Abstract
We show that minor capsid protein L2 is full length in clinical virion isolates and prepare furin-cleaved pseudovirus (fcPsV) as a model of the infectious intermediate for multiple human papillomavirus (HPV) types. These fcPsV do not require furin for in vitro infection, and are fully infectious in vivo. Both the γ-secretase inhibitor XXI and carrageenan block fcPsV infection in vitro and in vivo implying that they act after furin-cleavage of L2. Despite their enhanced exposure of L2 epitopes, vaccination with fcPsV particles fails to induce L2 antibody, although L1-specific responses are similar to PsV with intact L2. FcPsV can be applied in a simple, high-throughput neutralization assay that detects L2-specific neutralizing antibodies with >10-fold enhanced sensitivity compared with the PsV-based assay. The PsV and fcPsV-based assays exhibit similar sensitivity for type-specific antibodies elicited by L1 virus-like particles (VLP), but the latter improves detection of L1-specific cross-type neutralizing antibodies.
Keywords: L2, minor capsid protein, papillomavirus, furin, infectious intermediate, neutralization, human papillomavirus, HPV, heparin, carrageenan, gamma secretase
INTRODUCTION
Human Papillomavirus (HPV) is one of the most common sexually transmitted infections, and persistent infection with ~15 ‘high risk’ HPV genotypes (most often HPV16, HPV18, HPV31, HPV45) frequently can cause high-grade intraepithelial neoplasia. Left untreated, these high-grade lesions can progress to invasive carcinoma of the cervix and other anogenital regions and oropharynx. Indeed, HPV is the etiologic agent responsible for 5% of all cancer deaths worldwide, including 99% of cervical cancers (de Villiers et al., 2004; Parkin and Bray, 2006). As the two prophylactic HPV vaccines are licensed for protection against only two oncogenic HPV types (HPV16 and HPV18), the development of new inhibitors and second generation HPV vaccines has continued.
The study of papillomavirus has been technically difficult because completion of the PV life cycle requires squamous differentiation of the infected keratinocyte that is not replicated by standard tissue culture conditions. However, organotypic raft culture causes infected keratinocytes to undergo squamous differentiation and thus generates infectious PV (Conway et al., 2009b; McLaughlin-Drubin et al., 2004; Meyers et al., 1992). This method produces limited quantities of virions containing the authentic viral genome for which there is no simple per cell infectivity assay. An alternative approach is the production of PV pseudovirion (PsV) by the co-transfection of the 293TT cell line with codon-modified L1 and L2 expression vectors and a reporter plasmid genome(Buck et al., 2004) . The cells are lysed 48 hours later, and incubated overnight at 37°C (known as the maturation step) before being purified by density gradient ultra-centrifugation. These purified PsV can be readily used for surrogate infectious studies both in vitro and in vivo because they deliver a reporter construct, typically expressing luciferase or GFP, or alternatively the PV genomes can be encapsidated in this system to produce quasivirions (QV) (Buck et al., 2004; Culp et al., 2006; Pastrana et al., 2004; Pyeon et al., 2005; Roberts et al., 2007).
Residues 17-36 of minor capsid protein L2 are buried below the capsid surface of HPV16 PsV, inaccessible to the neutralizing monoclonal antibody RG1 (Gambhira et al., 2007), but become accessible to RG1 as early as four hours in the infectious process (Kines et al., 2009). For exposure of the RG1 epitope, PV must first undergo a conformational change and adopt an intermediate structure. This is triggered by binding of virions to heparan sulphate proteoglycans (HSPG) on the basement membrane (that has been revealed upon wounding the epithelium) and cleavage of the very amino terminus of L2 by furin at a conserved site. This conformational change in the capsid is also modeled in vitro by the association of PsV with extracellular matrix (ECM) produced by certain cell lines, e.g. HaCaT and MCF7, although not 293TT cells to which the PsV bind directly via HSPGs (Johnson et al., 2009; Kines et al., 2009). Importantly this difference in mechanism of L2 exposure upon binding of PsV to 293TT cells has been linked to poor sensitivity in L2-, but not L1 VLP-specific antibody-dependent in vitro neutralization assays using this cell line (Day et al., 2008a; Day et al., 2012a). Indeed, the discord between the low or undetectable neutralization titers measured using this system despite robust ELISA reactivity and protection upon passive transfer and PsV challenge of mice with the same L2-vaccinated sera, suggest the need for improved assays that use target cells other than 293TT to better replicate the uncloaking of L2 observed during infection in vivo.
Studies of the PsV production procedure show that HPV PsV particles which do not undergo the maturation step are more susceptible to neutralization by L2 antibodies, suggesting L2 is initially exposed during the early events of packaging and co-assembly with L1 capsomeres but is slowly “buried” in the capsid structure as the virus matures into a more stabilized form (Buck et al., 2005; Buck and Trus, 2012). In contrast, studies of organotypic raft culture-derived virions show more mature HPV virus particles (i.e. virions from a 20-day old raft) harvested from cornified layer, are more susceptible to neutralization by L2-specific antibodies compared to virions harvested from the suprabasal layer of the tissue rafts cultured for 10 days (Conway et al., 2011). These findings suggest differential exposure of L2 epitopes on the capsid during virion morphogenesis and elevation from the more reducing environment of the suprabasal layers to the upper oxidizing cornified layers in the differentiated tissue raft culture (Conway et al., 2009b; Conway et al., 2011). Whereas some studies analyzing L2 in virions purified from warts suggest L2 is full-length, others show L2 existing in a doublet by immunoblot (Doorbar and Gallimore, 1987; Jin et al., 1989; Rose et al., 1990). While the latter may reflect partial degradation during virion purification, it remains possible that a subset of wart-derived infectious virions exhibit variable degrees of L2 exposure and furin-cleavage.
There are important implications if a subset of L2 is already exposed and cleaved by furin even before transmission and encountering the host. Firstly, partially pre-cleaved HPV can infect both HSPG- or furin-deficient cell lines (Day et al., 2008b; Kines et al., 2009). This shows that changes in the conformation of the capsid associated with L2 exposure and cleavage allows HPV to become independent from cellular factors considered to be required for infection. Importantly, carrageenan (a type of sulfated polysaccharide extract from red algae which is used in sexual lubricants) as well as inhibitors of γ-secretase (e.g. XXI) (Huang et al., 2010; Karanam et al., 2010) were recently identified as potent inhibitors of mucosal trophic HPV types infection (Buck et al., 2006; Marais et al., 2011; Roberts et al., 2007). However, it is currently unknown if their potency would be compromised if a subset of, or the true infectious form of native virions is in the L2-cleaved conformation. A second implication is that potential differences in L2 cleavage within organotypic raft-derived virion and PsV preparations may account for reported differences in sensitivity to inhibitors and the impact of mutations in the RG1 epitope (Conway et al., 2009a; Cruz and Meyers, 2013).
Here we describe a method to generate milligram quantities of highly (~90% of L2) furin-cleaved pseudovirus (fcPsV), and examined its immunogenicity and the impact of carrageenan and furin and γ-secretase inhibitors upon infectivity of the furin-cleaved intermediate. To confirm if furin cleavage of L2 represents an infectious intermediate, we examined the cleavage status of L2 in several wart-derived virions of divergent PV genotypes. Further, we assessed if use of fcPsV for in vitro neutralization studies could enhance the sensitivity for L2-specific neutralizing antibodies in a high throughput format without compromising measurement of L1 VLP-specific antibody.
MATERIALS AND METHODS
Ethics Statement
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. All animal studies were performed with the prior approval of the Animal Care and Use Committee of Johns Hopkins University (protocol MO08M19). Human tissue samples were collected following informed consent of the patient or the patient's guardian in accordance to the Ethics Committee of the Medical University Vienna (ECS 1327/2012).
Plasmids
The plasmid vectors pShell expressing codon optimized L1 and L2 capsid genes of HPV16, 45 and 58 were kind gifts from John Schiller, NCI. Additional PsV genotypes HPV6, 11, 18, 31 and 33 codon optimized L1 and L2 capsid genes were sub-cloned into double expression vector pVITRO1-neo-mcs (Invivogen, San Diego CA). The human furin cDNA (NM_002569.2) was obtained from Sino Biological Inc and was sub-cloned into pIRESpuro2 (Clontech Laboratories Inc, USA) between the AflII and BstB1 restriction sites after PCR amplification using primers 5’-GAGAGACTTAAGATGGAGCTGAGGCCCTGG (Forward) and 5’-GACCGATTCGAATCATCAGAGGGCGCTCTGGTC (Reverse) to yield Furin-pIRESpuro2. Mouse papillomavirus (MmuPV1) plasmids pMusPV, pMuL2w, pMusSheLL and the SV40 T-antigen plasmid, pTIH were kind gifts from Christopher Buck, NCI. MmuPV1-L2 was sub-cloned into pet28a(+) vector (Novagen, San Diego, CA) between the BamH1 and Xho1 restriction sites after PCR amplification of codon optimized MmuPV1-L2 gene from pMuL2w using primers 5’-GAGAGAGGATCCATGGTCAGCGCCGATCGCTCA (Forward) and 5’ GACCGACTCGAGGTA GATCCTGTACTTCCGCTTGCGCTT to yield MmuPV1-L2-pet28a(+).
Cell culture and creation of cell lines 293TTF and LoVoT
All cell lines were maintained in DMEM supplemented with 10% FBS, 1X penicillin and streptomycin, 1X Non-essential amino acids and 1X Sodium Pyruvate (Gibco Life technologies, Grand Island NY). For the creation of 293TTF and LoVoT, 5×106 293TT or LoVo cells/well respectively were seeded into a 6 well plate the day before transfection. Individual wells were transfected with either no template (mock transfection) or either Furin-pIRESpuro2 into the 293TT cells or pTIH into the LoVo cells respectively using Mirus TransIT 2020 (Mirus Bio, Madison WI) according to the manufacturer's protocol. After 48 hours, the cells were harvested using 0.5% trypsin and each well re-seeded into individual 10cm2 plates. Both control and transfected cells were treated with complete growth media with either puromycin concentration of 2μg/mL for 293TT cells, or with 200 μg/mL Hygromycin B for LoVo cells. Fresh media was introduced every 4 days until all the control cells died and the appearance of clonal colonies in the transfected plate. Colonies were picked using cloning cylinders (Milipore) as per manufacturer's recommendations and expanded. The furin-deficient cell line FD11 (Gordon et al., 1995) and the FD11-F cell line in which furin expression is complemented by ectopic expression of the furin gene in FD11 cells, were both gifts from Tae Heung Kang and TC Wu (Johns Hopkins University, Bethesda, MD). The LoVo cell line (Drewinko et al., 1976) was a kind gift from Weijie Poh and James Herman Lab (Johns Hopkins University, Bethesda, MD). PGSA-745 cells (Esko et al., 1985) were a kind gift from John Schiller (National Cancer Institute, Bethesda, MD).
Pseudovirion (PsV) and Furin-cleaved Pseudovirion (fcPsV) Production
Standard PsV were generated in 293TT cells following the previously described production protocol (http://home.ccr.cancer.gov/Lco/pseudovirusproduction.htm). Firefly luciferase expression plasmid was employed as the reporter for PsV infection in both neutralization assays and for mice vaginal challenge studies. Furin-cleaved PsV (fcPsV) were generated by following the same standard PsV production protocol but with the following modifications: (1) 293TTF cells were used instead of 293TT cells; (2) Maturation buffer did not contain ammonium sulfate; (3) CaCl2 was added to the maturation buffer to 5mM; (4) Maturation was carried out for 48 hours instead of 24 hours. The protocol was also suitable for producing fcPsV encapsidating GFP or SEAP reporter and the former was used in time-course assays. Transmission electron microscopy (TEM) analysis on the purified virions were performed as described in (Buck et al., 2008)
Generation of infectious mouse papilloma virions (MmuPV1) and mouse papilloma tail lesions
The synthetic MmuPV1 genome plasmid (pMusPV) was excised from its vector backbone and re-ligated by adapting an earlier protocol (http://home.ccr.cancer.gov/lco/religation.htm). Co-transfection of pMusSheLL containing the codon optimized sequences of MmuPV1 L1, L2 and the re-ligated MmuPV1 genome into 293TT cells and following remaining steps of the standard PsV protocol to produce Infectious MmuPV1 virions. Five athymic nude mice (NCr-nu/nu) were subsequently challenged on their tails with 200μL of the purified MmuPV1 virion as described in (Cladel et al., 2013). Papilloma-like lesions were observed on the tails subsequently after 5 weeks. These mice were then euthanized and mouse papillomas were collected, snap frozen in liquid nitrogen and stored at −80°C until further use.
Western blot analysis and quantification
Primary antibodies used for L2 detection were mouse monoclonal antibody RG1 (Gambhira et al., 2007) to HPV16 L2 17-36, or rat monoclonal antibody WW1 that recognizes the same epitope and also neutralizes HPV16 (Wu et al., 28th International Papillomavirus Meeting, Puerto Rico, Nov 30, 2012, Abstract B06-086). Primary antibody for furin and T-antigen detection was respectively B-6 and PAB101 (sc-133142 and sc-147, Santa Cruz Biotechnology, Dallas, TX). Respective secondary antibodies include HRP-goat anti-mouse IgG light chain or HRP-donkey anti-rat IgG Heavy+Light chain (Jackson ImmunoResearch, West Grove PA). For 293TT and 293TTF supernatant analysis, cells were seeded in DMEM (10% FBS, 1X penicillin/streptomycin) at 500,000 cells/well in a 6 well plate in the presence or absence of puromycin (2 μg/mL). After 48 hours, the wells were washed 3 times with PBS and serum free media (plus puromycin 2μg/ml for 293TTF) was added for 24 hours. Subsequently, media was collected and spun at 1600 rpm for 4.5 minutes at room temperature to remove any debris. The supernatant was concentrated using 50kDa Centricon concentrators (Milipore, Billerica, MA) and subsequently used for western blot analysis. Intensity of bands was analyzed using the NCI software ImageJ (http://rsb.info.nih.gov/ij/index.html). For comparison of L2 bands in the various clinical warts samples, virions were extracted from the samples using the following protocol (http://home.ccr.cancer.gov/lco/VirionExtraction.htm). HPV26, HPV57 L2 was detected using WW1, HPV6 L2 was detected using L2 α(11-88)×8 rabbit serum made as described in (Jagu et al., 2013b), BPV-1 L2 was detected with mouse monoclonal antibody C6, and MmuPV1 L2 was detected with mouse anti-MmuPV1-L2 sera.
L1/L2 VLP vaccination and generation of MmuPV1-L2 anti-sera
Groups (n=10) of 8-10 week old female Balb/c mice were vaccinated s.c. three times at two week intervals with L1/L2 VLP (5 μg), or furin-cleaved L1/L2 VLP (5 μg) formulated with alum (50 μg) and MPL (5 μg), or 50 μL of Cervarix™, or PBS alone, or PBS with adjuvants alum (50 μg) and MPL(5 μg). Blood samples were collected two weeks after the second and third vaccination. For MmuPV1-L2 immunization, MmuPV1-L2 protein was synthesized by bacterial induction using the MmuPV1-L2-pet28a(+). Following protein induction and purification and dialysis (See (Jagu et al., 2013a) for full method details), five 8-10 week old female Balb/c mice were vaccinated s.c. with 25 μg of purified MmuPV1-L2 protein formulated with alum (50μg) and MPL (5μg) three times at two week intervals. Blood was collected a week after the first boost. In both immunization regimens, following blood collection, the blood samples were clotted o/n at 4 °C and serum was collected after centrifugation at 2,000g for 10 min at 4 °C.
ELISA
For analysis of antibody response against HPV16 L1-VLP and L2 full length protein, maxisorp microtiter 96-well plates (Thermo Scientific Nunc, Waltham MA) were coated with either L1-VLP or L2 protein at 500 ng in 100 μL PBS/well and incubated overnight at 4 °C. The next day, plates were blocked with PBS/1% BSA for 1 hour at 37 °C. Serum samples diluted 1:50 in PBS/1% BSA were then added to the plates for 1 hour at 37 °C. Following this, plates underwent 3 washes with washing buffer (0.01% Tween 20 in PBS) before HRP-sheep anti-mouse IgG diluted 1:5000 in 1% BSA was added to each well and plates were incubated for 1 hour at 37°C. After 3 further washes, 100 μL of ABTS solution, 2,2’Azinobis [3-ethylbenzothiazoline-6-sulfonic acid] (Roche, Basel Switzerland) was added to each well for development, and absorbance at 405nm read using a Benchmark Plus (Bio Rad, Hercules CA).
In vitro Neutralization Assays
Serum samples (4 μL) were serially diluted two-fold in culture media, and mixed with HPV PsV or fcPsV (0.1μg/μL of L1) carrying luciferase reporter plasmid. Mixtures were incubated at 37°C for two hours, added to 293TT, FD11 or LoVoT cells that had been plated at 15,000 cells/well one day prior. Approximately, 5-fold more fcPsV was required for assays using LoVoT and 50-fold for FD11cells as compared to 293TT cells e.g. at a 1:5000 or 1:1000 dilution of virus for 293TT and LoVoT respectively. These plates were incubated at 37°C. After 72 hours, cells were lysed with 30 μL of Cell Culture Lysis Reagent (Promega, Madison WI) for 15 min at room temperature on a rocking platform. The entire lysates were transferred to a 96-well black plate, and luciferase activity was measured by adding 50μl of luciferin substrate to each well (GloMax®-Multi Detection System, Promega, Madison WI). The same procedure was carried out for FD11 or LoVoT based neutralization assays.
Pull down assays
Immuno-precipitation studies were performed using Dynabeads® Protein G (Life Technologies, Grand Island NY) according to the manufacturer's protocol with some adjustments. Briefly, 10 μg of RG-1 monoclonal antibody was mixed with 1.5 mg of Dynabeads protein G and incubated on a rotator for 30 minutes at room temperature. Subsequently, equal amounts of purified fcPsV and PsV based on L1 content were added and the mixture incubated on a rotator overnight at 4 °C. Washing and elution of bound virions were performed as per the manufacturer's protocol. Elutes were subjected to western blot analysis.
Assay of Neutralization and Inhibition of infection
293TT, LoVoT or PGSA-745 cells were seeded at 15,000 cells/well in 100 μL of medium in a 96-well plate and incubated overnight. The next day, for neutralization assays, using another 96-well plate, the serum to be tested was serially 2-fold titrated across the plate in a total volume of 50 μL. Following this, 50μL of equivalent amounts of PsV and fcPsV based on L1 amount was added to make the antibody-virus mixture 100 μL. The plates were incubated at 37 °C for 2 hours before the mixture was added onto the pre-plated cells and incubated for 72 hours. The total volume of 200 μL resulted in the starting dilution for most L2 serum was at 1:50 while L1-serum was at 1:200. For inhibitor assays, using another 96-well plate, the relevant inhibitors in either sterile water or DMSO were subjected to a 2-fold serial dilution with the highest dilution starting at 256ug/ml. Titrated PsV in 50μL was then added to the diluted candidate inhibitor and this mixture (100 μL) was immediately added to the pre-plated cells. Regardless of assay type, the plates were incubated for 72 hours and cells were lysed with 30 μL of Cell Culture Lysis Reagent (Promega, Madison WI) for 15 min at room temperature on a rocking platform. The entire lysates were transferred to a 96-well black plate, and luciferase activity was measured by GloMax®-Multi Detection System (Promega, Madison WI) after adding 50 μL of luciferin substrate (Promega, Madison WI) to each well. IC50 values or titers were calculated using Prism (GraphPad Software, San Diego CA). Approximately, 5-fold more fcPsV was required for assays using LoVoT and PGSA-745 cells as compared to 293TT cells. For the time course infectivity assay, 293TT cells were pre-plated at 60,000 cells/well in a 24 well plate and incubated at 37 °C overnight. The next day, equal amounts of HPV16 PsV or fcPsV16 (based on L1 amount) encapsidating a GFP reporter plasmid was added to the cells. Following this, at time points 24,48 and 72 hours after addition of virus to the cells, the cells were harvested with trypsin-EDTA (1x) (Gibco, Life technologies, Grand Island NY) , and washed with 1mL of fluorescence-activated cell sorter (FACS) buffer (0.5% BSA in PBS, pH 7.4). The cell pellet was then resuspended in 300 μL of 1xFACS buffer and GFP expression as an indicator of infectivity was analyzed by flow cytometry with a Becton Dickinson FACSCalibur. The experiment was performed in triplicate and % infectivity data was analyzed using CellQuest software (Becton Dickinson Immunocytometry).
Mouse vaginal challenge studies
Four days before vaginal challenge, 8-10 weeks old Balb/c mice purchased from NCI were subcutaneously injected with 3 mg of medroxyprogesterone (Depo-Provera, Pfizer, New York NY). PsV or fcPsV stock amounts were standardized based on their L1 content. Each mouse was challenged with 2 μg of PsV or fcPsV (based on stock virus with L1 content of 0.2 μg/μL) which was pre-mixed for an hour with the relevant inhibitors or an equal volume of diluents with 3% (w/v) carboxymethyl cellulose (CMC) except for the 1% (w/v) carageenen for which CMC was omitted. The amount of Heparin used (based on previous data) was 1000-fold in excess to the L1 content (Johnson et al., 2009). Approximately, 200μM of Gamma secretase inhibitor XXI was used premixed for one hour with either PsV or fcPsV that were instilled into the vaginal vault before and after cytobrush treatment (15-20 rotations, alternating directions) while the mice were under isoflurane anesthesia. Forty eight hours after challenge, mice were anesthetized by isoflurane, and 20μL of luciferin substrate (7.8mg/ml, Promega, Madison WI) was delivered into the vaginal vault before imaging. Bioluminescence was acquired for 10 min with a Xenogen IVIS 100 (Caliper Life Sciences, Hopkinton MA) imager, and analysis was accomplished with Living Image 2.0 software. For γ-secretase inhibitor studies, imaging was performed 72 hours post-challenge.
Statistical Analysis of Neutralization assay titers
For comparison of 293TT assay against the FD11 assay (Table 1), individual mouse sera from (30) vaccinated with the respective L2 vaccine candidate titers were analyzed. Neutralization titers (the reciprocal of the dilution that causes 50% reduction in luciferase activity) were recorded for each individual serum and the sample average was calculated within each group. Nonparametric Wilcoxon signed-rank test was performed to detect statistical significance between the two groups. For comparison of 293TT assay against LoVoT assay (Table 2), the same mouse serum samples from (Jagu et al., 2013b) were pooled and underwent inter-assay triplicate comparison. Neutralization titers (EC50) reported are the mean of this triplicate testing. The non-linear model Y=Bottom + (Top-Bottom)/(1+10^((LogEC50-X)*HillSlope)) was fitted to log-transformed neutralization titers data. The estimated EC50s and their 95% confidence intervals are reported. Statistical calculations were performed using GraphPad Prism version 6.
Table 1.
Summary of neutralization assay titers against HPV16 comparing the conventional HPV16 PsV neutralization assay and using fcPsV in FD11 cells.
| Serum/monoclonal antibody used (replicates) | 293TT assay neutralization titers (mean) | FD11 assay neutralization titers (mean) | Approximate fold change | P-value |
|---|---|---|---|---|
| L2α(11-88)×5 (n=8) | 788 | 8150 | 10 | 0.0078 |
| L2α(11-88)×8 (n=10) | 1270 | 9480 | 11 | 0.0156 |
| Mouse monoclonal antibody, RG-1 (n=10) | 320 | 88320 | 276 | 0.0020 |
| Rat monoclonal antibody, WW1 (n=10) | 70 | 76800 | 1097 | 0.0020 |
| Cervarix™ (n=10) | 228280 | 143360 | −1.5 | 0.1055 |
Mean neutralization titers based on the reciprocal of the dilution that causes 50% reduction in luciferase activity is recorded. P-value is based on Wilcoxon matched-pairs signed rank test.
Table 2.
Summary of neutralization assay titers with a variety of HPV genotypes comparing the conventional 293TT neutralization assay and the new LoVoT assay.
| HPV Virus Studied | Serum/monoclonal antibody used | 293TT assay neutralization titers Mean [95% Confidence Interval] | LoVoT assay neutralization titers Mean [95% Confidence Interval] | Approximate fold change (LoVoT titer/293TT titer) |
|---|---|---|---|---|
| HPV 16 (Luciferase Reporter) | L2α(11-88)×5 | 1,236[944.3-1,617] | 13,893[4,920-39,266] | 11.2 |
| L2α(11-88)×8 | 1,252[865.4-1,812] | 31,609[15,732-63,509] | 25.0 | |
| Mouse MAb, RG-1 | 188[134-262] | 20,638[15,653-27,210] | 110 | |
| Rat MAb, WW1 | 3,978[2,784-5,684] | 32,768[17,147-62,619] | 8.0 | |
| Cervarix™ | 60,367[52,999-68,760] | 133,179[54,345-326,373] | 2.2 | |
| HPV 18 (Luciferase Reporter) | L2α(11-88)×5 | 380[314-462] | 9,229[4,791-17,778] | 24.2 |
| L2α(11-88)×8 | 4,685[3,963-5,539] | 52,158[56,117-110,171] | 11.1 | |
| Mouse MAb, RG-1 | 1,675[1,294-2,168] | 34,701[20,650-58,312] | 20.7 | |
| Rat MAb, WW1 | 503[392-644] | 26,209[16,953-40,519] | 52.0 | |
| Cervarix™ | 512,462[459,833-571,115] | 418,401[284,189-615,995] | −0.81 | |
| HPV 31 (Luciferase Reporter) | L2α(11-88)×5 | <50[N/A] | 305[109-850] | 12.2 |
| L2α(11-88)×8 | <50[N/A] | 1,438[670-3,086] | 28.8 | |
| Mouse MAb, RG-1 | <50[N/A] | <50[N/A] | N/A | |
| Rat MAb, WW1 | <50[N/A] | <50[N/A] | N/A | |
| Cervarix™ | 3,331[1,459-7,604] | 4,428[1,859-10,547] | 1.3 | |
| HPV 45 (Luciferase Reporter) | L2α(11-88)×5 | 52[29-90] | 3,013[1,746-5,197] | 57.9 |
| L2α(11-88)×8 | 176[125-248] | 15,497[10,207-23,530] | 87.6 | |
| Mouse MAb, RG-1 | <50[N/A] | <50[N/A] | N/A | |
| Rat MAb, WW1 | <50[N/A] | 8,033[4,601-14,024] | 321 | |
| Cervarix™ | <50[N/A] | 1,712[917-3,194] | 68.5 | |
| HPV 58 (Luciferase Reporter) | L2α(11-88)×5 | 68[36-126] | 4,025[1,555-10,424] | 58.9 |
| L2α(11-88)×8 | 416[286-604] | 7,562[4,788-11,945] | 18.2 | |
| Mouse MAb, RG-1 | <50[N/A] | <50[N/A] | NA | |
| Rat MAb, WW1 | <50 [N/A] | 830[194-3,538] | 27.0 | |
| Cervarix™ | <50 [N/A] | <50 [N/A] | NA | |
| HPV 6 (Luciferase Reporter) | L2α(11-88)×5 | 170[131-220] | 2,547[1,271-5,107] | 15.0 |
| L2α(11-88)×8 | 288[227-365] | 9,313[6595-13152] | 28 | |
| Mouse MAb, RG-1 | <50[N/A] | <50[N/A] | NA | |
| Rat MAb, WW1 | <50[N/A] | <50[N/A] | NA | |
| Cervarix™ | <50[N/A] | <50[N/A] | NA | |
| Gardasil™ | 92,623[71,929-119,271] | 73,113[49,166-108,723] | −1.2 |
Mean neutralization titers is based on the reciprocal of the dilution that causes 50% reduction in luciferase activity. Serum pooled from 10 mice vaccinated with either L2α(11-88)×5 or L2α(11-88)×8 or L2-specific monoclonal antibodies (MAb) was used by triplicate testing. These antisera were obtained from animals in which vaccination was previously shown to confer protection against in vivo mouse challenge with HPV6, 16, 18, 31, 45 and 58.
RESULTS
Generation of 293TTF, a clonal cell line that overexpresses enzymatically active furin
L2 in HPV16 PsV could be cleaved if furin is added during the maturation step of the standard HPV PsV protocol. However, the extent of L2 cleavage in the virions was only approximately 35% in these preparations(Day et al., 2008b). This prompted us to develop an alternative approach to reproducibly produce fully furin-cleaved PsV at high titer. To this end, a clonal 293TT cell line that over-expresses furin, termed 293TTF, was generated from 293TT cells that is used for conventional PsV production (Figure 1).
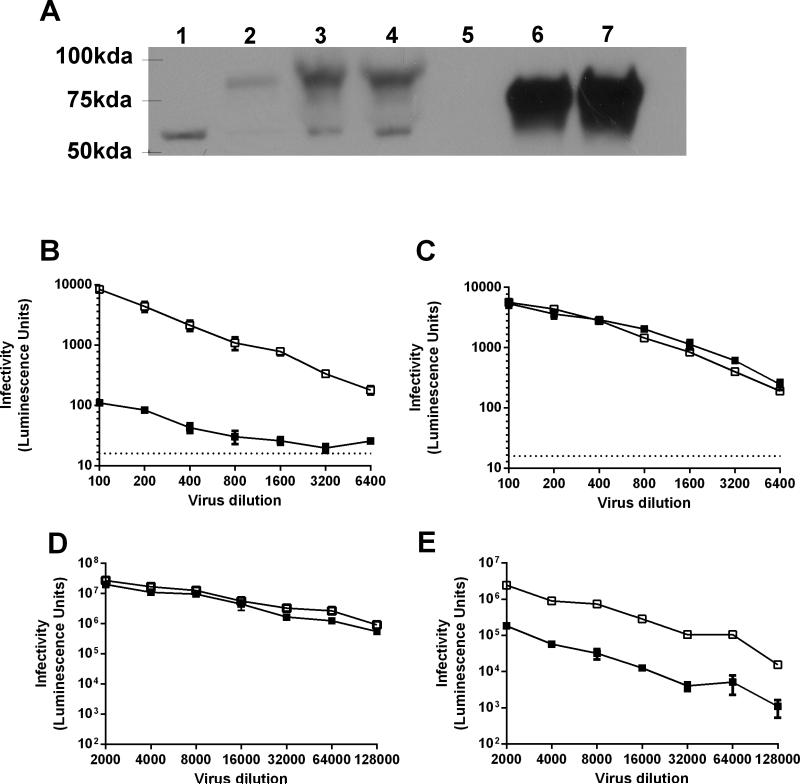
Figure 1. 293TTF cell line overexpresses furin and facilitates production of furin-cleaved PsV.
Expression of endogenous furin in parental 293TT cells (lane 1), 293TT transiently transfected with furin gene (lane 2), 293TTF cells stably transfected with furin in the presence (lane 3) and absence (lane 4) of puromycin (2μg/ml). Equivalent amounts of conditioned media supernatant of parental 293TT (lane 5) or 293TTF in the presence (lane 6) and absence (lane 7) of puromycin (2μg/ml) were used to assess furin secretion. Image analyses and relative densitometry using ImageJ indicate that for 293TTF cells endogenous furin expression and secretion was at least 150-fold higher compared to 293TT control cells. Band seen at ~60kDa was a furin splice variant as indicated in the material data sheet of the antibody used (A). Equivalent amounts of HPV16 PsV (solid squares) or HPV16 fcPsV (open squares) based on L1 content, were added onto pre-plated furin deficient cells FD11 (B) or FD11F (FD11 cells re-complemented with furin gene) (C), 293TT cells (D) and 293TT cells with 20μM of furin inhibitor (E). All experiments were performed in triplicate
Furin is present in 293TT cells but the amounts were below the limit of detection (Figure 1A, lane 1). This was consistent with the literature on furin being highly regulated and not readily detected via western blot methods (Bourne and Grainger, 2011; Thomas, 2002). However, Western blot analysis of 293TTF cells produced a prominent band of 90-100kDa, a size consistent with an unresolved doublet of the endogenous immature/pro-furin (96kDa) and mature furin (90kDa) (Figure 1A, lanes 3, 4). Image analysis by densitometry showed that the total amount of furin expressed in 293TTF was at least 150-fold higher than in parental 293TT cells. Another band was observed at ~60kDa which was reported in the antibody material data sheet as a furin splice variant (Figure 1A, lanes 1, 3, 4). The level of secreted furin released by 293TTF cells was also 200-fold higher compared to 293TT cells (Figure 1A, lane 5-7). Secreted furin has a lower molecular weight (~80kDa) than cell-associated furin due to cleavage of its C-terminal transmembrane region.
Production and analysis of HPV furin-cleaved pseudovirus (fcPsV)
To assess if 293TTF can act as a producer cell line for furin-cleaved pseudovirus (fcPsV), we performed the standard PsV production protocol using either 293TT or 293TTF. Particles purified from each preparation using Optiprep™ step gradients were morphologically indistinguishable when stained with uranyl acetate and viewed by transmission electron microscopy (Figure 2A-B).
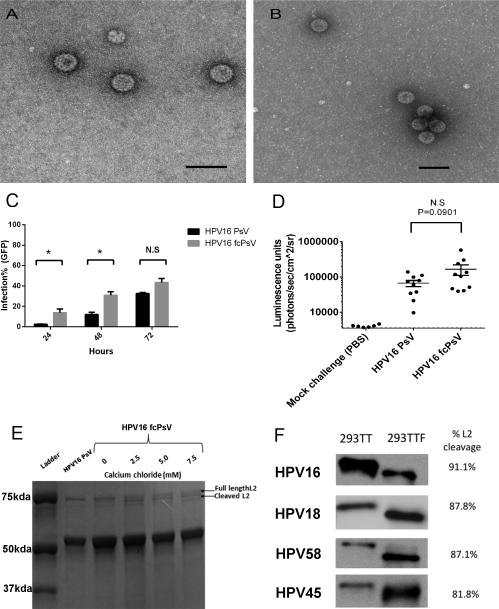
Figure 2. Furin-cleaved pseudovirus (fcPsV) exhibits 80-90% L2 cleavage without compromise of morphology or infectivity in vivo.
Transmission electron microscopy of HPV16 PsV (180,000x) (A) and fcPsV (135,000x) (B), black bar indicates 100nm. Infection time course of HPV16 PsV versus fcPsV encapsidating a GFP reporter was added to 293TT cells (60,000 cells/well) (significance was calculated using paired t-test: 24 hours P=0.0349, 48 hours P=0.0282, 72 hours P=0.0654) (C). In vivo vaginal challenge of mice for 72 hours with HPV16 PsV or fcPsV encapsidating a luciferase reporter (unpaired t-test, P=0.0901), or PBS as negative control (D). Commassie gel showing L2 cleavage status of HPV16 PsV using the standard PsV protocol or in 293TTF (fcPsV) matured in various calcium concentrations (0, 2.5, 5.0 and 7.5 mM) for 24 hours (E) Western blot analysis of L2 cleavage on HPV PsV genotypes 16, 18, 45, 58 made in either in 293TT with the standard HPV PsV protocol or in 293TTF with modified maturation timing of 48 hours and 5mM of calcium chloride (F)
To examine HPV16 fcPsV functionally, we tested whether fcPsV produced from 293TTF could bypass the requirement for furin and thus infect furin-deficient cell lines such as FD11 (Chinese hamster ovary-CHO cells with furin gene knocked out)(Gordon et al., 1995) and LoVo (a human colon adenocarcinoma line)(Drewinko et al., 1976). In FD11 cells, the infectivity of HPV16 fcPsV was 2 logs higher than HPV16 PsV (Figure 1B). Importantly, the infectivity of HPV16 PsV was restored to levels similar to that of fcPsV in FD11-F cells which are FD11 cells re-complemented for the wild type furin gene (Figure 1C). A similar trend was observed with LoVo cells versus LoVo supplemented with purified exogenous furin (data not shown). HPV16 fcPsV and PsV infectivity in 293TT was also tested with and without the presence of a furin inhibitor (20 μM). Both HPV16 fcPsV and PsV were similarly infectious in 293TT cells (Figure 1D), whereas upon furin inhibition, HPV16 PsV infection decreased dramatically by 2 logs. Interestingly, HPV16 fcPsV infection was alsoaffected whereby the infectivity decreased almost 1 log10-fold compared to fcPsV infection without inhibitor. This observation suggests that not all the virion particles in the 293TTF-made fraction were cleaved (Figure 1E).
We next performed a time course infectivity experiment with equal infectious units of GFP-encapsidated HPV16 PsV and fcPsV to assess if furin-cleaved viruses delivered the marker gene more rapidly since furin cleavage has been shown to be a rate-limiting step for PV infectivity. The infectivity of HPV16 fcPsV is higher at 24 and 48 hours, there was no significant difference from the PsV at 72 hours (Figure 2C), consistent with the wave of PsV infection catching up with the fcPsV. Similarly, the difference in infection of luciferase-encapsidated HPV16 fcPsV and PsV after 72 hours in vivo when tested in a murine vaginal challenge model was not significant (Figure 2D). These findings are consistent with fcPsV as an infectious intermediate, and suggest that the altered purification protocol does not damage the structure or infectious potential of fcPsV.
The furin inhibition results observed with HPV16 fcPsV in figure 1E prompted us to check the extent of L2 cleavage in HPV16 fcPsV made with 293TTF using the standard HPV PsV protocol. Densitometry results showed approximately half of the L2 of HPV16 fcPsV was cleaved after 24 hours maturation in these preparations from 293TTF cells, whereas cleavage of L2 in PsV prepared in 293TT was not observed (Supp Fig 1A). The partial cleavage of L2 supports the notion that not all virion particles are cleaved hence explaining why there is a small but detectable inhibition by furin seen in figure 1E. The partial cleavage of L2 despite 150-fold more furin in 293TTF (Figure 1A), suggested suboptimal conditions for furin proteolytic activity.
Studies of furin enzymology found that in vitro processing and activation of transfected furin in cell lysates was optimal at pH 6.5-7 with a calcium concentration of 5mM (Anderson et al., 1997; Thomas, 2002; Vey et al., 1994). To optimize the maturation conditions to obtain more fully cleaved L2 in fcPsV, we tested a range of calcium chloride concentrations (2.5, 5 or 7.5 mM) with 24 hours maturation (Figure 2E). Our results indicated that addition of CaCl2 beyond 5.0 mM calcium concentration was not beneficial. This was further substantiated in an infectivity test using 293T cells in which the infecitivity of fcPsV virions matured with 5mM CaCl2 was almost the same as its no furin-inhibitor counterpart. In contrast, the infecitivity results for HPV16 fcPsV matured without 5mM CaCl2 in the presence or absence of furin inhibitor was similar to the results seen in Figure 1E (Supplementary Fig1B).
To further increase the proportion of L2 cleaved in the pseudovirion preparations, we extended the maturation time to 48 hours.. The L2 cleavage of HPV16, HPV18, HPV45 and HPV58 fcPsV generated using48h maturation with 5mM CaCl2 supplementation was at least 80%, whereas L2 remained intact in PsV prepared using the standard protocol (Figure 2F). Likewise, the fcPsV of all four types were able to infect furin-deficient cells, whereas conventional PsV infected FD11 cells minimally (data not shown).
L2 is full length in natural papillomavirus virion isolates
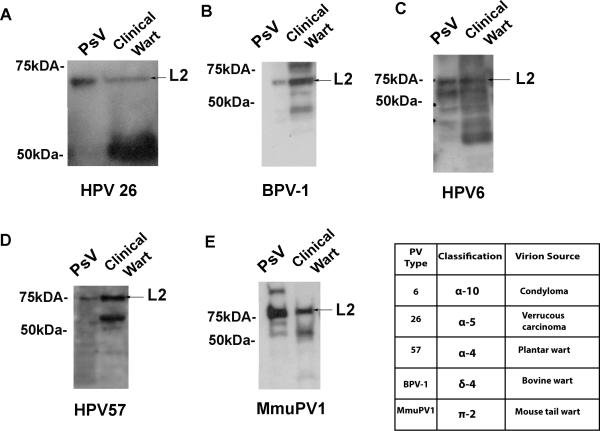
Whereas some studies analyzing L2 in virions purified from warts suggest that it is full-length, others show L2 existing in multiple forms by Western blot analysis (Doorbar and Gallimore, 1987; Jin et al., 1989; Rose et al., 1990). These lower molecular weight forms were explained as partial but unnatural degradation that occurred during the virion purification process. However, it is possible that a subset of wart-derived infectious virions contain furin cleavage that represents the fully mature form of the virus. Thus, to discern if furin-cleaved PV truly represents an intermediate conformation, we compared the molecular weight of L2 in regular PsV which are known to be uncleaved with L2 in PV derived from human and animal clinical wart samples. Side-by-side comparisons showed that PV L2 is uncleaved as only a single band of the same size was observed (Figure 3). In some preparations, significantly lower molecular weight L2 bands were also detected near the 50-55kda region (Figure 3C-D). However, based on our purified fcPsV experiments which distinguishes furin-cleaved and uncleaved L2, these lower L2 bands are not the furin-cleaved form of L2, but likely rather L2 degraded during the preparation. Thus the furin-cleaved form is an intermediate produced during the infectious process rather than the fully mature infectious form. Taken together, the results thus far indicate that fcPsV manufactured in 293TTF cells are consistent with an intermediate conformation and potentially useful to study its biology.
Figure 3. Absence of furin-cleaved L2 from papillomaviruses of natural isolates.
Western blot of papillomavirus pseudovirions (PsV) and PV virions L2 extracted from clinical isolates of warts of the same papillomavirus genotype; HPV26 (A) , BPV-1 (B), HPV6 (C), HPV57 (D), MmuPV1 (E) and table showing classification and clinical source of respective papillomavirus.
Impact of carrageenan and heparin on fcPsV infectivity
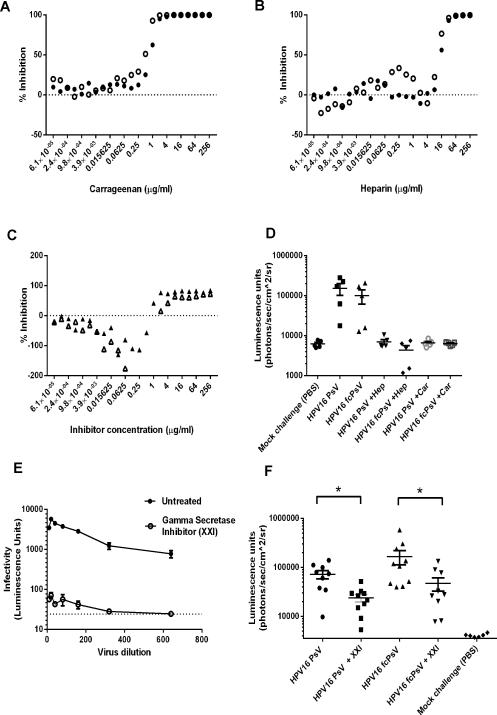
It was previously suggested that both heparin and carrageenan are highly inhibitory to HPV infection via blocking binding to and conformational changes in the virion on the ECM (Buck et al., 2006; Roberts et al., 2007). Since furin cleavage occurs after PV adopts in its intermediate structure, the use of fcPsV particles would allow us to assess whether these inhibitors act downstream of these events. Indeed, we hypothesized that neither carrageenan nor heparin would be inhibitory for HPV fcPsV infection as there is no further need of fcPsV to undergo primary binding to the extracellular matrix, as required to facilitate the conformational change and L2 cleavage events that fcPsV have already undergone. Unexpectedly, infection by fcPsV was still potently inhibited by both heparin and carrageenan in 293TT cells with similar IC50 values to PsV which were used as a control (Figure 4A-B). These IC50 values were also consistent with published literature using HPV PsV (Buck et al., 2006; Johnson et al., 2009) and the same degree/pattern of inhibition of infection was observed in the mouse vaginal challenge model using inhibitor concentrations that were previously published (Figure 4D)(Buck et al., 2006; Johnson et al., 2009).
Figure 4. Heparin, Carrageenan and γ-secretase inhibitor (XXI) inhibit both HPV16 PsV and fcPsV.
Inhibition assays were performed using equivilant amounts of HPV16 PsV (solid circles) or fcPsV (open circles) based on L1 content with varying concentrations of heparin (A), carrageenan (B) in 293TT cells. The same assay was performed using fcPsV16 infection of HSPG-deficient PGSA-745 cell line in the presence of either heparin (open triangles) or carrageenan (closed triangles) (C). In vivo mouse (n=5) vaginal challenge of HPV16 fcPsV or PsV in the presence or absence of heparin or carrageenan (D). Inhibition assay using HPV16 fcPsV in the presence or absence of XXI (500nM) in FD11 cells (furin-deficient CHO cells) (E). In vivo mouse (n=10) challenge with HPV16 fcPsV or PsV in the presence or absence of XXI. * indicates significance (P value= 0.0089 for HPV16 PsV comparison, P value= 0.0101 for HPV16 fcPsV comparison).
Both heparin and carrageenan could also inhibit fcPsV16 infection of PGSA-745 cells at high concentrations (Figure 4C). Interestingly, at lower concentrations of heparin or carrageenan, the infectivity of fcPsV16 was enhanced slightly in heparin-deficient PGSA 745 cells (Figure 4C) but not in 293TT cells (Figures 4A-B). This surprising phenomenon suggests that high quantities of either heparin or carrageenan completely coat the virion and block all receptor binding. Conversely, partial coating of fcPsV with heparin or carrageenan may either enhance secondary L1 conformational changes or bridge the interaction with HSPG-deficient cells but not cells with normal levels of HSPG. This observation is also consistent with recent evidence (Cerqueira et al., 2013; Richards et al., 2013; Surviladze et al., 2012) showing that soluble heparin and its associated factors can form complexes with PV to initiate cell entry and infection even in HSPG deficient cells.
γ-secretase is required for HPV infection after furin cleavage
We previously reported that PV infection requires γ-secretase and that inhibitors of γ-secretase such as XXI potently block HPV infection both in vitro and in vivo (31). To determine whether γ-secretase functions before or after furin-cleavage of L2, we assessed the infectivity of HPV16 fcPsV and PsV infection in the presence of XXI both in vitro and in vivo. XXI inhibited fcPsV and PsV infection similarly, both in vitro and in vivo, demonstrating that γ-secretase provides a critical function in HPV infection after furin cleavage of L2 (Figure 4E, F).
L2 exposure is enhanced in HPV16 fcPsV but it remains immunologically subdominant
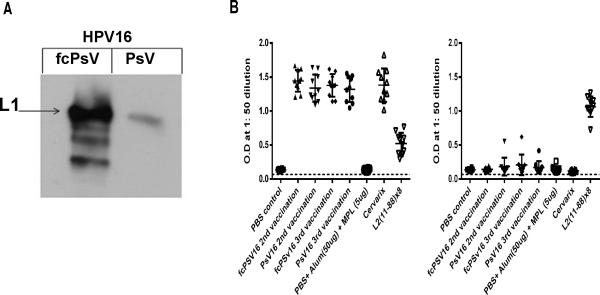
The majority of L2 is buried below the capsid surface of mature virions, including residues 17-36 that are recognized by the cross-neutralizing monoclonal antibody RG-1 (Gambhira et al., 2007). However, conformational changes in the capsid and furin cleavage of L2 during infection render the RG-1 epitope accessible once HPV16 adopts its intermediate structure. To determine if fcPsV also have this property, we performed immunoprecipitation of equal amounts of purified HPV16 PsV or fcPsV with the RG-1. RG-1 was able to pull down a significant amount of HPV16 fcPsV but not PsV (Figure 5A) indicating that furin cleavage during fcPsV production enhanced the exposure of L2 17-36 region upon the capsid surface, consistent with an intermediate conformation. Importantly, since fcPsV highly displays the L2 17-36 region (RG-1 epitope) on the capsid surface, whereas mature PsV do not (Figure 6E), we examined whether L2 in fcPsV was still immunologically sub-dominant to L1. Three groups of ten mice were vaccinated three times each at two week intervals with PBS, HPV16 PsV or fcPsV particles respectively, each formulated in alum and MPL adjuvant. Two additional groups of ten mice were also included in this study; one group was not vaccinated, while the other group was vaccinated with 0.1x of a human dose of Cervarix. Subsequently, full length HPV16 L2 peptide or HPV16 PsV ELISA studies were performed on sera obtained two weeks after the second vaccination or two weeks post the final vaccination. As a positive control for L2 ELISA responses, sera from a previous study with 10 mice vaccinated with L2α(11-88)X8 was used (Jagu et al., 2013b). These ELISA studies showed a similarly robust HPV16 L1 VLP-specific antibody response in sera of mice vaccinated with PsV, fcPsV or Cervarix after either two or three doses. However, negligible L2-specific antibody levels were detected L2-specific ELISA in either group, suggesting that L2 remains subdominant to L1 in fcPsV despite its surface display of the 17-36 epitope (Figure 5B).
Figure 5. Vaccination with HPV16 fcPsV particles which have enhanced RG-1 epitope exposure does not elicit an enhanced L2 immune response compared to PsV.
RG-1 Immunoprecipitation of equivalent amount of HPV16 fcPsV and PsV (based on L1 content) and immunoblot with antibody to L1. The arrow indicates amount of L1 pulled-down (A). ELISA results for mouse sera vaccinated with HPV16 PsV or fcPsV (plus alum-MPL adjuvant) using HPV16 PsV or full length HPV16 L2 peptide as antigens. (B)
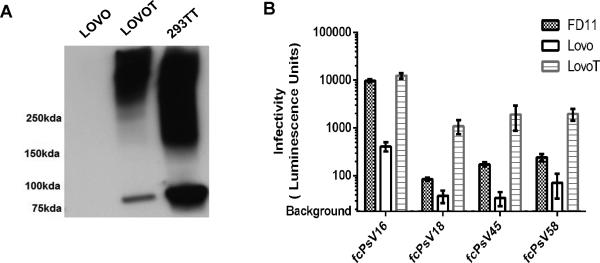
Figure 6. Generation of LoVoT cell line.
Stable expression of the SV40 T-antigen in LoVoT (lane 2) with parental LoVo cells (lane 1) and 293TT (lane3) as controls (A). Validation of LoVoT cell line demonstrating superior infectivity (luminescence signal) for the same input of HPV16 fcPsV using virus dilution of 1:8000 (B).
Use of fcPsV to enhance detection of L2-specific neutralizing antibodies
The ability to effectively detect L2-specific neutralizing antibodies is critical to the development of several second generation HPV vaccines. However considerable evidence suggests that the standard in vitro HPV PsV neutralization assay (now termed as 293TT- or L1-assay) developed by Pastrana and colleagues (Pastrana et al., 2004) is highly effective for L1-specific responses, but lacks sensitivity for L2-specific neutralizing antibodies. This lack of sensitivity was attributed to the failure of the 293TT assay to replicate the spacial-temporal separation between engagement with the primary and secondary receptors which limits the exposure of the dominant L2 cross-neutralizing epitopes (e.g. L2 17-36) to the antibodies. As the purified fcPsV already displays L2 17-36 on its surface, and we hypothesized that its use in the 293TT assay might therefore enhance sensitivity for the detection of L2-specific neutralizing antibodies without compromise of L1-specific measurements. Surprisingly, the substitution of HPV16 fcPsV into the framework of the existing 293TT assay failed to significantly increase the sensitivity of the assay (data not shown).
Studies have previously showed that HPV infection occurs whereby virions can bind directly to 293TT cells and readily enter the cell, thus avoiding the extended display of L2 by ECM-bound virus and limiting opportunity for neutralization by L2 antibodies in the milieu. Further, remaining uncleaved PsV in the fcPsV preparations can still infect 293TT cells, and are possibly cleaved by furin after their rapid uptake, rendering them resistant to L2-specific neutralizing antibodies. To eliminate the latter issue, 293TT cells were substituted with a furin-deficient line, FD11, as the target cell. While this approach (termed ‘FD11 assay’) was more sensitive in detecting L2-specific antibodies that neutralize HPV16 (Table 1), the lack of the T-antigen in FD11 resulted in the FD11 assays for several other HPV genotypes such as HPV18 PsV to display poor reporter signals. This resulted in difficulty establishing accurate 50% neutralization values. Furthermore, FD11 originates from hamster cells (CHO cells), and a human target cell would potentially be more appropriate.
To improve infection sensitivity while maintaining a requirement for furin cleavage, we decided to create a human cell line for detecting HPV neutralization via stably expressing the SV40 T-antigen (T-Ag) protein in LoVo cells, a human adenocarcinoma cell line that is furin-deficient and strongly expresses ECM. This new cell line, called LoVoT, retained the susceptibility for infection by fcPsV. Futher, the LoVoT cells expresses the SV40 large T-antigen that provides a higher reporter signal compared to FD11 or the parental LoVo cells suggesting that a lower viral inoculum can be utilized (Figure 6A-B). Importantly, we assessed several L2-specific sera and monoclonal antibodies using LoVoT as the target cell line for HPV in vitro neutralization assays (termed ‘LoVoT assay’) against furin-cleaved PsV of HPV types 16, 18, 31, 45, 58 and 6, in all cases, the detection sensitivity towards L2-based neutralization titers for these HPV genotypes was higher by at least 10-300 fold compared to titers detected by the 293TT assay (Table 2).
The LoVoT assay also demonstrated a similar sensitivity for HPV 16 (60,367, CI=52,999 to 68,760 for 293TT versus 133,179, CI= 54,345 to 326,373, for LoVoT) and HPV18 (512,462, CI=459,833 to 571,115 for 293TT versus 419,401, CI=284,189 to 615,995 for LoVoT) L1-specific neutralizing antibodies induced by vaccination of mice with Cervarix, the licensed L1-based vaccine made from HPV16 and HPV18 VLPs. Likewise, the LoVoT assay displayed similar sensitivity towards HPV6 L1-specific neutralizing antibodies elicited by Gardasil (92,623, CI= 71,929-119,271 for 293TT versus 73,113, CI=49,166-108,723 for LoVoT) (Table 2). Importantly, cross-neutralizing titers were detected with the LoVoT assay against both HPV31 and HPV45 in serum from mice vaccinated with Cervarix but the 293TT assay only detected low titers from HPV31 (3,331, CI=1859-10,547 for 293TT versus 4,428, CI=1,459-7,604 for LovoT) but not for HPV45 (<50 for 293TT versus 1712, CI=917-3194 for LoVoT,). We have shown previously that vaccination with Cervarix protects mice from vaginal challenge with HPV31 and HPV45 PsV (Jagu et al., 2013b), and this is also consistent with human data from clinical vaccine trials (56). These results suggest that our new format neutralization assay using fcPsV and the LoVoT line as target cells is a more sensitive assay compared to the conventional 293TT neutralization assay for detection of L2-specific and cross-neutralizing L1-specific antibodies while retaining the standard assay's high sensitivity for type-specific antibodies to L1 VLP, simplicity and throughput.
DISCUSSION
L2 17-36 epitope exposure occurs as early as four hours after addition of virus to cultured cells, and the infectious process is slow such that addition of antibody even 8 hours later is still neutralizing (Kines et al., 2009; Kreider et al., 1995), implying that HPV can exist in its secondary furin-cleaved conformation at the cell surface for several hours. This important insight may help explain why prophylactic HPV vaccination is so effective, and it likely impacts the measurement of L2-dependent neutralizing antibodies. In light of this, our ability to purify >80-90% furin-cleaved HPV PsV particles at milligram titers using our newly created cell line 293TTF provides the opportunity to study both basic and translational aspects of HPV in its furin-cleaved intermediate conformation in areas including structure, biology and immunology.
As expected, characteristics of furin-cleaved particles include furin-independent infection (Figure 1B-E). While some inhibition was noted in the infectivity of HPV fcPsV16 in 293TT cells in the presence of furin inhibitor (Figure 1E), we showed that this inhibition was due to the presence of uncleaved virions in the fcPsV preparation. Indeed, optimizing the conditions during the virion maturation period to enhance L2 cleavage increased the ratio of fcPsV to PsV and promote higher furin-independent infectivity (Figure 2 and Supplemental Figure 1). Given that fcPsV particles have already bypassed the rate-limiting furin cleavage step, they should also theoretically infect cells with a more rapid time course, and we observed evidence for this at early time points of 24 and 48 hours post infection. However the difference has narrowed by 72 hours post addition of virus to 293TT cells. Similarly, the in vivo infectivity of PsV and fcPsV was not significantly different at 72 hours post challenge (Figure 2C-D), suggesting sufficient time for HPV PsV to ‘catch up’ with the wave of fcPsV infection, as supported by our time-based observations in Figure 2C. Immuno-precipitation studies also showed that the L2 epitope for RG-1 is more exposed upon fcPsV than PsV (Figure 5), demonstrating that their capsid conformations are distinct but the morphological changes are too subtle to be resolved upon negative staining and transmission electron microscopy (Figure 2A-B). Taken together, these results further demonstrate that fcPsV have distinct properties compared to pre-infectious HPV virions (e.g HPV PsV) and are consistent with HPV in an infectious intermediate state that is characterized by the furin-cleaved L2 and has RG-1 epitope exposed.
Studies on the mechanisms and neutralization of HPV infection using PsV or virions derived from organotypic raft culture are occasionally conflicting, which might potentially reflect differences in their extent of furin L2 cleavage. Published studies of the state of PV L2 in clinical samples showed L2 sometimes existing in a doublet (Christensen et al., 1991; Doorbar and Gallimore, 1987; Jin et al., 1989; Rippe and Meinke, 1989; Rose et al., 1990) which might be consistent with furin cleavage rather than degradation. However, the in vivo significance of this doublet is controversial since in other studies, a single band of L2 is observed (Carter et al., 1991; Holmgren et al., 2005; Komly et al., 1986; Liu et al., 1997; Xi and Banks, 1991). This raises the possibility that the fully matured infectious form of papillomaviruses that is shed from the infected lesion is furin-cleaved. Here, our study of L2 in PV virions isolated from several human and animal papillomas did not detect any evidence to suggest the presence of furin-cleaved L2 (Figure 3). Although multiple sized bands of L2 were seen in certain blots (Figure 3C-D), the migration pattern of the lower bands did not correlate with the expected 8-9 amino acid loss in which furin-cleaved L2 would display, as seen in our furin cleavage experiments of purified PsV versus fcPsV in Figure 2E or 2F. Thus, the additional L2 bands observed are probably due to L2 degradation that occurs during the extraction and/or purification process. Importantly, our findings support the validity of the HPV pseudovirus system and that the furin-cleaved form of PV virions is an infectious intermediate rather than the fully mature infectious form.
Carrageenan has shown promise clinically as a vaginal microbicide for protection against sexual transmission of HPV (Marais et al., 2011). While studies using purified HPV PsV show convincingly that carrageenan is a potent inhibitor of infection by uncleaved PsV of various HPV types both in vitro and in mice (Buck et al., 2006; Roberts et al., 2007), it cannot be completely ruled out that a small proportion of HPV virions upon natural release is furin-cleaved (but not detected by our Western blot analysis). Hence, it is important to assess if L2 cleavage by furin renders this furin-cleaved form of HPV resistant to inhibition by heparin or carrageenan. Given that fcPsV is able to infect HSPG-deficient cell lines, we examined whether fcPsV overcoming heparin or carrageenan inhibition. Surprisingly, our studies both in vitro and in vivo using fcPsV demonstrated that the inhibitory potency of carrageenan or heparin was not compromised in HPV16 if L2 is cleaved (Figure 4A-D).
Interestingly, infection was enhanced at low concentrations of carrageenan or heparin when infectivity was tested in the HSPG-deficient PGSA-745 cells (Figure 4C). This likely reflects bridging between L1 and an HSPG-binding cell surface protein to anchor fcPsV to the surface of PGSA-745 cells and facilitate infection. However once there is sufficient carrageenan or heparin present to coat the virus and the surface of the HSPG-deficient PGSA-745 cells, infection is instead inhibited. Hence, this phenomenon (Figure 4C) which occurs in cells lacking HSPG is therefore likely irrelevant in vivo.
Recently, Richards et al identified two intermediate L1 conformational states prior to infectious entry. The first state is achieved after primary binding to HSPG specifically at Lys278 and Lys361 on L1 and is associated with L2 exposure and furin-cleavage. The characteristics of the next state remain ill-defined but it is driven by interactions between HSPG and other L1 residues. Achieving the second intermediate state was important for subsequent internalization and viral uncoating (Richards et al., 2013). It is unclear which L1 conformational state is exhibited by fcPsV but they do infect PGSA-745 cells, suggesting that further HSPG-mediated secondary conformational changes in L1 are not needed for subsequent internalization and uncoating in this situation.
Our results using fcPsV indicate that carrageenan remains as a promising topical HPV microbicide candidate and also further elucidate carrageenan's mechanism of action. Previously, Buck and colleagues suggested that the primary mechanism of inhibition is similar to heparin blocking the initial interaction between HSPGs and the viral capsid. Further, it was shown that carrageenan had a secondary inhibitory effect that was exerted after the virions were cell-bound. This secondary inhibition was termed as ‘HSPG-independent inhibition’ because it was reported using 50-fold more HPV PsV pre-bound to PGSA-754 cells which do not have HSPG. However, the exact mechanism of this secondary inhibitory mechanism was unclear, although two possible modes of actions may be considered. Either the secondary inhibitory effect could occur via preventing the interaction of HPV capsid with the unknown secondary receptor necessary for the remainder of the infectious process or carrageenan prevents the cell-bound HPV pseudovirus from further conformational change to initiate furin cleavage (Buck et al., 2006). Given that furin-cleaved HPV has most likely already undergone the first round of conformational change which includes surface display of the amino-terminus of L2), our results here suggest that the secondary inhibitory mechanism of carrageenan is most likely the masking of virus surfaces involved in binding to the secondary receptor and not the prevention of initial conformational changes of the capsid that lead to L2 exposure and furin cleavage. This may also explain why carrageenan is a more potent inhibitor of HPV compared to heparin, as the latter only inhibits the interaction with the primary receptor.
We recently discovered γ-secretase inhibitor XXI is potent inhibitor of HPV infection. While XXI prevents the escape of the viral DNA from the endosome, the proteolytic target for γ-secretase and how it relates to HPV infection remains unknown. Given that furin cleavage is a necessary intermediate step in infection, the inhibition of HPV16 fcPsV infection by XXI both in vitro and in vivo (Figure 4E-F) demonstrates that γ-secretase acts after furin during HPV infection.
Natural PV infection frequently triggers an antibody response to L1, but rarely to L2. Likewise, vaccination with virions or L1/L2 VLP induces a potent L1-specific response, whereas minimal titers are observed to L2, whereas vaccination with L2 alone does induce a strong L2-specific antibody response. The subdominance of L2 in the context of the capsid may reflect its low stoichiometry L1 (≤1:5) and wider spacing compared to L1, and that L2 is predominantly buried below the capsid surface (Roden et al., 2000). Indeed, L2 epitope display by insertion into an immunodominant epitope of the major capsid antigen for HPV and non-HPV VLPs does enhance its immunogenicity (Schellenbacher et al., 2009; Tumban et al., 2011). In contrast, fcPsV particles display the L2 17-36 epitope with an untethered end in the ordered array of the capsid surface (Buck et al., 2008). Nevertheless, no significant L2 antibody titer was detected after vaccination with fcPsV despite a robust L1-specific response (Figure 5B). The continued dominance of L1 responses for fcPsV may reflect both the ≥5:1 ratio of L1 to L2 in the capsid, and greater spacing between the L2 versus L1 epitopes. Further, since the amino terminus of L2 contains cross-type protective epitopes, we speculate that the sub-dominance of this region conveys evolutionary advantage by preventing a singly infected host from developing broad immunity to other PV infections.
L2 has received attention as a target antigen for second generation HPV vaccine development because of its potential to induce broad immunity. Clinical development of HPV vaccines requires an effective immune correlate. Given the central role of neutralizing antibodies in protection, it is critical to have an in vitro neutralization assay to measure the relevant immune correlate. While the classical PsV-based neutralization assay (293TT assay) has proven a useful correlate for L1 VLP vaccines, it is insensitive for detection of L2-specific neutralizing antibody and also cross-protective L1 responses in weaker sera. Recently an L2-specific neutralization assay has been described (Day et al., 2012b). This involves creating a basement membrane-like environment that generates furin-cleaved virions before the neutralization assay is carried out. Briefly, an ECM derived from a breast cancer cell line is first laid down before adding virions. Virion interaction with this environment triggers conformational change leading to exposure of L2. Supernatant from a separate furin secreting cell line is subsequently added to exert furin cleavage. Lastly, a heparin-deficient CHO cell line, PGSA-745 is added to the mixture as the targeted host cell and expression of the reporter GFP is detected by flow cytometry. While the assay has proven to be superior to the classical 293TT/L1-assay, several recognized limitations exist. Firstly, several cell lines need to be maintained and from a quality control perspective, it is also technically challenging to ensure consistently that equivalent amounts of furin from a cell supernatant is added each time. Secondly, PGSA-745 cells are of non-human origin and while the lack of HSPG in PGSA-745 will limit the infectivity to only furin cleaved virions, the absence of T-antigen in these cell lines suggests similar problems in reporter signal activity would be experienced with “weaker” HPV PsV.
To circumvent such issues and based upon the concepts illustrated by Day et al (Day et al., 2012b), we have developed an alternative high through-put neutralization assay via the use of fcPsV. Importantly, the fcPsV approach uses a format analogous to the classical 293TT assay. It is based upon the neutralization of fcPsV infection of a furin-deficient human cell line stably expressing the SV40 T-antigen (LoVoT). This fcPsV assay exhibits significantly improved sensitivity in measuring neutralization by L2-specific antisera and monoclonal antibodies by 10-300 fold, and yet maintains similar sensitivity for type-restricted responses to L1 VLP (Table 2).
Vaccination with Cervarix confers protection against HPV 31 and 45 infection in both people and mice (Malagon et al., 2012; Wheeler et al., 2012). However, using the 293TT assay, no cross-neutralization titers for HPV45 were detected in the sera of mice vaccinated with Cervarix although cross-neutralization titers were detected for HPV31. The use of the LoVoT assay however revealed similar levels of neutralizing titers against HPV31 in sera from mice vaccinated with Cervarix and even detected the presence of low titer neutralizing antibodies to HPV45 (Table 2). This ability to better detect low titer cross-neutralizing L1 antibody titers suggests that the conformation of fcPsV may better display these cross-protective L1 epitopes than mature PsV. Taken together, these results indicate that the LoVoT assay not only detects L2-specific antibodies with enhanced sensitivity, but potentially also cross-neutralizing L1-specific antibodies, without compromising the measurement of type-specific antibodies to L1 VLP (Table 2).
In summary, our results support the utility of fcPsV system to study several biological and immunological properties of HPV while in its furin-cleaved intermediate conformation. In addition, the LoVoT-based neutralization assay has promise as a tool in HPV vaccine development, especially for evaluating L2-specific and L1-cross neutralization.
Supplementary Material
HIGHLIGHTS.
Papillomavirus virions from clinical wart isolates contain full length L2
γ-secretase inhibitor and Carrageenan act after furin during HPV infection
Furin-cleaved HPV pseudovirus improves assay of L2 neutralizing antibodies
ACKNOWLEDGEMENTS
We gratefully acknowledge the help from Michael Delannoy and Barbara Smith from the JHMI microscopy core for their help in the transmission microscopy work. We also thank Weijie Poh and James Herman, Tae Heung Kang and TC Wu, Wai-Hong Wu (all at the Johns Hopkins University, Baltimore, MD), for the respective provision of LoVo cells, FD11 and FD11-F cells, WW-1 monoclonal antibody. We also acknowledge Christopher Buck, Patricia Day and John Schiller (all at the LCO, National Cancer Institute, Bethesda, MD) for provision of the mouse papillomavirus (MmuPV1) plasmids pMusPV, pMuL2w, pMusSheLL, the SV40 T-antigen plasmid pTIH, and PGSA-745 cells.
Funding: The study was funded by Public Health Service grant P50 CA098252, RO1 CA133749, and CA118790, the V foundation, and the Vienna Science and Technology Fund (WWTF; Life Science Call 2011: LS11-006). The funders had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: Richard Roden and Subhashini Jagu are inventors on L2 patents licensed to Shantha Biotechnics Ltd., GlaxoSmithKline, PaxVax, Inc. and Acambis, Inc. and Richard Roden holds equity in Papivax LLC and have received research funding from Sanofi Pasteur and GlaxoSmithKline. The terms of these arrangements are managed by Johns Hopkins University in accordance with its conflict of interest policies.
REFERENCES
- Anderson ED, VanSlyke JK, Thulin CD, Jean F, Thomas G. Activation of the furin endoprotease is a multiple-step process: requirements for acidification and internal propeptide cleavage. The EMBO journal. 1997;16:1508–1518. doi: 10.1093/emboj/16.7.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne GL, Grainger DJ. Development and characterisation of an assay for furin activity. Journal of immunological methods. 2011;364:101–108. doi: 10.1016/j.jim.2010.11.008. [DOI] [PubMed] [Google Scholar]
- Buck CB, Cheng N, Thompson CD, Lowy DR, Steven AC, Schiller JT, Trus BL. Arrangement of L2 within the papillomavirus capsid. Journal of virology. 2008;82:5190–5197. doi: 10.1128/JVI.02726-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck CB, Pastrana DV, Lowy DR, Schiller JT. Efficient intracellular assembly of papillomaviral vectors. Journal of virology. 2004;78:751–757. doi: 10.1128/JVI.78.2.751-757.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck CB, Thompson CD, Pang YY, Lowy DR, Schiller JT. Maturation of papillomavirus capsids. Journal of virology. 2005;79:2839–2846. doi: 10.1128/JVI.79.5.2839-2846.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck CB, Thompson CD, Roberts JN, Muller M, Lowy DR, Schiller JT. Carrageenan is a potent inhibitor of papillomavirus infection. PLoS pathogens. 2006;2:e69. doi: 10.1371/journal.ppat.0020069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck CB, Trus BL. The papillomavirus virion: a machine built to hide molecular Achilles’ heels. Advances in experimental medicine and biology. 2012;726:403–422. doi: 10.1007/978-1-4614-0980-9_18. [DOI] [PubMed] [Google Scholar]
- Carter JJ, Yaegashi N, Jenison SA, Galloway DA. Expression of human papillomavirus proteins in yeast Saccharomyces cerevisiae. Virology. 1991;182:513–521. doi: 10.1016/0042-6822(91)90592-y. [DOI] [PubMed] [Google Scholar]
- Cerqueira C, Liu Y, Kuhling L, Chai W, Hafezi W, van Kuppevelt TH, Kuhn JE, Feizi T, Schelhaas M. Heparin increases the infectivity of Human Papillomavirus Type 16 independent of cell surface proteoglycans and induces L1 epitope exposure. Cellular microbiology. 2013 doi: 10.1111/cmi.12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen ND, Kreider JW, Kan NC, DiAngelo SL. The open reading frame L2 of cottontail rabbit papillomavirus contains antibody-inducing neutralizing epitopes. Virology. 1991;181:572–579. doi: 10.1016/0042-6822(91)90890-n. [DOI] [PubMed] [Google Scholar]
- Cladel NM, Budgeon LR, Cooper TK, Balogh KK, Hu J, Christensen ND. Secondary Infections, Expanded Tissue Tropism, and Evidence for Malignant Potential in Immunocompromised Mice Infected with Mus musculus Papillomavirus 1 DNA and Virus. Journal of virology. 2013;87:9391–9395. doi: 10.1128/JVI.00777-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway MJ, Alam S, Christensen ND, Meyers C. Overlapping and independent structural roles for human papillomavirus type 16 L2 conserved cysteines. Virology. 2009a;393:295–303. doi: 10.1016/j.virol.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway MJ, Alam S, Ryndock EJ, Cruz L, Christensen ND, Roden RB, Meyers C. Tissue-spanning redox gradient-dependent assembly of native human papillomavirus type 16 virions. J Virol. 2009b;83:10515–10526. doi: 10.1128/JVI.00731-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway MJ, Cruz L, Alam S, Christensen ND, Meyers C. Cross-neutralization potential of native human papillomavirus N-terminal L2 epitopes. PloS one. 2011;6:e16405. doi: 10.1371/journal.pone.0016405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz L, Meyers C. Differential dependence on host cell glycosaminoglycans for infection of epithelial cells by high-risk HPV types. PloS one. 2013;8:e68379. doi: 10.1371/journal.pone.0068379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culp TD, Cladel NM, Balogh KK, Budgeon LR, Mejia AF, Christensen ND. Papillomavirus particles assembled in 293TT cells are infectious in vivo. Journal of virology. 2006;80:11381–11384. doi: 10.1128/JVI.01328-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day PM, Gambhira R, Roden RB, Lowy DR, Schiller JT. Mechanisms of human papillomavirus type 16 neutralization by l2 cross-neutralizing and l1 type-specific antibodies. Journal of virology. 2008a;82:4638–4646. doi: 10.1128/JVI.00143-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day PM, Lowy DR, Schiller JT. Heparan sulfate-independent cell binding and infection with furin-precleaved papillomavirus capsids. Journal of virology. 2008b;82:12565–12568. doi: 10.1128/JVI.01631-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day PM, Pang YY, Kines RC, Thompson CD, Lowy DR, Schiller JT. A human papillomavirus (HPV) in vitro neutralization assay that recapitulates the in vitro process of infection provides a sensitive measure of HPV L2 infection-inhibiting antibodies. Clinical and vaccine immunology : CVI. 2012a;19:1075–1082. doi: 10.1128/CVI.00139-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day PM, Pang YYS, Kines RC, Thompson CD, Lowy DR, Schiller JT. A Human Papillomavirus (HPV) In Vitro Neutralization Assay That Recapitulates the In Vitro Process of Infection Provides a Sensitive Measure of HPV L2 Infection-Inhibiting Antibodies. Clinical and Vaccine Immunology. 2012b;19:1075–1082. doi: 10.1128/CVI.00139-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Villiers EM, Fauquet C, Broker TR, Bernard HU, zur Hausen H. Classification of papillomaviruses. Virology. 2004;324:17–27. doi: 10.1016/j.virol.2004.03.033. [DOI] [PubMed] [Google Scholar]
- Doorbar J, Gallimore PH. Identification of proteins encoded by the L1 and L2 open reading frames of human papillomavirus 1a. Journal of virology. 1987;61:2793–2799. doi: 10.1128/jvi.61.9.2793-2799.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewinko B, Romsdahl MM, Yang LY, Ahearn MJ, Trujillo JM. Establishment of a human carcinoembryonic antigen-producing colon adenocarcinoma cell line. Cancer research. 1976;36:467–475. [PubMed] [Google Scholar]
- Esko JD, Stewart TE, Taylor WH. Animal cell mutants defective in glycosaminoglycan biosynthesis. Proceedings of the National Academy of Sciences of the United States of America. 1985;82:3197–3201. doi: 10.1073/pnas.82.10.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambhira R, Karanam B, Jagu S, Roberts JN, Buck CB, Bossis I, Alphs H, Culp T, Christensen ND, Roden RB. A protective and broadly cross-neutralizing epitope of human papillomavirus L2. Journal of virology. 2007;81:13927–13931. doi: 10.1128/JVI.00936-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon VM, Klimpel KR, Arora N, Henderson MA, Leppla SH. Proteolytic activation of bacterial toxins by eukaryotic cells is performed by furin and by additional cellular proteases. Infection and immunity. 1995;63:82–87. doi: 10.1128/iai.63.1.82-87.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren SC, Patterson NA, Ozbun MA, Lambert PF. The minor capsid protein L2 contributes to two steps in the human papillomavirus type 31 life cycle. J Virol. 2005;79:3938–3948. doi: 10.1128/JVI.79.7.3938-3948.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HS, Buck CB, Lambert PF. Inhibition of gamma secretase blocks HPV infection. Virology. 2010;407:391–396. doi: 10.1016/j.virol.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagu S, Kwak K, Karanam B, Huh WK, Damotharan V, Chivukula SV, Roden RB. Optimization of multimeric human papillomavirus L2 vaccines. PloS one. 2013a;8:e55538. doi: 10.1371/journal.pone.0055538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagu S, Kwak K, Schiller JT, Lowy DR, Kleanthous H, Kalnin K, Wang C, Wang HK, Chow LT, Huh WK, Jaganathan KS, Chivukula SV, Roden RB. Phylogenetic considerations in designing a broadly protective multimeric L2 vaccine. J Virol. 2013b;87:6127–6136. doi: 10.1128/JVI.03218-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin XW, Cowsert LM, Pilacinski WP, Jenson AB. Identification of L2 open reading frame gene products of bovine papillomavirus type 1 using monoclonal antibodies. The Journal of general virology. 1989;70(Pt 5):1133–1140. doi: 10.1099/0022-1317-70-5-1133. [DOI] [PubMed] [Google Scholar]
- Johnson KM, Kines RC, Roberts JN, Lowy DR, Schiller JT, Day PM. Role of heparan sulfate in attachment to and infection of the murine female genital tract by human papillomavirus. Journal of virology. 2009;83:2067–2074. doi: 10.1128/JVI.02190-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karanam B, Peng S, Li T, Buck C, Day PM, Roden RB. Papillomavirus infection requires gamma secretase. Journal of virology. 2010;84:10661–10670. doi: 10.1128/JVI.01081-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kines RC, Thompson CD, Lowy DR, Schiller JT, Day PM. The initial steps leading to papillomavirus infection occur on the basement membrane prior to cell surface binding. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:20458–20463. doi: 10.1073/pnas.0908502106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komly CA, Breitburd F, Croissant O, Streeck RE. The L2 open reading frame of human papillomavirus type 1a encodes a minor structural protein carrying type-specific antigens. Journal of virology. 1986;60:813–816. doi: 10.1128/jvi.60.2.813-816.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreider JW, Cladel NM, Patrick SD, Welsh PA, DiAngelo SL, Bower JM, Christensen ND. High efficiency induction of papillomas in vivo using recombinant cottontail rabbit papillomavirus DNA. Journal of virological methods. 1995;55:233–244. doi: 10.1016/0166-0934(95)00062-y. [DOI] [PubMed] [Google Scholar]
- Liu WJ, Gissmann L, Sun XY, Kanjanahaluethai A, Muller M, Doorbar J, Zhou J. Sequence close to the N-terminus of L2 protein is displayed on the surface of bovine papillomavirus type 1 virions. Virology. 1997;227:474–483. doi: 10.1006/viro.1996.8348. [DOI] [PubMed] [Google Scholar]
- Malagon T, Drolet M, Boily MC, Franco EL, Jit M, Brisson J, Brisson M. Cross-protective efficacy of two human papillomavirus vaccines: a systematic review and meta-analysis. The Lancet infectious diseases. 2012;12:781–789. doi: 10.1016/S1473-3099(12)70187-1. [DOI] [PubMed] [Google Scholar]
- Marais D, Gawarecki D, Allan B, Ahmed K, Altini L, Cassim N, Gopolang F, Hoffman M, Ramjee G, Williamson AL. The effectiveness of Carraguard, a vaginal microbicide, in protecting women against high-risk human papillomavirus infection. Antiviral therapy. 2011;16:1219–1226. doi: 10.3851/IMP1890. [DOI] [PubMed] [Google Scholar]
- McLaughlin-Drubin ME, Christensen ND, Meyers C. Propagation, infection, and neutralization of authentic HPV16 virus. Virology. 2004;322:213–219. doi: 10.1016/j.virol.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Meyers C, Frattini MG, Hudson JB, Laimins LA. Biosynthesis of Human Papillomavirus from a Continuous Cell-Line Upon Epithelial Differentiation. Science. 1992;257:971–973. doi: 10.1126/science.1323879. [DOI] [PubMed] [Google Scholar]
- Parkin DM, Bray F. Chapter 2: The burden of HPV-related cancers. Vaccine 24 Suppl. 2006;3:S3/11–25. doi: 10.1016/j.vaccine.2006.05.111. [DOI] [PubMed] [Google Scholar]
- Pastrana DV, Buck CB, Pang YY, Thompson CD, Castle PE, FitzGerald PC, Kruger Kjaer S, Lowy DR, Schiller JT. Reactivity of human sera in a sensitive, high-throughput pseudovirus-based papillomavirus neutralization assay for HPV16 and HPV18. Virology. 2004;321:205–216. doi: 10.1016/j.virol.2003.12.027. [DOI] [PubMed] [Google Scholar]
- Pyeon D, Lambert PF, Ahlquist P. Production of infectious human papillomavirus independently of viral replication and epithelial cell differentiation. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:9311–9316. doi: 10.1073/pnas.0504020102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards KF, Bienkowska-Haba M, Dasgupta J, Chen XS, Sapp M. Multiple heparan sulfate binding site engagements are required for the infectious entry of human papillomavirus type 16. Journal of virology. 2013;87:11426–11437. doi: 10.1128/JVI.01721-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rippe RA, Meinke WJ. Identification and characterization of the BPV-2 L2 protein. Virology. 1989;171:298–301. doi: 10.1016/0042-6822(89)90543-6. [DOI] [PubMed] [Google Scholar]
- Roberts JN, Buck CB, Thompson CD, Kines R, Bernardo M, Choyke PL, Lowy DR, Schiller JT. Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nature medicine. 2007;13:857–861. doi: 10.1038/nm1598. [DOI] [PubMed] [Google Scholar]
- Roden RB, Yutzy W.H.t., Fallon R, Inglis S, Lowy DR, Schiller JT. Minor capsid protein of human genital papillomaviruses contains subdominant, cross-neutralizing epitopes. Virology. 2000;270:254–257. doi: 10.1006/viro.2000.0272. [DOI] [PubMed] [Google Scholar]
- Rose RC, Bonnez W, Strike DG, Reichman RC. Expression of the full-length products of the human papillomavirus type 6b (HPV-6b) and HPV-11 L2 open reading frames by recombinant baculovirus, and antigenic comparisons with HPV-11 whole virus particles. The Journal of general virology. 1990;71(Pt 11):2725–2729. doi: 10.1099/0022-1317-71-11-2725. [DOI] [PubMed] [Google Scholar]
- Schellenbacher C, Roden R, Kirnbauer R. Chimeric L1-L2 virus-like particles as potential broad-spectrum human papillomavirus vaccines. Journal of virology. 2009;83:10085–10095. doi: 10.1128/JVI.01088-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surviladze Z, Dziduszko A, Ozbun MA. Essential roles for soluble virion-associated heparan sulfonated proteoglycans and growth factors in human papillomavirus infections. PLoS pathogens. 2012;8:e1002519. doi: 10.1371/journal.ppat.1002519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas G. Furin at the cutting edge: from protein traffic to embryogenesis and disease. Nature reviews. Molecular cell biology. 2002;3:753–766. doi: 10.1038/nrm934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumban E, Peabody J, Peabody DS, Chackerian B. A pan-HPV vaccine based on bacteriophage PP7 VLPs displaying broadly cross-neutralizing epitopes from the HPV minor capsid protein, L2. PloS one. 2011;6:e23310. doi: 10.1371/journal.pone.0023310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vey M, Schafer W, Berghofer S, Klenk HD, Garten W. Maturation of the trans-Golgi network protease furin: compartmentalization of propeptide removal, substrate cleavage, and COOH-terminal truncation. The Journal of cell biology. 1994;127:1829–1842. doi: 10.1083/jcb.127.6.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler CM, Castellsague X, Garland SM, Szarewski A, Paavonen J, Naud P, Salmeron J, Chow SN, Apter D, Kitchener H, Teixeira JC, Skinner SR, Jaisamrarn U, Limson G, Romanowski B, Aoki FY, Schwarz TF, Poppe WA, Bosch FX, Harper DM, Huh W, Hardt K, Zahaf T, Descamps D, Struyf F, Dubin G, Lehtinen M. Cross-protective efficacy of HPV-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by non-vaccine oncogenic HPV types: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. The lancet oncology. 2012;13:100–110. doi: 10.1016/S1470-2045(11)70287-X. [DOI] [PubMed] [Google Scholar]
- Xi SZ, Banks LM. Baculovirus expression of the human papillomavirus type 16 capsid proteins: detection of L1-L2 protein complexes. The Journal of general virology. 1991;72(Pt 12):2981–2988. doi: 10.1099/0022-1317-72-12-2981. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.