
- 110.Jenks BH, McKee RW, Swendseid ME, Faraji B, Figueroa WG, Clemens RA. Methylated niacin derivatives in plasma and urine after an oral dose of nicotinamide given to subjects fed a low-methionine diet. Am J Clin Nutr. 1987;46:496–502. doi: 10.1093/ajcn/46.3.496. [DOI] [PubMed] [Google Scholar]
- 111.Houston JB, Levy G. Modification of drug biotransformation by vitamin C in man. Nature. 1975;255:78–79. doi: 10.1038/255078a0. [DOI] [PubMed] [Google Scholar]
- 112.Regulus P, Desilets JF, Klarskov K, Wagner JR. Characterization and detection in cells of a novel adduct derived from the conjugation of glutathione and dehydroascorbate. Free Radic Biol Med. 2010;49:984–991. doi: 10.1016/j.freeradbiomed.2010.05.029. [DOI] [PubMed] [Google Scholar]
- 113.Birringer M. Analysis of vitamin E metabolites in biological specimen. Mol Nutr Food Res. 2010;54:588–598. doi: 10.1002/mnfr.200900457. [DOI] [PubMed] [Google Scholar]
- 114.Parkinson A. Biotransformation of xenobiotics, in Casarett and Doull’s Toxicology: The Basic Science of Poisons. 6th ed (ed. C.D. Klaasen) McGraw Hill: New York; 2001. pp. 133–224. [Google Scholar]
- 115.Back DJ, Breckenridge AM, MacIver M, Orme ML, Purba H, Rowe PH. Interaction of ethinyloestradiol with ascorbic acid in man. Br Med J (Clin Res Ed) 1981;282:1516. doi: 10.1136/bmj.282.6275.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dunne JW, Davidson L, Vandongen R, Beilin LJ, Tunney AM, Rogers PB. The effect of ascorbic acid on the sulphate conjugation of ingested noradrenaline and dopamine. Br J Clin Pharmacol. 1984;17:356–360. doi: 10.1111/j.1365-2125.1984.tb02354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sun WP, Li D, Lun YZ, Gong XJ, Sun SX, Guo M, Jing LX, Zhang LB, Xiao FC, Zhou SS. Excess nicotinamide inhibits methylation-mediated degradation of catecholamines in normotensives and hypertensives. Hypertens Res. 2012;35:180–185. doi: 10.1038/hr.2011.151. [DOI] [PubMed] [Google Scholar]
- 118.Pipili C, Cholongitas E, Ioannidou D. The diagnostic importance of photosensitivity dermatoses in chronic alcoholism: report of two cases. Dermatol Online J. 2008;14:15. [PubMed] [Google Scholar]
- 119.Lavebratt C, Almgren M, Ekström TJ. Epigenetic regulation in obesity. Int J Obes (Lond) 2012;36:757–765. doi: 10.1038/ijo.2011.178. [DOI] [PubMed] [Google Scholar]
- 120.Lillycrop KA, Burdge GC. Epigenetic changes in early life and future risk of obesity. Int J Obes (Lond) 2011;35:72–83. doi: 10.1038/ijo.2010.122. [DOI] [PubMed] [Google Scholar]
- 121.Latham KE, Sapienza C, Engel N. The epigenetic lorax: gene-environment interactions in human health. Epigenomics. 2012;4:383–402. doi: 10.2217/epi.12.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Robertson KD. DNA methylation and human disease. Nat Rev Genet. 2005;6:597–610. doi: 10.1038/nrg1655. [DOI] [PubMed] [Google Scholar]
- 123.Li D, Tian YJ, Guo J, Sun WP, Lun YZ, Guo M, Luo N, Cao Y, Cao JM, Gong XJ, et al. Nicotinamide supplementation induces detrimental metabolic and epigenetic changes in developing rats. Br J Nutr. 2013;110:2156–2164. doi: 10.1017/S0007114513001815. [DOI] [PubMed] [Google Scholar]
- 124.Tian YJ, Luo N, Chen NN, Lun YZ, Gu XY, Li Z, Ma Q, Zhou SS. Maternal nicotinamide supplementation causes global DNA hypomethylation, uracil hypo-incorporation and gene expression changes in fetal rats. Br J Nutr. 2014:Feb 10; Epub ahead of print. doi: 10.1017/S0007114513004054. [DOI] [PubMed] [Google Scholar]
- 125.Raubenheimer D, Lee KP, Simpson SJ. Does Bertrand’s rule apply to macronutrients. Proc Biol Sci. 2005;272:2429–2434. doi: 10.1098/rspb.2005.3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ivers DJ, Veum TL. Effect of graded levels of niacin supplementation of a semipurified diet on energy and nitrogen balance, growth performance, diarrhea occurrence, and niacin metabolite excretion by growing swine. J Anim Sci. 2012;90:282–288. doi: 10.2527/jas.2011-4035. [DOI] [PubMed] [Google Scholar]
- 127.Shibata K, Shimada H, Taguchi H. Fate of nicotinamide differs due to an intake of nicotinamide. Biosci Biotechnol Biochem. 1996;60:1204–1206. doi: 10.1271/bbb.60.1204. [DOI] [PubMed] [Google Scholar]
- 128.Winter SL, Boyer JL. Hepatic toxicity from large doses of vitamin B3 (nicotinamide) N Engl J Med. 1973;289:1180–1182. doi: 10.1056/NEJM197311292892208. [DOI] [PubMed] [Google Scholar]
- 129.Rader JI, Calvert RJ, Hathcock JN. Hepatic toxicity of unmodified and time-release preparations of niacin. Am J Med. 1992;92:77–81. doi: 10.1016/0002-9343(92)90018-7. [DOI] [PubMed] [Google Scholar]
- 130.Dalton TA, Berry RS. Hepatotoxicity associated with sustained-release niacin. Am J Med. 1992;93:102–104. doi: 10.1016/0002-9343(92)90689-9. [DOI] [PubMed] [Google Scholar]
- 131.Coppola A, Brady PG, Nord HJ. Niacin-induced hepatotoxicity: unusual presentations. South Med J. 1994;87:30–32. doi: 10.1097/00007611-199401000-00007. [DOI] [PubMed] [Google Scholar]
- 132.Kimmons JE, Blanck HM, Tohill BC, Zhang J, Khan LK. Multivitamin use in relation to self-reported body mass index and weight loss attempts. MedGenMed. 2006;8:3. [PMC free article] [PubMed] [Google Scholar]
- 133.Major GC, Doucet E, Jacqmain M, St-Onge M, Bouchard C, Tremblay A. Multivitamin and dietary supplements, body weight and appetite: results from a cross-sectional and a randomised double-blind placebo-controlled study. Br J Nutr. 2008;99:1157–1167. doi: 10.1017/S0007114507853335. [DOI] [PubMed] [Google Scholar]
- 134.Brown BG, Zhao XQ, Chait A, Fisher LD, Cheung MC, Morse JS, Dowdy AA, Marino EK, Bolson EL, Alaupovic P, et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med. 2001;345:1583–1592. doi: 10.1056/NEJMoa011090. [DOI] [PubMed] [Google Scholar]
- 135.Green PS, Vaisar T, Pennathur S, Kulstad JJ, Moore AB, Marcovina S, Brunzell J, Knopp RH, Zhao XQ, Heinecke JW. Combined statin and niacin therapy remodels the high-density lipoprotein proteome. Circulation. 2008;118:1259–1267. doi: 10.1161/CIRCULATIONAHA.108.770669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Davis RE, Icke GC, Hilton JM. High serum thiamine and the sudden infant death syndrome. Clin Chim Acta. 1982;123:321–328. doi: 10.1016/0009-8981(82)90177-2. [DOI] [PubMed] [Google Scholar]
- 137.Darlow BA, Buss H, McGill F, Fletcher L, Graham P, Winterbourn CC. Vitamin C supplementation in very preterm infants: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed. 2005;90:F117–F122. doi: 10.1136/adc.2004.056440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA. 2007;297:842–857. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- 139.Koo WW, Hockman EM. Posthospital discharge feeding for preterm infants: effects of standard compared with enriched milk formula on growth, bone mass, and body composition. Am J Clin Nutr. 2006;84:1357–1364. doi: 10.1093/ajcn/84.6.1357. [DOI] [PubMed] [Google Scholar]
- 140.Gariani K, Philippe J, Jornayvaz FR. Non-alcoholic fatty liver disease and insulin resistance: from bench to bedside. Diabetes Metab. 2013;39:16–26. doi: 10.1016/j.diabet.2012.11.002. [DOI] [PubMed] [Google Scholar]


