Abstract
Individual participants vary greatly in their ability to estimate and discriminate intervals of time. This heterogeneity of performance may be caused by reliance on different time perception networks as well as individual differences in the activation of brain structures utilized for timing within those networks. To address these possibilities we utilized event-related functional magnetic resonance imaging (fMRI) while human participants (n=25) performed a temporal or color discrimination task. Additionally, based on our previous research, we genotyped participants for DRD2/ANKK1-Taq1a, a single-nucleotide polymorphism associated with a 30-40% reduction in striatal D2 density and associated with poorer timing performance. Similar to previous reports, a wide range of performance was found across our sample; crucially, better performance on the timing versus color task was associated with greater activation in prefrontal and sub-cortical regions previously associated with timing. Furthermore, better timing performance also correlated with increased volume of the right lateral cerebellum, as demonstrated by voxel-based morphometry. Our analysis also revealed that A1 carriers of the Taq1a polymorphism exhibited relatively worse performance on temporal, but not color discrimination, but greater activation in the striatum and right dorsolateral prefrontal cortex, as well as reduced volume in the cerebellar cluster. These results point to the neural bases for heterogeneous timing performance in humans, and suggest that differences in performance on a temporal discrimination task are, in part, attributable to the DRD2/ANKK1 genotype.
1. Introduction
Individuals vary greatly in their ability to estimate and discriminate intervals of time (Carlson & Feinburg, 1968; Brown, Newcomb & Kahrl, 1995). This variability may arise from multiple factors including memory and decision-making processes (Buhusi & Meek, 2005). Between-subject variance in time perception has been largely ignored until recently. Here we explore the neural and genetic factors that contribute to heterogeneous timing performance across individuals.
Human neuroimaging studies of timing demonstrate a wide degree of heterogeneity in the neural regions that become activated during a given timing task. Recently, we characterized this variability with a quantitative meta-analysis of the likelihood of activation of any given neural structure during different time perception tasks. Our results demonstrated that the likelihood of activation differed, depending on the temporal context (Wiener, Turkeltaub & Coslett, 2010). Generally, subcortical structures, such as the basal ganglia and cerebellum, were more likely to be activated during sub-second intervals, whereas cortical regions, such as the prefrontal cortex, were more likely to be activated during supra-second intervals. Furthermore, the right inferior frontal gyrus (rIFG) and supplementary motor area (SMA) were highly likely to be active across all timing tasks. An additional finding from our meta-analysis was that the pattern of basal ganglia activation likelihood differed depending on the temporal context; given the proposed involvement of regions of the basal ganglia (i.e. caudate, putamen) in different cognitive functions (Grahn, Parkinson & Owen, 2008), and the central role of the basal ganglia in current models of timing (Matell & Meek, 2004), this differential pattern of activity may be particularly relevant.
Although the results of our meta-analysis provided some clarification of the heterogeneity of neuroimaging findings for timing, they are based on inferences from group performance. A shortcoming of group averaging of fMRI performance is that individual differences in activation patterns will not be detected (Fedorenko, Behr & Kanwisher, 2011). For example, the SMA may be implicated across most timing studies, but this does not guarantee that every subject activates the SMA to the same extent, or, indeed, at all (Ferrandez, et al. 2003). In a recent study combining transcranial magnetic stimulation (TMS) and electroencephalography (EEG) (Wiener, et al. 2012), we found that the behavioral effect of TMS to the right supramarginal gyrus differed substantially between subjects, with respect to both the ability to alter timing performance and the polarity of contingent negative variation (CNV), a waveform that is in part mediated by the SMA (Nagai, et al. 2004). Similar findings have been demonstrated within the working memory literature, where substantial differences between group and individual-based fMRI and EEG responses have been found (Feredoes & Postle, 2007; Vogel & Awh, 2008) with only individual-based regions predicting behavioral disruptions from TMS (Feredoes, Tononi & Postle, 2007). As such, group differences in fMRI can tell us the regions most likely to be activated during time perception, but not whether those regions are differentially activated in individual subjects.
One explanation for individual differences in activation of timing networks is that different timing procedures may be employed as a function of task demands or subject strategy (Wiener, Matell & Coslett, 2011). One example of the effects of strategy comes from recent neuroimaging evidence demonstrating that networks of activated structures differ both within and between subjects as a function of whether subjects employ beat-based (Grahn & McAuley, 2009) or counting strategies (Hinton, et al. 2004) during timing.
Another factor that may account, at least in part, for individual differences in temporal processing is basic personality profiles. Numerous studies have demonstrated differences between different personality indices and time perception abilities (see Rammsayer, 1997 for a brief review). Consistent among these differences is the notion that the rate of an internal pacemaker varies between individuals leading to a “faster” clock for some and “slower” clock for others.
Finally, several investigators have reported data that genetic factors influence temporal processing. We demonstrated that timing performance differs between individuals with single-nucleotide polymorphisms of genes affecting dopamine function on temporal perception (Wiener, Lohoff & Coslett, 2011) and production (Balci, et al. 2012). Such differences have also been found for dopamine genes in different cognitive domains, such as working memory (Berryhill, et al. 2013), learning (Klein, et al. 2006) and task switching (Stelzel, et al. 2010). Additionally, differences as a function of genotype have been found in fMRI responses to a variety of cognitive tasks (Green, et al. 2008). These differences may be used as intermediate phenotypes between genetic differences and the behavioral manifestation of different psychiatric disorders (Winterer & Weinberger, 2006).
Within the neuroimaging literature, two recent studies have focused specifically on individual differences in the brain mechanisms recruited for time perception. Tipples, Brattan and Johnston (2013) utilized fMRI while subjects performed a sub-second temporal bisection task with face stimuli or an orthogonal gender identification task in a blocked design. The bisection point, a measure of accuracy, when regressed against activation, revealed a correlation with activity in the SMA and rIFG, with greater activity associated with overestimation of durations. A second study by Gilaie-Dotan, Kanai and Rees (2011) examined differences in structural morphometry associated with performance on supra-second discrimination tasks. Significant positive correlations were found between discriminability and gray matter differences in right primary auditory and secondary somatosensory cortices for longer (12s) durations; negative correlations were also found between discriminability and bilateral parahippocampal volume. Shorter (2s) durations did not correlate with any region when correcting for whole-brain significance levels, although primary visual cortices (positively) and SMA volume (negatively) did correlate at uncorrected thresholds.
Additional studies, not focusing directly on individual differences, have also noted correlations between subject performance and activation. Wencil and colleagues (2010), utilizing a between-subject covariate for accuracy on a sub- to supra-second temporal discrimination task noted positive correlations between performance and activation within bilateral inferior frontal gyrus. In contrast, Coull and colleagues (2008) noted that activity within the left putamen positively correlated with sub- to supra-second temporal discrimination accuracy; notably this correlation was only found for encoding, as opposed to retrieval. As a further difference, Harrington and colleagues (2004a) noted a positive correlation between supra-second bisection points and right parahippocampal activation. Notably, some of these studies only examined correlational activity post-hoc, in regions that had already been activated in group-level contrasts.
In order to elucidate the neural mechanisms associated with differences across individuals, we conducted a study using event-related fMRI to measure activity within brain regions correlating with inter-individual differences in behavior. Additionally, we used voxel-based morphometry (VBM) to address the question of morphological, as well as functional differences. Finally, in order to investigate the contribution of genetic predisposition on individual differences in brain network recruitment during temporal perception, we separated subjects on the basis of a well-known genetic polymorphism (DRD2/ANKK1-Taq1a) previously implicated in temporal perception. We hypothesized that individual differences in timing ability would be associated with differential activation of frontostriatal circuitry commonly activated in studies of temporal perception (Wiener, et al. 2010). Additionally, we expected to find that A1 allele carriers of the DRD2/ANKK1-Taq1a polymorphism would demonstrate impaired timing performance, but only with durations in the sub-second range (Wiener, et al. 2011), and not on a control task. We further hypothesized that this difference in performance would also be associated with a difference in activation within the brain regions we identified. However, we note that we were agnostic as to the direction (over- or under-activation) of this effect, as alterations in the dopamine system may lead to either increased (Jahanshahi, et al. 2010) or decreased (Coull, et al. 2012) levels of activity during timing along with decreases in performance.
A common and vexing issue in neuroimaging studies of time perception is the choice of an appropriate control task. In any timing paradigm, the duration of the stimulus cannot be known until the interval is over; thus, unlike many other stimulus features (i.e. size, pitch, intensity, etc.) that may be classified with very brief presentation, processing of a temporal interval necessarily extends for the duration of the stimulus. For our analysis of individual differences in brain activation, we therefore chose to use the well-known time-color behavioral paradigm (Coull, et al. 2004). This task, utilized by a number of fMRI researchers (Coull, et al., 2004; 2008; 2012; Livesey, et al. 2007; Morillon, et al., 2009), surmounts the above issue by presenting subjects with two sequentially presented, rapidly flickering colored stimuli; in the timing condition, subjects must judge the relative duration of both stimuli, whereas in the color condition they must judge the overall color of both stimuli by integrating information from the entire exposure. In this way, subjects cannot make a judgment regarding the colored stimulus until it has extinguished. The use of this task has been previously demonstrated to provide adequate control of the attentional and working memory demands in temporal discrimination, as both tasks use identical stimulus conditions (Coull et al, 2004).
In order to investigate the role of individual differences in time processes, we chose to use the relative difference in performance between time and color tasks within subjects, rather than raw accuracy on each task. This decision was motivated by the fact that the time and color tasks share many of the same task requirements (e.g., sustained attention, visual processing). Thus, although performances on the color and time tasks are not correlated (Gilaie-Dotan, et al. 2011), the signed difference between them is likely to reflect the relative differences in task-specific ability. As such, a large difference indicates that an individual is better at leveraging timing (or color) related circuitry than color (or timing) circuitry. Support for this approach is provided by pharmacological studies (Coull, et al. 2009; 2012) utilizing the time-color paradigm that demonstrated impairments in timing with preserved color processing. We believe that raw accuracy scores would be less informative for regressing against hemodynamic responses, as differences in performance may reflect discrepancies in non task-related processing. We hypothesized that subjects who are better are leveraging timing-related regions than color-related regions will also show greater activation in timing-related regions than those with little or no difference.
2. Materials and Methods
2.1. Subjects
Twenty-five right-handed subjects participated in the experiment (14 females, mean age 25 years (SD 3.8 years). All subjects had normal or corrected-to-normal vision and provided written consent, as approved by the local institutional review board. All subjects were screened for personal and familial neurologic, psychiatric, and other medical conditions as well as drug use and abuse; female subjects were screened for premenstrual tension. Subjects were recruited from two larger behavioral studies of temporal processing and genotype (Wiener, et al., 2011; Balci, et al., 2012). Twenty subjects were Caucasian, three were of African descent and one was of Asian descent. Fifteen subjects (7 female) were identified as having at least one copy of the A1 allele of the DRD2/ANKK1-Taq1a genotype (hereafter, A1+); ten subjects (7 female) did not have a copy of the A1 allele (hereafter, A1-). The A1 allele of the DRD2/ANKK1-Taq1a polymorphism has previously been associated with a 30-40% reduction in the density of striatal D2 receptors (Jönsson, et al. 1999), with relatively preserved density at extra-striatal sites (Hirvonen, et al. 2009).
2.2. Task
Subjects performed a temporal or color discrimination task. Subjects began by viewing a white fixation cross on a dark background. Positioned above or below the fixation cross was the word COLOR or TIME, respectively; this cue signaled to subjects which task they would be performing on the subsequent trial. The task cue extinguished after 1000ms, during which subjects viewed the fixation point alone for a jittered interval (1400-2600ms). Following this, two 4×4cm squares were presented consecutively in the center of the screen, separated by an inter-stimulus interval (ISI) of 2500ms; the color of the square rapidly alternated (50ms) between different admixtures of red and blue. The duration of the first square (S1) persisted for either 500 or 2000ms, whereas the second square (S2) persisted for one of six different durations, depending on the length of S1. If S1 was 500ms, S2 persisted for 350, 400, 450, 550, 600, or 650ms; if S1 was 2000ms, S2 persisted for 1400, 1600, 1800, 2200, 2400, or 2600ms. Following S2, the word RESPOND appeared on the screen for 500ms. An inter-trial-interval, consisting of a dark screen, persisted for 2000ms until the onset of the next task cue.
On trials marked by the word TIME, subjects were required to judge the duration of the S2 relatile to S1 and respond whether S2 was longer or shorter than S1. For trials marked by the word COLOR, subjects were required to judge the average shade of S2 relative to S1 and respond whether S2 was more or less red than S1. Responses were made on a fORP (Current Designs) fiber optic button box, held in both hands; both trials used the same button/response-mapping (longer = more red/ shorter = less red). The laterality of hand responses was also counterbalanced between subjects, with 13 subjects using their right hand for responding longer/more red and their left hand for responding shorter/less red, and 12 subjects using the opposite mapping. Hence, all stimulus features remained identical between each task type, with the task cue signaling the relevant dimension to be attended (color, or time).
2.3. Protocol
All subjects performed a 10-minute practice version of the task immediately prior to entering the scanner. Upon entering the scanner, subjects lay supine facing a rear-projection mirrored display. Subjects performed four runs of the task lasting eight minutes and fifty-four seconds each. Each run contained 48 trials, with equal counterbalancing between all twelve intervals (six S2 intervals per each S1) and two tasks (Time & Color). Trials within each run were presented in a pseudo-randomized, permuted order, for an event-related design (Josephs & Henson, 1999). There were a total of 192 trials across four runs, with 96 trials for each task; 16 trials with each interval were presented, with 8 trials per interval in each task.
2.4. fMRI acquisition
A 3.0 Tesla Siemens TIM Trio with an eight-channel coil was used to acquire all functional and structural volumes. All subjects initially received a high resolution, T1-weighted 3-D magnetization prepared rapid gradient echo (MPRAGE) scan (TR= 1620ms, TE= 3ms, TI= 950ms, matrix size 192 × 256, voxel size 0.9766 × 0.9766 × 0.9766mm) for anatomical localization and morphometric analysis. For subsequent functional runs, T2* weighted echo-planar, blood-oxygenation level dependent (BOLD) images (TR= 3000ms, TE= 30ms, matrix size 64 × 64, voxel size 3.4 × 3.4 × 3 mm) were acquired in an interleaved order. Fifty slices were acquired in the axial plane (3 mm). Head motion was minimized by foam padding and on-line prospective motion correction. The first three volumes of each run were discarded to allow for steady-state magnetization.
2.5. Behavioral Data Analysis
Reaction time (RT) and accuracy (proportion correct) for both time and color tasks were determined. Responses were collected during the response cue and inter-trial-interval. Mean performance measures were determined and the data were analyzed in SPSS (IBM) as separate, mixed-model ANOVAs for RT and accuracy data, with task (COLOR or TIME) and duration (sub-second or supra-second) as within-subject factors and genotype (A1-, A1+) as a between-subject factor. Additionally, as we hypothesized differences as a function of duration, planned paired-samples t-tests were carried out as within-genotype analyses, similar to our previous investigation (Wiener, et al. 2011), by separately comparing accuracy and RT measures for A1+ and A1- subjects between both tasks and duration ranges.
2.6. fMRI Data Analysis
Image pre-processing and data analysis was carried out with SPM8 (http://www.fil.ion.ucl.ac.uk/spm/; Friston, et al. 1995) and Matlab (Mathworks). Slice-timing acquisition correction was applied for all volumes, which were realigned to the first volume of the first run, then normalized into standard stereotaxic (Montreal Neurological Institute) space. All volumes were then smoothed with a 8mm full-width at half-maximum (FWHM) Gaussian kernel.
At the first-level, functional data of each subject were evaluated whereby trial-types, time-locked to the onset of S1, were separately convolved with a canonical hemodynamic response function (Friston, et al. 1998). Responses were then estimated using the general linear model at each voxel, modeling the activation effects for TIME and COLOR trials. A high-pass filter (128s cutoff) was applied to remove noises (e.g., slow scanner drifts) unrelated to the experimental task. Additionally, six motion parameters were included as regressors to account for movement-related signals. Finally, contrast maps were separately derived for TIME or COLOR only, and TIME vs COLOR.
Subsequently, these single contrast maps were entered into a second-level, random-effects analysis (RFX). Whereas one-sample t-tests were used for assessing the main effect of TIME and COLOR, a multiple regression analysis was conducted for [TIME vs. COLOR] with subjects’ behavioral accuracy difference scores (Time – Color) as a covariate. The multiple regression analysis permitted us to interrogate whether activation at a given voxel was modulated by relative behavioral differences in time perception over color perception across subjects; accordingly, a positive relationship would indicate that better relative performance on the time task over the color task was associated with greater activation in a given region. A statistical threshold was set to p<0.05, corrected for Family-Wise Error (FWE) at the cluster-level (height threshold p<0.001, uncorrected); this threshold was used to interrogate voxels across the whole brain and at predetermined regions of interest with small volume correction (SVC). Regions of interest were selected on the basis of results from our prior meta-analysis of 41 fMRI studies of timing. Those regions included the basal ganglia (caudate, putamen), SMA, bilateral prefrontal cortex (superior, middle and inferior frontal gyrus), and inferior parietal cortex and were defined using the WFU pickatlas (Maldjian, et al. 2003) with automated anatomical labeling (Tzourio-Mazoyer et al. 2002). Similar differences for the color task were also evaluated by examining negative loadings on the multiple regression factor in SPM. In this analysis, SVC was applied to color-specific regions of interest, as defined by previous studies utilizing this exact same paradigm (Coull, et al. 2012; 2008; 2005; Morillon, et al. 2009; Livesey, et al. 2007) and including bilateral V4, inferior frontal gyrus and inferior parietal cortex. We note this analysis is very similar in many respects to a recent investigation of timing and dopamine challenge by Coull and colleagues (2012).
The influence of DRD2/ANKK1 genotype on individual timing performance and activation was also evaluated by investigating functional activations at the group level. Activation was evaluated as a two-sample t-test between A1- and A1+ groups for the main effects of TIME and COLOR conditions separately. For this exploratory analysis, candidate voxels were chosen from the result of [TIME vs. COLOR] regression with behavioral difference scores (uncorrected p<0.005, k = 10) in order to compare the genotype effect within the regions that were differentially modulated by the relative degree of behavioral timing over color performance. For color processing, we used the result of the [COLOR vs. TIME] regression analysis (uncorrected p<0.01) as an inclusive mask for the main effect of COLOR in the two-sample t-test between A1- and A1+ groups; we used a lower threshold for this mask as no suprathreshold voxels were detected with p<0.005 in this contrast.
Finally, we conducted an exploratory analysis to investigate any differences in activation associated with differences in the duration of intervals (Coull, et al. 2012; Wiener, et al. 2011). Due to design constraints, there was insufficient time to fully separate the hemodynamic responses for sub-second and supra-second interval conditions. Nevertheless, the first-level analysis for each subject also included a covariate for duration range (sub-second or supra-second) in order to explore any potential differences that may exist between these conditions in another first level analysis. Separate single-subject contrasts were derived for the main effect of TIME at sub-second or supra-second levels; these contrasts were separately fed into second-level, independent samples t-tests between A1+ and A1- groups for each duration range, and masked with the [TIME vs. COLOR] regression contrast as described above.
2.7. VBM Analysis
Individual T1-weighted structural images were segmented into gray matter, white matter and cerebro-spinal fluid using the segmeimWRn functions in SPM8. Diffeomorphic anatomical registration through integrated lie algebra was performed using DARTEL functions (Ashburner, 2007) with the VBM toolbox (http://dbm.neuro.uni-jena.de/vbm/). Individual structural images were further smoothed with a 8mm full-width at half-maximum Gaussian kernel. The evaluation of individual differences was conducted in a similar manner to the fMRI data; a multiple regression tested for differences in gray matter volume between subjects by utilizing difference scores for the accuracy of [TIME vs. COLOR] conditions as a covariate. Regional effects were evaluated at the whole-brain level and included subcortical structures such as the basal ganglia, using the same threshold as the fMRI analysis (p<0.05 cluster threshold FWE, p<0.001 height threshold); as few studies have examined morphometric differences in timing, we attempted to examine the whole-brain rather than few a-priori candidate regions. An absolute threshold of 0.1 was used as a mask for the resulting statistical images. The same statistical threshold used for functional data analysis was also applied to the structural data analysis. We note that, as morphometric data provide only a single set to analyze, we did not have an orthogonal set with which to evaluate the genotypic influence on individual differences in behavior. However, we evaluated differences in significant regions in a post-hoc manner by segregating regression data between A1- and A1+ groups; as this does not fully address the issue of circularity, we stress caution in the interpretation of these data and have included it as an exploratory measure.
For differences between sub-second and supra-second measures, we separated accuracy difference scores between duration ranges and included them as separate covariates in our multiple regression analysis. This allowed us to measure whether morphometric differences were differentially associated with sub- or supra-second duration ranges. We chose to utilize difference scores in our VBM analysis to make the results more compatible with our fMRI multiple regression analysis, which used the same behavioral covariates.
3. Results
3.1. Behavioral Data
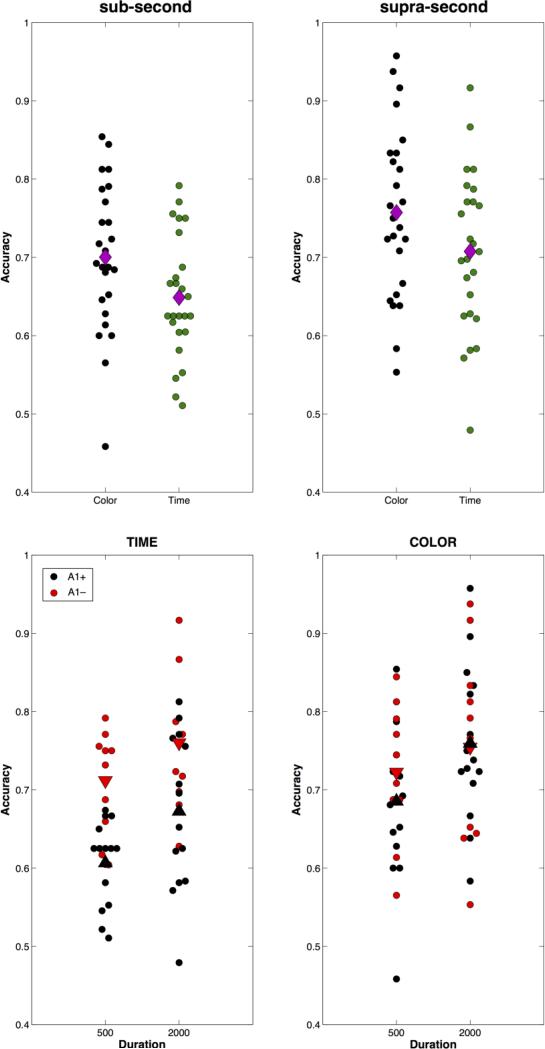
For the TIME and COLOR tasks, a substantial difference between individual performances was found (Figure 1). We found that subjects performed better on COLOR as opposed to TIME tasks (main effect of task F(1, 23) = 5.455, p= 0.029, ηp2 = 0.192), and better on supra-second, as opposed to sub-second versions (main effect of duration: F(1,23) = 16.583, p = 0.0004, ηp2 = 0.419). However, no interaction between the two effects was found [F(1,23) = 0.020, p> 0.05, ηp2 = 0.001], indicating that there was no differential effect of duration range on a particular task. Analysis of genotypic differences revealed a significant main effect of genotype [F(1,23) = 4.841, p= 0.038, ηp2 = 0.174], as well as a within-subject interaction between genotype and task [F(1,23) = 4.812, p= 0.039, ηp2 = 0.173]. Post-hoc analysis showed that DRD2 genotype influenced performance only for TIME, as opposed to COLOR tasks, at both sub-second [t(23) = 4.426, p= 0.0001, η2 = 0.459] and supra-second [t(23) = 2.3, p= 0.031, η2 = 0.187] duration ranges (Figure 1). Within-genotype analyses demonstrated that A1+ subjects performed significantly worse for sub-second than supra-second durations on both the TIME [t(14) = −2.241, p= 0.042, η2 = 0.264] and COLOR [t(14) = −3.352, p = 0.005, η2 = 0.445] tasks, whereas A1- subjects did not differ in performance as a function of duration for either task [TIME: t(9) = −1.833, p> 0.05, η2 = 0.272; COLOR: t(9) = −1.002, p> 0.05, η2 = 0.1]. No other significant effects or interactions were found for this analysis, or for the analysis of RT scores (all p> 0.05).
Figure 1.
Behavioral performance for individual subjects on TIME and COLOR tasks. Top: Accuracy data (proportion correct) for TIME and COLOR tasks, separated by duration range. Each circle represents an individual subject; diamonds indicate the group mean. Subjects performed better on the COLOR task than the TIME task at both interval ranges, but overall performed better with supra-second intervals. Bottom: Accuracy data for subjects genotyped for DRD2/ANKK1-Taq1a polymorphism. Triangles indicate group mean for each genotype (A1-, A1+). Subjects with the A1 allele performed worse on the TIME, but not COLOR tasks at both duration ranges.
We examined the consistency of performance across color and duration conditions, in the manner reported by Gilaie-Dotan et al, (2011). Like these investigators, we found no correlation between subject performance (accuracy) across the TIME and COLOR tasks, for either sub-second [Pearson r= 0.116, p> 0.05] or supra-second [r= 0.305, p> 0.05] ranges. However, we found a strong correlation between performance with sub- and supra-second durations on the COLOR task [r= 0.594, p = 0.002]; performance with sub- and supra-second TIME stimuli correlated at a trend level [r= 0.383, p = 0.058]; this trend was not found with a non-parametric spearman correlation [ρ = 0.318, p= 0.121].
We also note that a number of participants exhibited very poor performance, with one subject performing below chance on the sub-second COLOR task and supra-second TIME task. As the goal of our study is to characterize the causes of individual differences in timing, this subject was included; we note, however, that removing this subject did not alter the effects reported. Another point of interest is that the majority of subjects reported the COLOR task to be more difficult than the TIME task, even though they were more accurate on the former.
3.2. fMRI Data
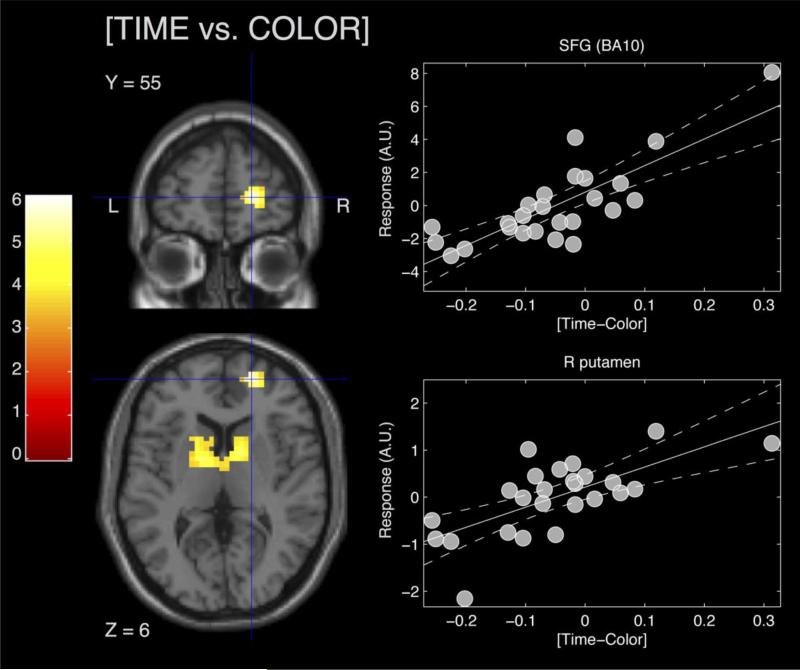
The results of all experimental fMRI analyses are summarized in both Figures 2 and 3, and Table 1 (Also see supplementary materials, for summary of main effect for TIME and COLOR conditions and the behavioral difference scores). Our primary regression analysis yielded activation within the bilateral basal ganglia, peaking in the right putamen (MNI x,y,z=21, 0, 21), right dorsolateral prefrontal cortex, encompassing the superior frontal gyrus (Brodmann Area, BA10) and middle frontal gyrus (BA 11), with a peak in the superior frontal gyrus (MNI x,y,z = 21, 55, 6), and a small separate cluster in the right premotor cortex (BA 6) (MNI ,y,z =: 31, 14, 63), indicating a greater activation for time vs. color can be predicted by better behavioral performance in the time than in color task (Figure 2). Post-hoc examination revealed that these effects were significant when examined with a non-parametric Spearman rank correlation [right superior frontal gyrus: ρ = 0.741, p< 0.0001; right putamen: ρ = 0.631, p< 0.001; right middle frontal gyrus (BA6): ρ = 0.56, p< 0.005] (Schwarzkopf, de Haas & Rees, 2012), ruling out the possible influence from outliers. However, this effect was only found when regressing with difference-scores from the supra-second interval range. No significant peaks or clusters were found when regressing sub-second difference scores at a matched threshold of activation. Additionally, we note that several regions not implicated by our prior meta analysis also exhibited significant correlation (p<0.001, uncorrected) with performance, yet did not survive FWE cluster-wise correction. These areas included the left superior (BA 22) and right inferior (BA20) temporal gyri (left MNI x,y,z: −52, 41, −91 [ρ = 0.578, p< 0.005]; right: 52, −31, −12 [ρ = 0.424, p< 0.05]), and the left parahippocampal gyrus (BA 28) (MNI x,y,z: −28, −21, −3 [ρ = 0.541, p< 0.005]). No significant peaks or clusters were found when examining the reverse contrast of [COLOR vs. TIME] associated with better performance on the COLOR than TIME task, at either corrected or uncorrected thresholds.
Figure 2.
Timing specific differenpwun activation tied to subject performance. Individual differences were examined ay regressing the difference in accuracy between TIME and COLOR tasks with the difference in activation. Activation in right prefrontal cortex and bilateral basal ganglia were associated with better performance on the TIME relative to the COLOR task. Right panels indicate significant correlations between behavioral difference scores from the supra-second version of the task and parameter estimates (β values) in the right superior frontal gyrus (SFG) and right putamen, where basal ganglia differences peaked. Both correlations were also significant with non-parametric Spearman correlations, suggesting that neither effect crucially relied on outliers. Effects displayed are significant at a height threshold of p<0.001, uncorrected, and a cluster threshold of p<0.05 FWE corrected with SVC in regions of interest. A.U. = Arbitrary Units.
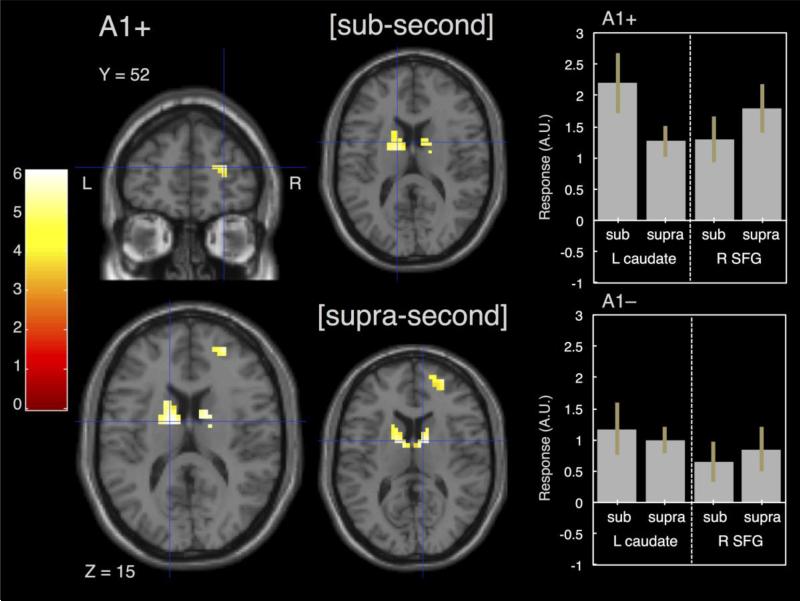
Figure 3.
Effect of genotype on activation during the TIME condition. In a comparison between A1+ and A1- genotypes, only A1+ subjects exhibited significant activation, exclusively in the bilateral basal ganglia and right dorsolateral prefrontal cortex. When dividing this contrast into sub and supra-second duration intervals, A1+ subjects exhibited basal ganglia activation for both ranges, but only right prefrontal activation for supra-second intervals. A1- subjects did not exhibit any differences at either range. All contrasts were masked by the TIME vs. COLOR regression contrast and are significant at p<0.001, uncorrected. Right panels display mean parameter estimates (β values) for A1+ and A1- groups in left caudate and SFG for sub and supra-second intervals, error bars indicate standard error.
Table 1.
Brain regions differentially activated by individual subject performance. Task accuracy effect represents the regression analysis on the TIME vs. COLOR contrast with supra-second behavioral difference scores. Asterisks indicate regions that did not survive cluster-wise correction (FWE p<0.05 in combination with a voxel-wise threshold at P<0.001, uncorrected), either within or outside regions of interest.
| Location | hemisphere | x | y | z | t-score | Volume |
|---|---|---|---|---|---|---|
| Task accuracy effect [TIME - COLOR] | ||||||
| Superior Frontal Gyrus (BA 10) | R | 21 | 55 | 6 | 6.02 | 82 |
| Middle Frontal Gyrus (BA 11) | R | 28 | 41 | −9 | 5.34 | |
| Superior Temporal Gyrus (BA 22)* | L | −52 | −7 | −6 | 4.99 | 12 |
| Putamen | R | 21 | 0 | 21 | 4.83 | 249 |
| Putamen | L | −14 | 0 | 6 | 4.81 | |
| Lateral Globus Pallidus | R | 14 | 3 | 6 | 4.81 | |
| Middle Frontal Gyrus (BA 6) | R | 31 | 14 | 63 | 4.43 | 13 |
| Parahippoca mpal Gyrus (BA 28)* | L | −28 | −21 | −3 | 4.2 | 10 |
| Inferior Temporal Gyrus (BA 20)* | R | 52 | −31 | −12 | 4.09 | 18 |
Subsequently, the effect of genotype the (i.e., DRD2/ANKK1-Taq1a polymorphism) on time processing was examined. To this end, candidate brain regions were delineated from the results of the regression analysis described above. We found that subjects with the A1+ genotype exhibited significantly greater activation in the bilateral basal ganglia, centered in the left caudate (MNI x,y,z = −14, −3, 15), and right superior frontal gyrus (MNI x,y,z = 28, 52, 15), whereas A1- subjects exhibited no significant increases in these regions (Figure 3). For the main effect of COLOR, we used a contrast from the multiple regression analysis examining voxels with increased activation associated with better performance on the COLOR than TIME task, with a very relaxed threshold (height p<0.05) in order to find significant voxels, as a mask for genotype effects. No significant differences were found for the main effect of COLOR for A1+ or A1- subjects.
For our exploratory analysis of duration range effects on genotype, we repeated the above analysis for the main effect of TIME, but only in those voxels that were modulated by our duration range covariate for either sub-second or supra-second intervals. When separately examining the main effect of TIME for A1+ and A1- subjects at sub-second afcl supra-second ranges, we found that the basal ganglia clusters were significant for A1+ subjects in both the sub-second and supra-second conditions (Figure 3), with peaks in the left putamen (MNI x,y,z : −21, 0, 21) and right caudate (MNI x,y,z : 10, 0, 9,); in contrast, the right dorsolateral prefrontal cortex, across superior and middle frontal gyri (peak MNI coordinate: 31, 52, 0) was found for the supra-second and not sub-second duration range. No significant increases in either region were found for A1-carriers. No significant increases were found for A1+ carriers relative to A1- carriers or vice versa.
3.3. VBM Data
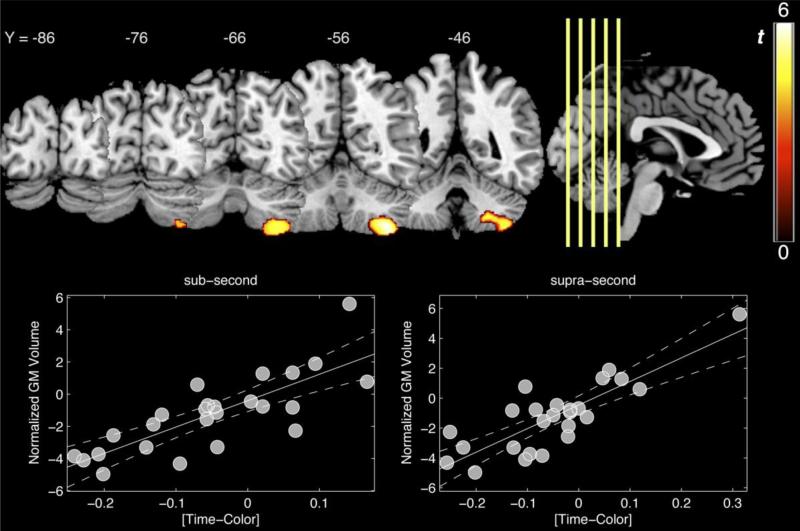
The results of the VBM analysis revealed a single, significant cluster in the right cerebellum (Figure 4). The peak of this cluster [MNI x,y,z: 38, −51, −57, t-score = 5.49, volume = 1620] was located in the lobule Villa according to the probabilistic cerebellar atlas of Diedrichsen and colleagues (2009) [probability = 0.72]. Accordingly, larger relative cerebellar volume in this cluster was associated with greater performance on the TIME relative to the COLOR task. When comparing sub-second and supra-second durations, performance at both duration ranges were significantly associated with greater cerebellar volume (Figure 4); both effects were significant with Spearman rank correlations [sub-second: ρ = 0.78, p< 0.0001; supra-second: ρ = 0.727, p< 0.0001]. No significantly greater effects were found for better COLOR relative to TIME performance.
Figure 4.
Differences in voxel-based morphometry associated with better relative performance on the timing as compared to the color task. A single cluster in the right cerebellum, centered on lobule Villa, exhibited a significant relationship between gray matter (GM) volume and performance. Bottom panels indicate significant correlations between normalized GM volume and behavioral difference scores. GM values in this region were positively associated with better timing performance at both duration ranges. Both correlations were also significant with non-parametric Spearman correlations, suggesting that neither effect crucially relied on outliers. Significant voxels are displayed at a height threshold of p<0.001, uncorrected, and a cluster threshold of p<0.05 FWE corrected.
For our exploratory analysis of genotypic effects, subjects were segregated according to DRD2/ANKK1-Taq1a status; A1+ subjects exhibited lower cerebellar volume than A1- subjects in the cluster identified by the regression analysis [t(24) = 2.194,p= 0.039, η2 = 0.173]. To summarize, A1+ exhibited both reduced behavioral performance on the timing relative to the color task at both duration ranges, and lower cerebellar volume in an area associated with better performance on the timing task relative to the color control task.
4. Discussion
4.1. Main Findings
We investigated the neural regions associated with heterogeneous performance on a perceptual timing task. Substantial differences in performance were observed, with some subjects performing well, and others performing close to chance. Furthermore, on the basis of behavioral and neural data suggesting differential processing as a function of interval duration range (Lewis & Miall, 2003; Wiener, et al. 2010), we employed both sub- and supra-second intervals; we found that subjects performed better on both the perceptual timing and color control task when the interval range was long (>1s). When regressing the difference between timing and color tasks against differences in BOLD signal derived from a standard contrast of timing and control task activity, we found bilateral regions of the basal ganglia (caudate and putamen) and a cluster in the right dorsolateral prefrontal cortex that covaried with performance. Furthermore, these differences were only found for performance at longer duration ranges. In a similar analysis of structural differences, we found that better performance on the timing task than the color task at both duration ranges correlated with volume within a large cluster of the right cerebellum, despite the fact that no BOLD differences were observed in this region. These results provide greater contextualization of the regions likely to be active across different temporal task contexts, and provide further evidence regarding brain regions that are crucial for timing.
4.2. Behavioral heterogeneity
The present study employed a task that has been successful at disentangling working memory and attentional effects from time perception processes in neuroimaging (Coull, et al. 2004; 2008; 2012; Livesey, et al. 2007; Morillon, et al. 2009). The time-color task is useful in that both tasks require the subject to sustain attention and integrate information over time in order to reach a decision, using the same perceptual stimuli in both conditions; as such, non-temporal demands are equal between both tasks. Our experiment differed from previous work, however in that we employed a different range of durations, centered on two local maxima (500 and 2000 ms). Previous studies utilizing the time-color task have either used exclusively supra-second (Livesey, et al. 2007; Gilaie-Dotan, et al. 2011) intervals, or collapsed across sub and supra-second ranges (Coull, et al. 2004; 2008; 2009; 2012; Morillon, et al. 2009); as such, our study is the first of which we are aware to examine performance on the time-color task at separate duration ranges. Like previous investigators (e.g., Gilaie-Dotan, et al. 2011), we found that performance on the time and color tasks do not correlate, suggesting that time and color perception are at least partially independent. Consistent with this claim we found a strong correlation between performance with sub and supra-second intervals for the color task, but only a trend for correlation between timing performance at different intervals. These findings suggest that performance on the color task relies on a similar mechanism for sub- and supra-second intervals, whereas the timing task may involve separate mechanisms (Wiener, et al. 2011; Gooch, et al. 2011).
4.3. Neural heterogeneity
In the present experiment, when covarying for subject performance on the time minus color task with the fMRI contrast for the same conditions, we found significant voxels in a large cluster covering the basal ganglia bilaterally, as well as the right dorsolateral prefrontal cortex, covering the superior and middle frontal gyri. Both of these regions have been strongly implicated in time perception processes, across a wide variety of task contexts (Wiener, et al. 2010). Intriguingly, activation in these regions was only revealed when covarying for activity in the supra-second version of the task, rather than the sub-second. It is important to note that there are three key differences between the supra and sub-second conditions. First, subjects performed less well on the sub-second as compared to the supra-second condition, thereby reducing power to observe an effect. Secondly, whereas the duration was varied every 200 ms among the six intervals for the supra-second condition, it was varied only every 50 ms for the sub-second condition. Third, the overall duration of a trial was shorter for the sub-second than supra-second condition, thereby potentially reducing BOLD signal in this condition (Pfeuffer, et al. 2003). Together, these factors may have obscured the differential activity in response to the sub-second condition. Future studies are warranted to directly compare supra vs. sub-second timing differences.
Activation of the basal ganglia and right prefrontal cortex in the present study is consistent with previous work demonstrating activation in these regions tied to temporal processing. We found that activation across bilateral caudate and putamen correlated with greater accuracy on the timing than color task, with peak activation in the right putamen, (cf, Coull, et al. 2008). The basal ganglia have been afforded a critical role in many models of time perception (Buhusi & Meek, 2005; Coull, Cheng & Meek, 2010). Matell and Meck's (2004) Striatal Beat Frequency (SBF) model posits the striatum as a coincidence-detection mechanism that tracks the similarity of oscillating cortical input to previously experienced durations. Consistent with this theory, diseases of the basal ganglia, such as Parkinson's and Huntington's disease show disruptions on temporal perception tasks, with greater impairments found with greater disease progression (Allman & Meek, 2012). In a recent study utilizing the same time-color paradigm used here, Coull and colleagues (2012) demonstrated that dopamine precursor depletion via APTD similarly reduced striatal activation and impaired behavioral performance. The correlation with accuracy and basal ganglia activity in the present study may thus relate to the ability of the striatum to retain temporal intervals in memory.
In addition to striatal activation, we also found a significant cluster in the right dorsolateral prefrontal cortex, encompassing the middle and superior frontal gyri (BA 10/11), and a second cluster in the right middle frontal gyri, across the premotor cortex (BA 6) where greater activation was associated with better performance on the timing task relative to the color task. Activation in these regions has been demonstrated on time-color tasks previously (Coull, et al. 2012; Morillon, et al., 2009; Livesey, et al., 2007). The right dorsolateral prefrontal cortex may be related to decision-making components for temporal information; Morillon and colleagues (2009), using the time-color task, found activation in this region corresponding to an interaction between duration length and correct decisions on the timing task, which they termed the ‘counting’ system. Similarly, Brunia and colleagues (2000), using positron emission tomography (PET), observed right prefrontal activation associated with feedback on a time estimation task, suggesting that this region corrects and improves temporal responses. Finally, using a temporal discrimination task with both sub- and supra-second stimuli and a carefully designed covariate for temporal comparison activity, we (Wencil et al, 2010) found prefrontal activation associated with better performance. Similarly, lesions of the right prefrontal cortex found in stroke patients have been associated with poor perceptual timing performance (Gooch, et al. 2011) and TMS of the right, but not left, dorsolateral prefrontal cortex disrupts temporal reproduction abilities specifically related to comparison processes (Jones, et al. 2004). The right premotor cortex (BA6), has also been implicated in perceptual timing tasks (Wiener, et al. 2010) – Coull and colleagues (2012) found activation within this same region during the measurement of comparison duration stimuli in the time-color task. However, we note that this cluster was not significant when tested with a non-parametric correlation, and so should be interpreted with caution.
In addition to timing regions explicitly investigated, we note that several other regions were also activated in the regression contrast with timing performance. These areas included bilateral regions of the temporal cortex, including the left superior temporal gyrus and right inferior temporal gyrus, as well as a small sub-cortical cluster approximately in the left parahippocampal gyrus; although these regions were outside our a-priori regions of interest and only significant at a lower cluster threshold, these regions warrant discussion in the context of time perception performance. Notably, these regions have also been implicated in time perception when covarying for subject performance. Harrington and colleagues (2004a) noted a post-hoc positive correlation between accuracy on a temporal bisection task and activity in the right parahippocampal gyrus. In contrast, Gilaie-Dotan and colleagues (2011) noted a negative correlation between temporal discrimination accuracy and gray-matter volume in the bilateral parahippocampal gyrus, as well as a positive correlation between accuracy and gray-matter volume in the right primary auditory cortex; this effect was found for both auditory and visual stimuli. The involvement of the parahippocampal gyrus suggests the recruitment of mnemonic processes during temporal performance. The hippocampus has also received renewed attention in animal studies of temporal processing, due to the existence of so-called ‘time-cells’ that appear to track delay lengths and may provide a metric for measuring time (MacDonald et al. 2011). Similarly, in a previous neuroimaging study employing the same time-color task used here, activity was reported in the left parahippocampal gyrus that parametrically varied with increases in stimulus duration (Morillon, et al. 2009). The association of the left parahippocampal gyrus in the present experiment with subject performance may thus be related to the ability of individual subjects to track the remembered duration of presented stimuli. For the auditory cortex activations also observed in the present study, we note that these regions have also been found in previous neuroimaging studies using the time-color task (Morillon, et al. 2009; Coull, et al. 2008; 2012). The involvement of auditory cortex regions may at first seem unexpected given the visual nature of both tasks. However, recent studies have suggested that the auditory cortex may serve as a supramodal timing system. Consistent with this are findings that auditory cortex disruption with TMS impacts both visual and auditory temporal perception (Kanai, et al. 2011), and that auditory stimuli dominate temporal percepts when both auditory and visual information are present (Guttmarm, Gilroy & Blake, 2005). Activation in these regions correlated with accuracy on the timing task may thus relate to the ability of subjects to leverage this supramodal system for inputting duration information.
Notably absent in the present study is any association with accuracy and activation of the SMA. Most studies utilizing the time-color task have demonstrated SMA activation during timing (Coull, et al. 2004; 2008; 2012; Morillon, et al. 2009), and the SMA is most commonly activated during time perception tasks (Wiener, et al. 2010). The SMA has been suggested to serve as a temporal accumulator that indexes the elapsed passage of time during an interval (Casini & Vidal, 2011), and is related to the speed of a hypothetical internal clock (Gibbon, Church & Meek, 1984). One possibility for the absence of an effect of SMA in the present study is the nature of our analysis. In the present study, we covaried differences between subjects in behavioral performance with neural activation. Our use of a difference score tested for areas corresponding to better timing-specific performance over color-specific performance. As such, differential activation in the SMA between subjects may not have been predictive of the relative difference in performance between these two tasks and across individuals. Indeed, Coull and colleagues (2012) found that APTD-induced decreases in timing performance on the time-color task decreased SMA activity within subjects on the time task. Similarly, we have recently demonstrated that TMS to a parietal region shown to increase subjective duration within-subject is also associated with larger frontocentral negativity in EEG recordings, consistent with an increase in accumulator output (Wiener, et al. 2012). While this difference may still be difficult to reconcile, we note that our findings using difference scores are consistent with many other neuroimaging studies of timing that exhibit frontostriatal activity in conjunction with time perception (Wiener, et al. 2010).
We also note that the rIFG, a region also highly linked to time perception activity and performance, was not active in the present study. It remains possible that the rIFG activity implicated in our meta-analysis and the superior/middle frontal gyrus activity in the present study represent the same cognitive process; future studies will be necessary to determine the similar (or different) roles of these regions.
A second goal of our study was to examine morphometric differences that may account for individual differences in behavior. In recent years, voxel-based morphometry has been utilized to demonstrate associations between gray-matter volume and individual variations in a number of distinct cognitive and perceptual functions (Kanai & Rees, 2011; Schwartz et al, 2011, 2012). In the present study, we found a single large cluster associated with performance in the right lateral cerebellum (lobule VIIIa). Within this cluster, there was an association between larger cerebellar volume and better timing as compared to color performance at both sub-second and supra-second duration ranges. The cerebellum has long been implicated in timing performance (Ivry & Keele, 1989), although its exact role remains controversial (Harrington, et al. 2004b). Previous neuroimaging (Wiener, et al. 2010; Aso, et al. 2010) evidence has suggested that the cerebellum is activated during both motor and perceptual timing studies, and is exclusively activated during sub-second processing. However, recent work has demonstrated that patients with cerebellar stroke also exhibit supra-second timing deficits (Gooch, et al. 2010), particularly for shorter supra-second stimuli (~2s). In a recent study examining the effects of training on a time perception task, Bueti and colleagues (2012) demonstrated that four weeks of training led to increases in cerebellar volume in a region of the right lateral cerebellum very close to the one reported here (lobule Vila); these increases paralleled improvements in timing performance with training, and suggest that larger cerebellar volume relates directly to the ability of an individual to perform a timing task. Also similar to the present results, Bueti and colleagues (2012) found no activation differences in BOLD signal in the cerebellum. However, we note that a possible explanation for the discrepancy between fMRI and VBM results in the present study is that we had a lower BOLD signal quality in the lateral regions of the cerebellum, where the VBM result was located (supplementary materials). VBM, which is based on T1-weighted structural images, may thus be more sensitive to differences in the cerebellum. Future fMRI studies may focus exclusively on the cerebellum to interrogate differences between BOLD and VBM results (Spencer, et al. 2007). We also note that another previous VBM study of time perception did not find cerebellar differences associated with performance (Gilaie-Dotan, et al. 2011); however, in this study the authors did not use a difference measure as in the present study. Differences in cerebellar volume may thus relate to the ability to perform better on a timing task than a control task. Our findings suggest that cerebellar volume may be a useful metric for predicting differences in timing performance in disorders such as autism and attention deficit hyperactivity disorder (Allman & Meek, 2012).
4.4. Genetic influence on timing
As described above, numerous factors contribute to timing performance. We have previously demonstrated that functional variants in genes regulating dopamine homeostasis are associated with inter-individual differences in perceptual timing performance (Wiener, et al. 2011). The DRD2/ANKK1-Taq1a polymorphism was associated with a decrease in temporal precision for sub-second, but not supra-second stimuli. Given that the Taq1a polymorphism regulates the encoding of striatal D2 receptors, with a single copy of the A1 allele leading to a 30-40% reduction in striatal D2 density (Jönsson, et al. 1999), we assessed in the present experiment, the implications of the Taq1a polymorphism for interval timing. When our sample was divided into A1- and A1+ groups, we found a number of differences in both behavioral and neural measures.
We found that A1+, but not A1-, subjects performed worse with respect to accuracy on the time as compared to the color task; A1+ subjects thus predominantly exhibited negative difference scores in the time-color behavioral contrast. This finding is similar to previous pharmacological work showing that APTD-induced decreases in dopamine precursors (Coull, et al. 2012), as well as ketamine administration (Coull, et al. 2009) reduce performance only on the time, but not color condition of the time-color task, further suggesting that, although both tasks use identical stimuli, the attention to one dimension or another crucially relies on separate circuits. We further note that the over-representation of A1+ subjects in our sample may explain the difference between performance ontB^tmie and color tasks; that is, as the 15 A1+ subjects performed less well with time as compared to color processing, their performance may have biased the group results. Consistent with our earlier findings (Wiener, et al. 2011), A1+ subjects performed relatively worse at sub-second as opposed to supra-second intervals. However, A1+ subjects also performed worse than their A1- counterparts at both sub- and supra-second durations. This result may appear contradictory to our earlier finding that A1+ subjects are preferentially disrupted at sub-second intervals (Wiener, et al. 2011). However, we note that, in the present study, subjects performed both time and color trials, with both duration ranges, within the same block and in pseudo-random order, and so were required to rapidly switch between both tasks, whereas our previous study only tested one task and duration range at a time. Recent research has demonstrated that A1 carriers of the Taq1a polymorphism are impaired in task-switching ability that crucially relies on frontostriatal activity (Stelzel, et al. 2010). Furthermore, A1+ subjects exhibit increased caudate activation during task-switching behavior; as such, one possible explanation for poorer performance across both sub and supra-second intervals is impaired task-switching. However, as A1+ subjects were not impaired on the color task, a task-switching deficit cannot fully account for the timing deficit. This issue may be investigated further in future studies.
For the neural measures, when examining differences between A1- and A1+ subjects in those regions modulated by subject performance and on timing-related activation, we found that A1+ subjects significantly activated the bilateral basal ganglia, centered on the left caudate, and the right superior frontal gyrus (BA10), whereas A1- subjects did not. When duration length was used as a covariate to separate timing-related responses, A1+ subjects exhibited activity in the basal ganglia for sub- and supra-second stimuli but right superior frontal gyrus activation for supra-second durations only. This finding is consistent with Coull and colleagues’ (2012) recent study in which APTD-induced decreases in timing performance also showed a difference in basal ganglia activation specifically for a sub-second interval (540ms). However, we note that APTD led to a decrease in striatal activity, whereas A1+ subjects in the present study exhibited increased activation. One explanation may stem from the difference between an acute pharmacological administration and a lifelong developmental polymorphism – each may affect the same system but in different ways. In a similar vein, patients with Parkinson's disease when scanned in an off-medication state also exhibit greater striatal activation in both motor and perceptual timing tasks, that is attenuated when returned to an on-medication state (Harrington, et al. 2011; Jahanshahi, et al. 2010). Jahanshahi and colleagues (2010) suggested that excessive inhibitory pallidal outflow in the off-medication state led to reduced timing performance that was later renormalized on-medication. Although D2 receptors are inhibitory, and so a decrease in D2 density would be expected to lead to a decrease in pallidal outflow, evidence suggests that the Taq1a polymorphism is specific to D2 autoreceptors projecting from the substantia nigra (Laakso, et al. 2005); a reduction in D2 autoreceptors would thus lead to an increase in dopamine availability at D2 post-synaptic receptors and thus increase inhibitory activation similar to that observed in Parkinson's disease. Similarly, A1+ subjects also exhibit slower mean tapping rates on a motor tempo task (Wiener, et al. 2011).
We acknowledge that the fMRI findings of A1+ subjects may be difficult to reconcile with the multiple regression analysis at first; the regression analysis demonstrated that defter performance on the timing task was associated with greater activation in the basal ganglia and prefrontal cortex, yet A1+ subjects, who are worse at the timing task, show increased activation in these regions. To clarify, we note that the regression analysis examined the correlation between differences in performance on the time and color tasks with the activation difference between the time and color tasks; the A1+ effects examined activation in this group for the main effect of time, without subtracting color task activation. Accordingly, the regression analysis examined relative differences in activation, whereas the genotype analysis examined absolute differences in activation. As such, A1+ subjects show greater absolute levels of activity in these regions for timing, whereas greater relative levels of activity compared to color processing are necessary within these regions for better performance.
The observation that A1+ subjects exhibit increased activation in both striatal and prefrontal cortex for supra-second stimuli suggests that both systems are involved in the processing of longer duration stimuli. This finding is consistent with Hellstrom & Rammsayer's (2002) dual-systems theory, that postulates separate systems for sub- and supra-second timing, but stipulates that the sub-second system will also be utilized during supra-second processing (but not vice-versa). The coinciding involvement of striatum and prefrontal cortex also mirrors well-known connectivity between both regions, as well as their implication in timing processes (Jones & Jahanshahi, 2011). One explanation for increased prefrontal activation during supra-second processing in A1+ subjects is that these subjects required greater activation of this system to compensate for inefficient temporal processing resulting from sub-second striatal dysfunction.
For the morphometric results, A1+ subjects exhibited lower cerebellar volume than A1- subjects within the right cerebellar cluster associated with timing performance for both sub-second and supra-second ranges. Thissurprising result suggests that the effects of the Taq1a polymorphism extend beyond striatal differences, and have long-range effects that impact cerebellar morphometry. Of course, it is difficult to ascribe directional consequences with our data alone, as cerebellar volume may be reduced in these subjects as a consequence of poor timing abilities, rather than a direct genetic link. Nevertheless, disruption of D2 receptor functioning has been shown to have a direct influence on glucose metabolism in the cerebellum (Volkow, et al. 1997), and Parkinson's disease patients show a decrease in cerebellar activation when receiving dopaminergic medication and performing a motor timing task (Jahanshahi, et al. 2010). We suggest that the A1+ findings here demonstrate a widespread impact of reduced D2 function on both activation and morphometry in time perception regions.
We note that, in the present study, we utilized a modest sample size (25 subjects). However, the primary goal of our study was to investigate the neural effects underlying individual differences in timing behavior, for which we believe our sample size is adequate. However, for comparing the results between different genotypes, we encourage caution in extrapolating our results to much larger sample sizes without further testing. Nevertheless, our results do fit within the larger and predicted framework of temporal processing, and provide additional testable hypothesis that may be further evaluated. Furthermore, we note that the false-positive rate in imaging genetics is relatively low, suggesting that standard significance-correction techniques in neuroimaging can control for spurious findings when comparing genetic polymorphisms (Meyer-Lindenberg, et al. 2008); additionally, we note that the sample size used in the present study is within the range of other neuroimaging studies investigating the DRD2/ANKK1 Taq1a polymorphism that found similar results (Stelzel, et al. 2010; Jocham, et al. 2009; Klein, et al. 2007; Fossella, et al. 2006).
4.5. Conclusions
Understanding individual differences is a crucial challenge for cognitive neuroscience. Central to this is fractionating inter-individual variance into separable components. In the present study, we demonstrate that the wide variety of performance seen in perceptual timing may be tied to individual differences in the recruitment of frontostriatal circuitry, as well as the morphometry of the lateral cerebellum. Furthermore, we demonstrate here for the first time that developmental genetic polymorphisms affecting the dopamine system are associated with differences in frontostriatal activation and cerebellar volume. Furthermore, these differences may have different implications for sub- and supra-second temporal processing. These findings contribute to the understanding of the neural mechanisms utilized for temporal processing, and provide possible connections to neuropsychological and psychiatric disorders in which timing disruptions are present.
Supplementary Material
Highlights.
We investigated the neural bases of individual differences in time perception.
Individuals were additionally genotyped for the DRD2/ANKK1-Taq1a polymorphism.
Activity in striatum and prefrontal cortex was associated with better performance.
Greater cerebellar volume was also associated with better performance.
Differences within these regions were found for Taq1a carriers.
Table 2.
Results of the genotype analysis on the main effect of TIME are shown for combined, and individual sub- and supra-second interval conditions. Asterisks indicate regions that did not survive cluster-wise correction (FWE p<0.05 in combination with a voxel-wise threshold at P<0.001, uncorrected), either within or outside regions of interest.
| Location | hemisphere | x | y | z | t-score | Volume |
|---|---|---|---|---|---|---|
| Main effect of TIME | ||||||
| A1+ vs A1- | ||||||
| Caudate Body | L | −14 | −3 | 15 | 5.65 | 136 |
| Putamen | R | 21 | 3 | 18 | 5.62 | |
| Putamen | L | −21 | 0 | 18 | 5.34 | |
| Superior Frontal Gyrus (BA 10) | R | 28 | 52 | 15 | 5.02 | 14 |
| Superior Frontal Gyrus (BA 10) | R | 28 | 58 | 6 | 4.2 | |
| Parahippoca mpal Gyrus (BA 28)* | L | −24 | −21 | −6 | 4.99 | 10 |
| sub-second A1+ vs A1- | ||||||
| Putamen | L | −21 | 0 | 21 | 5.75 | 47 |
| Caudate Body | L | −14 | −3 | 15 | 5.1 | |
| Lentiform Nucleus Lateral Globus Pallidu | L | −14 | −3 | 6 | 4.74 | |
| Putamen | R | 21 | 3 | 18 | 5.46 | 33 |
| Thalamus Ventral Anterior Nucleus | R | 17 | −7 | 15 | 4.31 | |
| Caudate Body | R | 14 | 10 | 12 | 3.9 | |
| supra-second A1+ vs A1- | ||||||
| Caudate Body | R | 10 | 0 | 9 | 5.92 | 179 |
| Caudate Body | L | −14 | 3 | 15 | 5.63 | |
| L | −7 | −3 | 0 | 5.39 | ||
| Middle Frontal Gyrus (BA 10) | R | 31 | 52 | 0 | 5.9 | 38 |
| Superior Frontal Gyrus (BA 10) | R | 28 | 52 | 15 | 5.09 | |
| Superior Frontal Gyrus (BA 10) | R | 21 | 58 | 12 | 4.21 | |
| Inferior Temporal Gyrus (BA 20)* | R | 52 | −31 | −15 | 5.17 | 16 |
| Sub-Gyral Hippocampus* | L | −31 | −21 | −6 | 4.79 | 10 |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen EA, Erhardt EB, Calhoun VD. Data Visualization in the Neurosciences: Overcoming the Curse of Dimensionality. Neuron. 2012;74(4):603–608. doi: 10.1016/j.neuron.2012.05.001. doi:10.1016/j.neuron.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allman MJ, Meek WH. Pathophysiological distortions in time perception and timed performance. Brain. 2012;135(3):656–677. doi: 10.1093/brain/awr210. doi:10.1093/brain/awr210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. Neurolmage. 2007;38(1):95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Aso K, Hanakawa T, Aso T, Fukuyama H. Cerebro-cerebellar interactions underlying temporal information processing. Journal of Cognitive Neuroscience. 2010;22(12):2913–2925. doi: 10.1162/jocn.2010.21429. [DOI] [PubMed] [Google Scholar]
- Balci F, Wiener M, Cavdaroglu B, Coslett HB. Epistasis effects of dopamine genes on interval timing and reward magnitude in humans. Neuropsychologia. 2013;51(2):293–308. doi: 10.1016/j.neuropsychologia.2012.08.002. [DOI] [PubMed] [Google Scholar]
- Berryhill ME, Wiener M, Stephens JA, Lohoff FW, Coslett HB. Zhang H, editor. COMT and ANKK1-Taq-Ia Genetic Polymorphisms Influence Visual Working Memory. PLoS ONE. 2013;8(1):e55862. doi: 10.1371/journal.pone.0055862. doi: 10.137 l/journal.pone.0055862.g004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunia CHM, de Jong BM, van den Berg-Lenssen MMC, Paans AMJ. Visual feedback about time estimation is related to a right hemisphere activation measured by PET. Experimental Brain Research. 2000;130(3):328–337. doi: 10.1007/s002219900293. doi:10.1007/s002219900293. [DOI] [PubMed] [Google Scholar]
- Brown SW, Newcomb DC, Kahrl KG. Temporal-signal detection and individual differences in timing. Perception. 1995;24(5):525–538. doi: 10.1068/p240525. [DOI] [PubMed] [Google Scholar]
- Buhusi CV, Meek WH. What makes us tick? Functional and neural mechanisms of interval timing. Nature Reviews Neuroscience. 2005;6(10):755–765. doi: 10.1038/nrn1764. doi:10.1038/nrnl764. [DOI] [PubMed] [Google Scholar]
- Bueti D, Lasaponara S, Cercignani M, Macaluso E. Learning about time: plastic changes and interindividual brain differences. 2012;75(4):725–737. doi: 10.1016/j.neuron.2012.07.019. [DOI] [PubMed] [Google Scholar]
- Carlson VR, Feinberg I. Individual variations in time judgment and the concept of an internal clock. Journal of Experimental Psychology. 1968;77(4):631–640. doi: 10.1037/h0026048. [DOI] [PubMed] [Google Scholar]
- Coull JT, Vidal F, Nazarian B, Macar F. Functional anatomy of the attentional modulation of time estimation. Science. 2004;303:1506–1508. doi: 10.1126/science.1091573. [DOI] [PubMed] [Google Scholar]
- Coull JT, Nazarian B, Vidal F. Timing, storage, and comparison of stimulus duration engage discrete anatomical components of a perceptual timing network. Journal of Cognitive Neuroscience. 2008;20:2185–2197. doi: 10.1162/jocn.2008.20153. [DOI] [PubMed] [Google Scholar]
- Coull JT, Cheng R-K, Meek WH. Neuroanatomical and Neurochemical Substrates of Timing. Neuropsychopharmacology. 2010;36(1):3–25. doi: 10.1038/npp.2010.113. doi:10.1038/npp.2010.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull JT, Hwang HJ, Leyton M, Dagher A. Dopamine Precursor Depletion Impairs Timing in Healthy Volunteers by Attenuating Activity in Putamen and Supplementary Motor Area. Journal of Neuroscience. 2012;32(47):16704–16715. doi: 10.1523/JNEUROSCI.1258-12.2012. doi:10.1523/JNEUROSCI.1258-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull JT, Morgan H, Cambridge VC, Moore JW, Giorlando F, Adapa R, Corlett PR, et al. Ketamine perturbs perception of the flow of time in healthy volunteers. Psychopharmacology. 2011;218(3):543–556. doi: 10.1007/s00213-011-2346-9. doi:10.1007/s00213-0112346-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E, Behr MK, Kanwisher N. Functional specificity for high-level linguistic processing in the human brain. Proceedings of the National Academy of SciencMSA. 2011;108(39):16428–16433. doi: 10.1073/pnas.1112937108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feredoes E, Postle BR. Localization of load sensitivity of working memory storage: Quantitatively and qualitatively discrepant results yielded by single-subject and group-averaged approaches to fMRI group analysis. Neurolmage. 2007;35(2):881–903. doi: 10.1016/j.neuroimage.2006.12.029. doi: 10.1016/j.neuroimage.2006.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feredoes E, Tononi G, Postle BR. The Neural Bases of the Short-Term Storage of Verbal Information Are Anatomically Variable across Individuals. Journal of Neuroscience. 2007;27(41):11003–11008. doi: 10.1523/JNEUROSCI.1573-07.2007. doi: 10.1523/JNEUROSCI.1573-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossella J, Green AE, Fan J. Evaluation of a structural polymorphism in the ankyrin repeat and kinase domain containing 1 (ANKK1) genet and the activation of executive attention networks. Cogn Affect Behav Neurosci. 2006;6(1):71–78. doi: 10.3758/cabn.6.1.71. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Poline J-B, Grasby PJ, Williams SCR, Frackowiak RSJ, Turner R. Analysis of fMRI Time-Series Revisited. Neurolmage. 1995;2(1):45–53. doi: 10.1006/nimg.1995.1007. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Josephs O, Rees G, Turner R. Nonlinear event-related responses in fMRI. Magnetic Resonance In Medicine. 1998;39(1):41–52. doi: 10.1002/mrm.1910390109. [DOI] [PubMed] [Google Scholar]
- Gibbon J, Church RM, Meek WH. Scalar timing in memory. Annals of the New York Academy of Sciences. 1984;423:52–77. doi: 10.1111/j.1749-6632.1984.tb23417.x. [DOI] [PubMed] [Google Scholar]
- Gilaie-Dotan S, Kanai R, Rees G. Anatomy of human sensory cortices reflects inter-individual variability in time estimation. Frontiers in Integrative Neuroscience. 2011;5(76) doi: 10.3389/fnint.2011.00076. doi: 10.3389/fnint.2011.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooch CM, Wiener M, Wencil EB, Coslett HB. Interval timing disruptions in subjects with cerebellar lesions. Neuropsychologia. 2010;48(4):1022–1031. doi: 10.1016/j.neuropsychologia.2009.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooch CM, Wiener M, Hamilton CA, Coslett HB. Temporal discrimination of sub- and suprasecond time intervals: a voxel-based lesion mapping analysis, Frontiers in Integrative. Neuroscience. 2011:1–10. doi: 10.3389/fnint.2011.00059. doi: 10.3389/fnint.2011.00059/abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AE, Munafo MR, DeYoung CG, Fossella JA, Fan J, Gray JR. Using genetic data in cognitive neuroscience: from growing pains to genuine insights. Nature Reviews Neuroscience. 2008;9:710–720. doi: 10.1038/nrn2461. [DOI] [PubMed] [Google Scholar]
- Grahn JA, McAuley JD. Neural bases of individual differences in beat perception. Neurolmage. 2009;47(4):1894–1903. doi: 10.1016/j.neuroimage.2009.04.039. [DOI] [PubMed] [Google Scholar]
- Grahn JA, Parkinson JA, Owen AM. The cognitive functions of the caudate nucleus. Progress in Neurobiology. 2008;86:141–155. doi: 10.1016/j.pneurobio.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Guttman SE, Gilroy LA, Blake R. Hearing what the eyes see. Auditory encoding of visual temporal sequences. Psychological Sciences. 2005;16(3):228–235. doi: 10.1111/j.0956-7976.2005.00808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock PA. On the left hand of time. American Journal of Psychology. 2011;124(2):177–188. doi: 10.5406/amerjpsyc.124.2.0177. [DOI] [PubMed] [Google Scholar]
- Harrington DL, Boyd LA, Mayer AR, Sheltraw DM, Lee RR, Huang M, Rao SM. Neural representation of interval encoding and decision making. Cognitive Brain Research. 2004a;21(2):193–205. doi: 10.1016/j.cogbrainres.2004.01.010. doi:10.1016/j.cogbrainres.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Harrington DL, Lee RR, Boyd LA, Rapcsak SZ, Knight RT. Does the representation of time depend on the cerebellum? Effect of cerebellar stroke. Brain. 2004b;127:561–574. doi: 10.1093/brain/awh065. [DOI] [PubMed] [Google Scholar]
- Harrington DL, Zimbelman JL, Hinton SC, Rao SM. Neural Modulation of Temporal Encoding, Maintenance, and Decision Processes. Cerebral Cortex. 2010;20(6):1274–1285. doi: 10.1093/cercor/bhp194. doi:10.1093/cercor/bhpl94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington DL, Castillo GN, Greenberg PA, Song DD, Lessig S, Lee RR, Rao SM. Neurobehavioral mechanisms of temporal processing deficits in Parkinson's disease. PLoS One. 2011;6(2):el7461. doi: 10.1371/journal.pone.0017461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellström A, Rammsayer TH. Mechanisms behind discrimination of short and long auditory durations. In: Da Silva JA, Matsushima EH, Ribeiro Filho NP, editors. Fechner Day 2002. Eighteenth annual meeting of the international society for psychophysics. International Society for Psychophysics; Rio de Janeiro, Brazil: 2002. [Google Scholar]
- Hinton SC, Harrington DL, Binder JR, Durgerian S, Rao SM. Neural systems supporting timing and chronometric counting: an FMRI study. Cognitive Brain Research. 2004;21(2):183–192. doi: 10.1016/j.cogbrainres.2004.04.009. doi:10.1016/j.cogbrainres.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Hirvonen MM, Lumme V, Hirvonen J, Pesonen U, Nagren K, Vahlberg T, Scheinin H, et al. C957T polymorphism of the human dopamine D2 receptor gene predicts extrastriatal dopamine receptor availability in vivo. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2009;33(4):630–636. doi: 10.1016/j.pnpbp.2009.02.021. doi:10.1016/j.pnpbp.2009.02.021. [DOI] [PubMed] [Google Scholar]
- Ivry RB, Keele SW. Timing functions of the cerebellum. Journal of Cognitive Neuroscience. 1989;1:136–152. doi: 10.1162/jocn.1989.1.2.136. [DOI] [PubMed] [Google Scholar]
- Jahanshahi M, Jones CR, Zijlmans J, Katzenschlager R, Lee L, Quinn N, Frith CD, Lees AJ. Dopaminergic modulation of striato-frontal connectivity during motor timing in Parkinson's disease. Brain. 2010;133(3):727–745. doi: 10.1093/brain/awq012. [DOI] [PubMed] [Google Scholar]
- Jocham G, Klein TA, Neumann J, von Cramon DY, Reuter M, Ullsperger M. Dopamine DRD2 polymorphism alters reversal learning and associated neural activity. J. Neurosci. 2009;29(12):3695–3704. doi: 10.1523/JNEUROSCI.5195-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CG, Rosenkranz K, Rothwell J, Jahanshahi M. The right dorsolateral prefrontal cortex is essential in time reproduction: an investigation with repetitive transcranial magnetic stimulation. Experimental Brain Research. 2004;158(3) doi: 10.1007/s00221-004-1912-3. doi: 10.1007/s00221-004-1912-3. [DOI] [PubMed] [Google Scholar]
- Jones CR, Jahanshahi M. Dopamine modulates striato-frontal functioning during temporal processing. Frontiers in Integrative Neuroscience. 2011;5:70. doi: 10.3389/fnint.2011.00070. doi: 10.3389/fnint.2011.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jönsson EG, Nothen MM, Grunhage F, Farde L, Nakashima Y, Propping P, Sedvall GC. Polymorphisms in the dopamine D2 receptor gene and their relationships to striatal dopamine receptor density of healthy volunteers. Molecular Psychiatry. 1999;4:290–296. doi: 10.1038/sj.mp.4000532. [DOI] [PubMed] [Google Scholar]
- Josephs O, Henson RN. Event-related functional magnetic resonance imaging: modeling, inference and optimization. Philosophical Transactions of the Royal Society of London B Biological Sciences. 1999;354(1387):1215–1228. doi: 10.1098/rstb.1999.0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai R, Rees G. The structural basis of inter-individual differences in human behaviour and cognition. 2011:1–12. doi: 10.1038/nrn3000. doi:10.1038/nrn3000. [DOI] [PubMed] [Google Scholar]
- Kanai R, Lloyd H, Bueti D, Walsh V. Mfcdally-independent role of the primary auditory cortex in time estimation. Experimental Brain Research. 2011;209(3):465–471. doi: 10.1007/s00221-011-2577-3. doi: 10.1007/s00221-011-2577-3. [DOI] [PubMed] [Google Scholar]
- Klein TA, Neumann J, Reuter M, Hennig J, von Cramon DY, Ullsperger M. Genetically determined differences in learning from errors. Science. 2007;318:1642–1645. doi: 10.1126/science.1145044. [DOI] [PubMed] [Google Scholar]
- Laakso A, Pohjalainen T, Bergman J, Kajander J, Haaparanta M, Solin O, Syvalahti E, Hietala J. The Al allele of the human D2 dopamine receptor gene is associated with increased activity of stratal L-amino acid decarboxylase in healthy subjects. 2005. [DOI] [PubMed]
- Lewis PA, Miall RC. Distinct systems for automatic and cognitively controlled time measurement: evidence from neuroimaging. Current Opinion in Neurobiology. 2003;13(2):250–255. doi: 10.1016/s0959-4388(03)00036-9. doi:10.1016/S0959-4388(03)00036-9. [DOI] [PubMed] [Google Scholar]
- Livesey AC, Wall MB, Smith AT. Time perception: Manipulation of task difficulty dissociates clock functions from other cognitive demands. Neuropsychologia. 2007;45(2):321–331. doi: 10.1016/j.neuropsychologia.2006.06.033. doi:10.1016/j.neuropsychologia.2006.06.033. [DOI] [PubMed] [Google Scholar]
- Macaluso DBSLMCE, Lasaponara S, Cercignani M, Macaluso E. Learning about Time: Plastic Changes and Interindividual Brain Differences. Neuron. 2012;75(4):725–737. doi: 10.1016/j.neuron.2012.07.019. doi:10.1016/j.neuron.2012.07.019. [DOI] [PubMed] [Google Scholar]
- MacDonald CJ, Lepage KQ, Eden UT, Eichenbaum H. Hippocampal “Time Cells”” Bridge the Gap in Memory for Discontiguous Events. Neuron. 2011;71(4):737–749. doi: 10.1016/j.neuron.2011.07.012. doi: 10.1016/j.neuron.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic anad cytoarchitectonic atlas-based interrogation of fMRI data sets. Neurolmage. 2003;19(3):1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Matell MS, Meek WH. Cortico-striatal circuits and interval timing: coincidence detection of oscillatory processes. Cognitive Brain Research. 2004;21(2):139–170. doi: 10.1016/j.cogbrainres.2004.06.012. doi:10.1016/j.cogbrainres.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Nicodemus KK, Egan MF, Callicott JH, Mattay V, Weinberger DR. False positives in imaging genetics. NeuroImage. 2008;40(2):655–661. doi: 10.1016/j.neuroimage.2007.11.058. doi: 10.1016/j.neuroimage.2007.11.058. [DOI] [PubMed] [Google Scholar]
- Morillon B, Kell CA, Giraud AL. Three Stages and Four Neural Systems in Time Estimation. Journal of Neuroscience. 2009;29(47):14803–14811. doi: 10.1523/JNEUROSCI.3222-09.2009. doi: 10.1523/JNEUROSCI.3222-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai Y, Critchley HD, Featherstone E, Fenwick PBC, Trimble MR, Dolan RJ. Brain activity relating to the contingent negative variation: an fMRI investigation. Neurolmage. 2004;21(4):1232–1241. doi: 10.1016/j.neuroimage.2003.10.036. doi: 10.1016/j.neuroimage.2003.10.036. [DOI] [PubMed] [Google Scholar]
- Pfeuffer J, McCullough JC, Van de Moortele P-F, Ugurbil K, Hu X. Spatial dependence of the nonlinear BOLD response at short stimulus duration. Neurolmage. 2003;18:990–1000. doi: 10.1016/s1053-8119(03)00035-1. [DOI] [PubMed] [Google Scholar]
- Rammsayer TH. On the relationship between personality and time estimation. Personality and Individual Differences. 1997;23(5):739–744. [Google Scholar]
- Schwartz MF, Kimberg DY, Walker GM, Brecher A, Faseyitan OK, Dell G, Mirman D, Coslett HB. Neuroanatomical dissociation for taxonomic and thematic knowledge in the human brain. Proceedings of the National Academy of Science. 2011;108:8520–8524. doi: 10.1073/pnas.1014935108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MF, Faseyitan OK, Kim J, Coslett HB. The dorsal stream contribution to phonological retrieval in object naming. Brain. 2012;135:3799–3814. doi: 10.1093/brain/aws300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzkopf DS, De Haas B, Rees G. Better ways to improve standards in brain-behavior correlation analysis. Frontiers in Human Neuroscience. 2012;6(200) doi: 10.3389/fnhum.2012.00200. doi: 10.3389/fnhum.2012.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelzel C, Basten U, Montag C, Reuter M, Fiebach CJ. Frontostriatal Involvement in Task Switching Depends on Genetic Differences in D2 Receptor Density. Journal of Neuroscience. 2010;30(42):14205–14212. doi: 10.1523/JNEUROSCI.1062-10.2010. doi: 10.1523/JNEUROSCI.1062-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipples J, Brattan V, Johnston P. Sinigaglia C, editor. Neural Bases for Individual Differences in the Subjective Experience of Short Durations (Less than 2 Seconds). PLoS ONE. 2013;8(1):e54669. doi: 10.1371/journal.pone.0054669. doi:10.1371/journal.pone.0054669.t001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix B, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neurolmage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Vidai F, Casini L. The SMAs: neural substrate of the temporal accumulator? Frontiers in Integrative Neuroscience. 2011:1–3. doi: 10.3389/fnint.2011.00035. doi:10.3389/fnint.2011.00035/abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel EK, Awh E. Using individual differences to constrain cognitive theory. Current Directions in Psychological Science. 2008;17(2):171–176. [Google Scholar]
- Volkow ND, Wang G-J, Fowler JS, Logan J, Angrist B, Hitzemann R, Lieberman J, Pappas N. Effects of methylphenidate on regional brain glucose metabolism in humans: relationship to dopamine D2 receptors. American Journal of Psychiatry. 1997;154(1):50–55. doi: 10.1176/ajp.154.1.50. [DOI] [PubMed] [Google Scholar]
- Wencil EB, Coslett HB, Aguirre GK, Chatterjee A. Carving the Clock at Its Component Joints: Neural Bases for Interval Timing. Journal of Neurophysiology. 2010;104(1):160–168. doi: 10.1152/jn.00029.2009. doi: 10.1152/jn.00029.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiener M, Turkeltaub PE, Coslett HB. The Image of Time: A voxel-wise meta-analysis. Neurolmage. 2010;49:1728–1740. doi: 10.1016/j.neuroimage.2009.09.064. [DOI] [PubMed] [Google Scholar]
- Wiener M, Lohoff FW, Coslett HB. Double dissociation of dopamine genes and timing in humans. Journal of Cognitive Neuroscience. 2011;23(10):2811–2821. doi: 10.1162/jocn.2011.21626. [DOI] [PubMed] [Google Scholar]
- Wiener M, Matell MS, Coslett HB. Multiple mechanisms for temporal process. Frontiers Integrative Neuroscience. 2011:1–3. doi: 10.3389/fnint.2011.00031. doi: 10.3389/fnint.2011.00031/abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiener M, Kliot D, Turkeltaub PE, Hamilton RH, Wolk DA, Coslett HB. Parietal Influence on Temporal Encoding Indexed by Simultaneous Transcranial Magnetic Stimulation and Electroencephalography. Journal of Neuroscience. 2012;32(35):12258–12267. doi: 10.1523/JNEUROSCI.2511-12.2012. doi:10.1523/JNEUROSCI.2511-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterer G, Weinberger DR. Genes, dopamine and cortical signal-to-noise ratio in schizophrenia. Trends in Neuroscience. 2004;27:683–690. doi: 10.1016/j.tins.2004.08.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.