Abstract
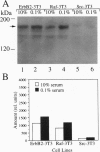
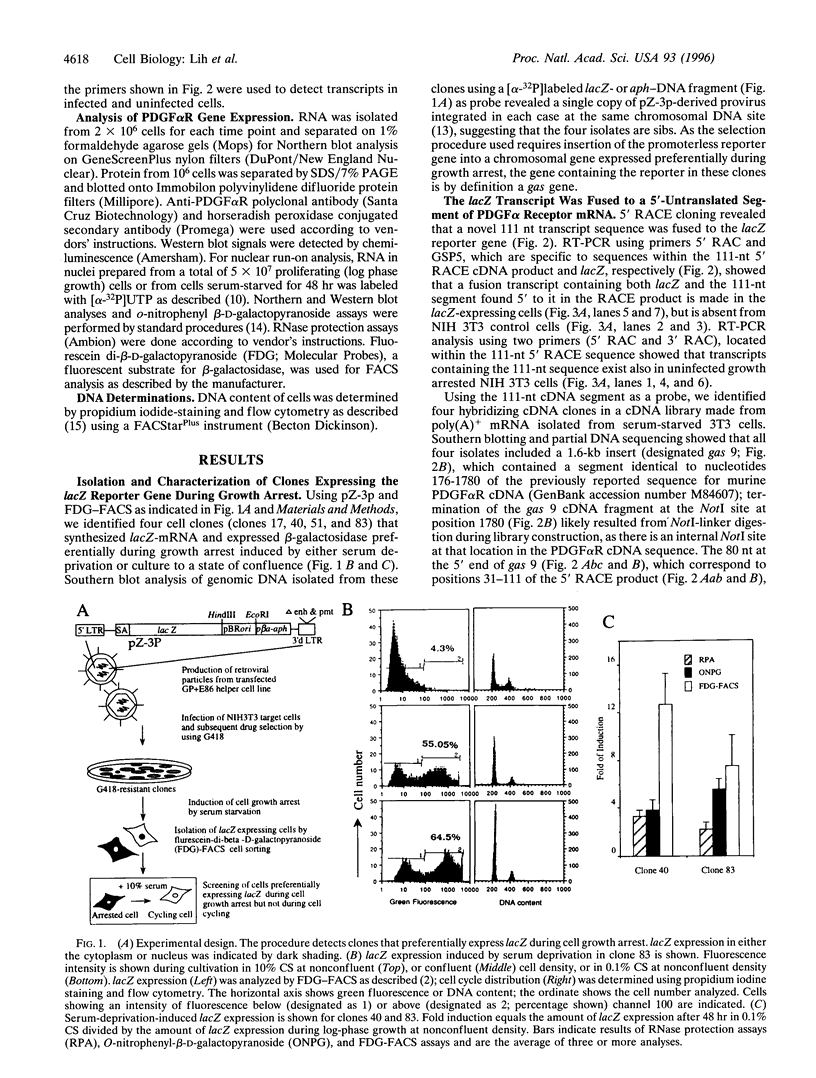
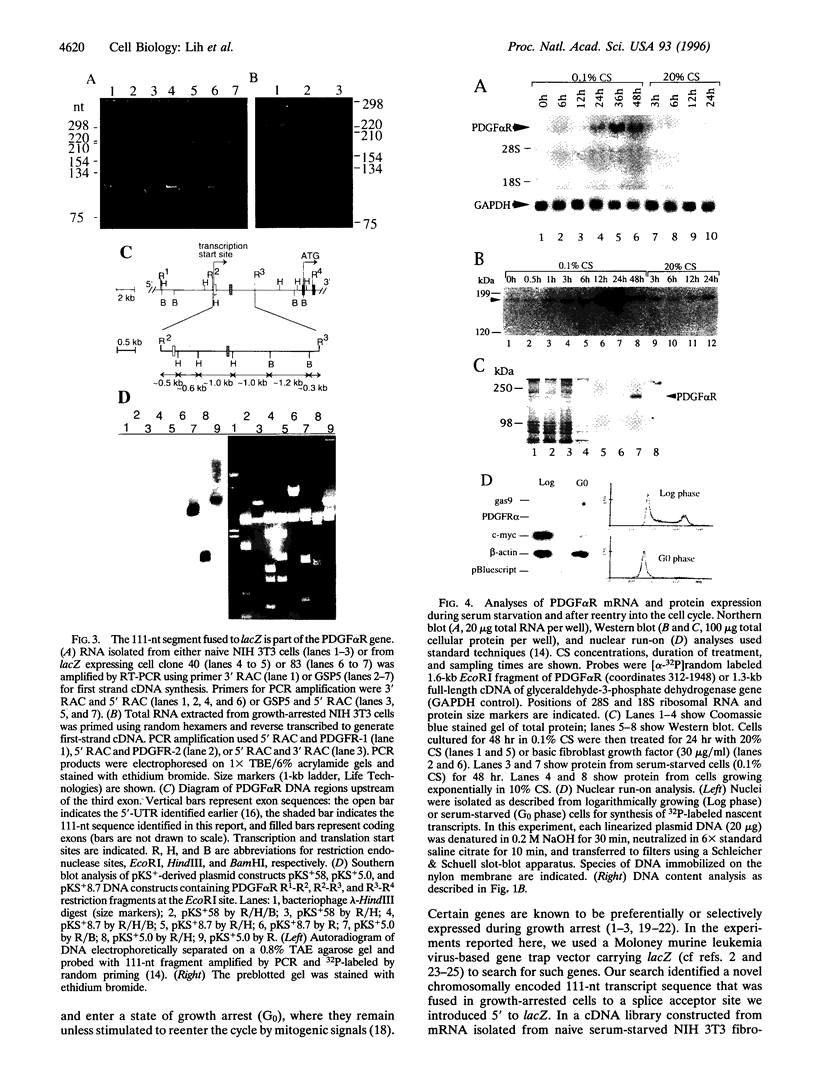
Using the Escherichia coli lacZ gene to identify chromosomal loci that are transcriptionally active during growth arrest of NIH 3T3 fibroblasts, we found that an mRNA expressed preferentially in serum-deprived cells specifies the previously characterized alpha-receptor (alphaR) for platelet-derived growth factor (PDGF), which mediates mitogenic responsiveness to all PDGF isoforms. Both PDGFalphaR mRNA, which was shown to include a 111-nt segment encoded by a DNA region thought to contain only intron sequences, and PDGFalphaR protein accumulated in serum-starved cells and decreased as cells resumed cycling. Elevated PDGFalphaR gene expression during serum starvation was not observed in cells that had been transformed with oncogenes erbB2, src, or raf, which prevent starvation-induced growth arrest. Our results support the view that products of certain genes expressed during growth arrest function to promote, rather than restrict, cell cycling. We suggest that accumulation of the PDGFalphaR gene product may facilitate the exiting of cells from growth arrest upon mitogenic stimulation by PDGF, leading to the state of "competence" required for cell cycling.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowen-Pope D. F., Vogel A., Ross R. Production of platelet-derived growth factor-like molecules and reduced expression of platelet-derived growth factor receptors accompany transformation by a wide spectrum of agents. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2396–2400. doi: 10.1073/pnas.81.8.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brancolini C., Benedetti M., Schneider C. Microfilament reorganization during apoptosis: the role of Gas2, a possible substrate for ICE-like proteases. EMBO J. 1995 Nov 1;14(21):5179–5190. doi: 10.1002/j.1460-2075.1995.tb00202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner D. G., Lin-Chao S., Cohen S. N. Analysis of mammalian cell genetic regulation in situ by using retrovirus-derived "portable exons" carrying the Escherichia coli lacZ gene. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5517–5521. doi: 10.1073/pnas.86.14.5517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J., Pardee A. B. Post-transcriptional control of the onset of DNA synthesis by an insulin-like growth factor. Mol Cell Biol. 1984 Sep;4(9):1807–1814. doi: 10.1128/mcb.4.9.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claesson-Welsh L. Platelet-derived growth factor receptor signals. J Biol Chem. 1994 Dec 23;269(51):32023–32026. [PubMed] [Google Scholar]
- Del Sal G., Ruaro M. E., Philipson L., Schneider C. The growth arrest-specific gene, gas1, is involved in growth suppression. Cell. 1992 Aug 21;70(4):595–607. doi: 10.1016/0092-8674(92)90429-g. [DOI] [PubMed] [Google Scholar]
- Fornace A. J., Jr, Alamo I., Jr, Hollander M. C. DNA damage-inducible transcripts in mammalian cells. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8800–8804. doi: 10.1073/pnas.85.23.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornace A. J., Jr, Nebert D. W., Hollander M. C., Luethy J. D., Papathanasiou M., Fargnoli J., Holbrook N. J. Mammalian genes coordinately regulated by growth arrest signals and DNA-damaging agents. Mol Cell Biol. 1989 Oct;9(10):4196–4203. doi: 10.1128/mcb.9.10.4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich G., Soriano P. Promoter traps in embryonic stem cells: a genetic screen to identify and mutate developmental genes in mice. Genes Dev. 1991 Sep;5(9):1513–1523. doi: 10.1101/gad.5.9.1513. [DOI] [PubMed] [Google Scholar]
- Godowski P. J., Mark M. R., Chen J., Sadick M. D., Raab H., Hammonds R. G. Reevaluation of the roles of protein S and Gas6 as ligands for the receptor tyrosine kinase Rse/Tyro 3. Cell. 1995 Aug 11;82(3):355–358. doi: 10.1016/0092-8674(95)90424-7. [DOI] [PubMed] [Google Scholar]
- Gossler A., Joyner A. L., Rossant J., Skarnes W. C. Mouse embryonic stem cells and reporter constructs to detect developmentally regulated genes. Science. 1989 Apr 28;244(4903):463–465. doi: 10.1126/science.2497519. [DOI] [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Leof E. B., Van Wyk J. J., O'Keefe E. J., Pledger W. J. Epidermal growth factor (EGF) is required only during the traverse of early G1 in PDGF stimulated density-arrested BALB/c-3T3 cells. Exp Cell Res. 1983 Aug;147(1):202–208. doi: 10.1016/0014-4827(83)90285-9. [DOI] [PubMed] [Google Scholar]
- Lih C. J., Wu S. M., Lin-Chao S. Rapid identification and isolation of transcriptionally active regions from mouse genomes. Gene. 1995 Oct 27;164(2):289–294. doi: 10.1016/0378-1119(95)00452-c. [DOI] [PubMed] [Google Scholar]
- Manfioletti G., Brancolini C., Avanzi G., Schneider C. The protein encoded by a growth arrest-specific gene (gas6) is a new member of the vitamin K-dependent proteins related to protein S, a negative coregulator in the blood coagulation cascade. Mol Cell Biol. 1993 Aug;13(8):4976–4985. doi: 10.1128/mcb.13.8.4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manfioletti G., Ruaro M. E., Del Sal G., Philipson L., Schneider C. A growth arrest-specific (gas) gene codes for a membrane protein. Mol Cell Biol. 1990 Jun;10(6):2924–2930. doi: 10.1128/mcb.10.6.2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi K., Nagata K., Toshima J., Nakano T., Arita H., Tsuda H., Suzuki K., Mizuno K. Stimulation of sky receptor tyrosine kinase by the product of growth arrest-specific gene 6. J Biol Chem. 1995 Sep 29;270(39):22681–22684. doi: 10.1074/jbc.270.39.22681. [DOI] [PubMed] [Google Scholar]
- Pledger W. J., Stiles C. D., Antoniades H. N., Scher C. D. An ordered sequence of events is required before BALB/c-3T3 cells become committed to DNA synthesis. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2839–2843. doi: 10.1073/pnas.75.6.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pledger W. J., Stiles C. D., Antoniades H. N., Scher C. D. Induction of DNA synthesis in BALB/c 3T3 cells by serum components: reevaluation of the commitment process. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4481–4485. doi: 10.1073/pnas.74.10.4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rake J. B., Quiñones M. A., Faller D. V. Inhibition of platelet-derived growth factor-mediated signal transduction by transforming ras. Suppression of receptor autophosphorylation. J Biol Chem. 1991 Mar 15;266(8):5348–5352. [PubMed] [Google Scholar]
- Reddy S., DeGregori J. V., von Melchner H., Ruley H. E. Retrovirus promoter-trap vector to induce lacZ gene fusions in mammalian cells. J Virol. 1991 Mar;65(3):1507–1515. doi: 10.1128/jvi.65.3.1507-1515.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatteman G. C., Morrison-Graham K., van Koppen A., Weston J. A., Bowen-Pope D. F. Regulation and role of PDGF receptor alpha-subunit expression during embryogenesis. Development. 1992 May;115(1):123–131. doi: 10.1242/dev.115.1.123. [DOI] [PubMed] [Google Scholar]
- Schneider C., King R. M., Philipson L. Genes specifically expressed at growth arrest of mammalian cells. Cell. 1988 Sep 9;54(6):787–793. doi: 10.1016/s0092-8674(88)91065-3. [DOI] [PubMed] [Google Scholar]
- Snipes G. J., Suter U., Welcher A. A., Shooter E. M. Characterization of a novel peripheral nervous system myelin protein (PMP-22/SR13). J Cell Biol. 1992 Apr;117(1):225–238. doi: 10.1083/jcb.117.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreyer P., Kuhn G., Hanemann C. O., Gillen C., Schaal H., Kuhn R., Lemke G., Müller H. W. Axon-regulated expression of a Schwann cell transcript that is homologous to a 'growth arrest-specific' gene. EMBO J. 1991 Dec;10(12):3661–3668. doi: 10.1002/j.1460-2075.1991.tb04933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varnum B. C., Young C., Elliott G., Garcia A., Bartley T. D., Fridell Y. W., Hunt R. W., Trail G., Clogston C., Toso R. J. Axl receptor tyrosine kinase stimulated by the vitamin K-dependent protein encoded by growth-arrest-specific gene 6. Nature. 1995 Feb 16;373(6515):623–626. doi: 10.1038/373623a0. [DOI] [PubMed] [Google Scholar]
- Vaziri C., Faller D. V. Repression of platelet-derived growth factor beta-receptor expression by mitogenic growth factors and transforming oncogenes in murine 3T3 fibroblasts. Mol Cell Biol. 1995 Mar;15(3):1244–1253. doi: 10.1128/mcb.15.3.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vindeløv L. L., Christensen I. J. A review of techniques and results obtained in one laboratory by an integrated system of methods designed for routine clinical flow cytometric DNA analysis. Cytometry. 1990;11(7):753–770. doi: 10.1002/cyto.990110702. [DOI] [PubMed] [Google Scholar]
- Vogel U., Denecke B., Troyanovsky S. M., Leube R. E., Böttger E. C. Transcriptional activation of psoriasis-associated cytokeratin K17 by interferon-gamma. Analysis of gamma-interferon activation sites. Eur J Biochem. 1995 Jan 15;227(1-2):143–149. doi: 10.1111/j.1432-1033.1995.tb20370.x. [DOI] [PubMed] [Google Scholar]
- Wang C., Stiles C. D. Platelet-derived growth factor alpha receptor gene expression: isolation and characterization of the promoter and upstream regulatory elements. Proc Natl Acad Sci U S A. 1994 Jul 19;91(15):7061–7065. doi: 10.1073/pnas.91.15.7061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welcher A. A., Bitler C. M., Radeke M. J., Shooter E. M. Nerve growth factor binding domain of the nerve growth factor receptor. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):159–163. doi: 10.1073/pnas.88.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C. L., Prochownik E. V., Imperiale M. J., Jove R. Attenuation of serum inducibility of immediate early genes by oncoproteins in tyrosine kinase signaling pathways. Mol Cell Biol. 1993 Apr;13(4):2011–2019. doi: 10.1128/mcb.13.4.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan Q., Lord K. A., Alamo I., Jr, Hollander M. C., Carrier F., Ron D., Kohn K. W., Hoffman B., Liebermann D. A., Fornace A. J., Jr The gadd and MyD genes define a novel set of mammalian genes encoding acidic proteins that synergistically suppress cell growth. Mol Cell Biol. 1994 Apr;14(4):2361–2371. doi: 10.1128/mcb.14.4.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoidl G., Blass-Kampmann S., D'Urso D., Schmalenbach C., Müller H. W. Retroviral-mediated gene transfer of the peripheral myelin protein PMP22 in Schwann cells: modulation of cell growth. EMBO J. 1995 Mar 15;14(6):1122–1128. doi: 10.1002/j.1460-2075.1995.tb07095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]