SUMMARY
RNA polymerase II (RNAPII) lies at the core of dynamic control of gene expression. Using 53 RNAPII point mutants, we generated a point mutant epistatic miniarray profile (pE-MAP) comprising ~60,000 quantitative genetic interactions in Saccharomyces cerevisiae. This analysis enabled functional assignment of RNAPII subdomains and uncovered connections between individual regions and other protein complexes. Using splicing microarrays and mutants that alter elongation rates in vitro, we found an inverse relationship between RNAPII speed and in vivo splicing efficiency. Furthermore, the pE-MAP classified fast and slow mutants that favor upstream and downstream start site selection, respectively. The striking coordination of polymerization rate with transcription initiation and splicing suggests that transcription rate is tuned to regulate multiple gene expression steps. The pE-MAP approach provides a powerful strategy to understand other multifunctional machines at amino acid resolution.
INTRODUCTION
Alterations within a genome often cause specific as well as global phenotypic changes to a cell. Combining two alterations in the same cell allows for measurement of the genetic interaction between them: negative genetic interactions (synthetic sick/lethal) arise when two mutations in combination cause a stronger growth defect than expected from the single mutations. This is often observed for factors participating in redundant pathways or as nonessential components of the same essential complex. In contrast, positive interactions occur when the double mutant is either no sicker (epistatic) or healthier (suppressive) than the sickest single mutant (Beltrao et al., 2010) and may indicate that the factors are components of a nonessential complex and/or that the factors perform antagonizing roles in the cell. However, single genetic interactions are often difficult to interpret in isolation; an interaction pattern for a given mutation can be more informative, as it reports on the phenotype in a large number of mutant backgrounds (Schuldiner et al., 2005; Tong et al., 2004). These genetic profiles provide highly specific readouts that can be used to identify genes that are functionally related (Beltrao et al., 2010).
One of the first organisms to be genetically interrogated on a large scale was Saccharomyces cerevisiae (S. cerevisiae), in which nonquantitative genetic interaction data could be collected using the SGA (synthetic genetic array) (Tong et al., 2004) or dSLAM (heterozygous diploid-based synthetic lethality analysis on microarrays) (Pan et al., 2004) approaches. We developed a technique termed epistatic miniarray profile (E-MAP) (Collins et al., 2010; Schuldiner et al., 2005; Schuldiner et al., 2006), which utilizes the SGA methodology and allows for the quantitative collection of genetic interaction data on functionally related subsets of genes, including those involved in chromatin regulation (Collins et al., 2007b), RNA processing (Wilmes et al., 2008), signaling (Fiedler et al., 2009), or plasma membrane function (Aguilar et al., 2010). However, the vast majority of systematic genetic screening interrogates deletions of nonessential genes or hypomorphic knockdown alleles of essential genes. Because many genes, especially essential ones, are multifunctional, these methods perturb all activities associated with a given gene product.
Here, we describe an important advance of the E-MAP approach, which allows us to address higher levels of complexity by examining the genetic interaction space of point mutant alleles of multifunctional genes in a technique that we term point mutant E-MAP (pE-MAP). This method greatly increases the resolution achievable by gene function analysis, as it allows assignment of specific genetic relationships to individual residues and domains. In this study, we have used the pE-MAP approach to functionally dissect RNAPII using alteration-of-function alleles in five different subunits of the enzyme. Using the genetic data, we assign transcriptional activity and specific functions to different residues and regions of RNAPII. By examining the relationship between transcription rate and genetic interaction partners, transcription-rate-sensitive factors were revealed. Through the characterization of multiple stages of gene regulation, including start site selection, transcription elongation rate, and mRNA splicing, the pE-MAP technique has provided both global and specific insight into structure-function relationships of RNAPII. We propose this strategy as a useful paradigm for the high-resolution interrogation of any multifunctional protein.
RESULTS
A Set of Alleles for the Functional Dissection of RNAPII
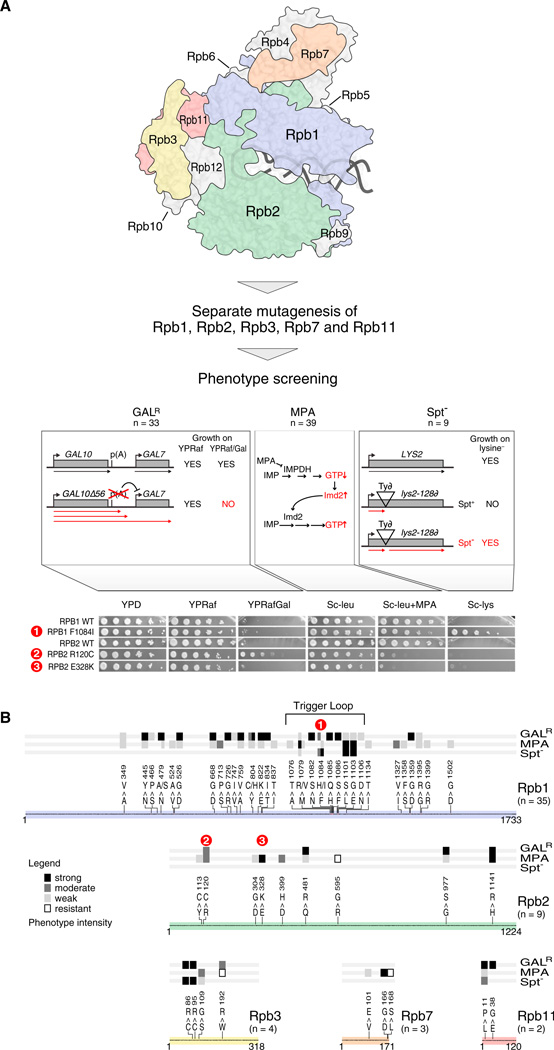
To identify residues that are important for transcriptional regulation in vivo, we isolated RNAPII alleles that confer one or more of the following transcription-related phenotypes: suppression of galactose sensitivity in gal10Δ56 (GalR) (Greger and Proudfoot, 1998; Kaplan et al., 2005), the Spt− phenotype (Winston and Sudarsanam, 1998), or mycophenolic acid (MPA) sensitivity (Shaw and Reines, 2000) (for additional details, see Figure 1A and the Experimental Procedures). Each of these phenotypes relates to a gene-specific transcription defect that can be monitored using plate assays (Figure 1A). Random mutagenesis by PCR was carried out on the entire coding regions of RNAPII subunit genes RPB2, RPB3, RPB7, and RPB11 and most of RPO21/RPB1 (Experimental Procedures). These genes encode the essential subunits that are unique to RNAPII (Rpb5, Rpb6, Rpb8, Rpb10, and Rpb12 are shared with RNAPI and RNAPIII, and Rpb4 and Rpb9 are nonessential) (Archambault and Friesen, 1993). In total, 53 single point mutants were identified that exhibit at least one of these phenotypes (Kaplan et al., 2012) (Figure 1B and Figure S1 and Table S1 available online).
Figure 1. Generation and Selection of RNAPII Point Mutants.
(A) RNAPII point mutants were screened for three transcription-related phenotypes. (GalR, left) Deletion of the major GAL10 p(A) site (gal10Δ56) results in RNAPII readthrough and interference with initiation at GAL7, causing a Gal-sensitive phenotype. GalR mutants increase GAL10 3′ end formation/termination, thereby rescuing GAL7 expression. (MPA, middle) Mycophenolic acid (MPA) inhibits IMP-dehydrogenase (IMPDH)-dependent GTP synthesis but is counteracted by upregulation of an MPA-resistant form of IMPDH, IMD2. Transcriptional defects that are sensitive to low GTP levels or reduce IMD2 expression render cells sensitive to MPA. (Spt−, right) Insertion of a Ty retrotransposon into LYS2 (lys2-128∂) results in a lysine auxotrophy due to transcription block. Certain mutants suppress lys2-128∂ and allow expression of LYS2 due to activation of an internal promoter. Spot tests to identify each phenotype for three representative mutants are displayed.
(B) Positions, mutations, and phenotypes of the 53 single point mutants analyzed in the pE-MAP. Colored lines represent subunit sequences, with mutations denoted by residue numbers and single letter amino acid codes for WT and mutant.
Analysis of the distribution of phenotypes relative to the RNAPII structure suggested that our alleles might be diverse in their functions. GalR and MPA-sensitive mutations were broadly distributed, whereas those with the Spt− phenotype were less common and more localized (Figure 1B and Table S1). The screens identified mutations in highly conserved residues and structural domains known to be important for RNAPII activity, including the Rpb1 trigger loop, the Rpb1 bridge helix, and the Rpb2 lobe and protrusion (Cramer et al., 2001; Gnatt et al., 2001; Kaplan, 2013) (Table S1). Quantitatively measuring the genetic interactions of specific residues might provide insight into the functions of these RNAPII regions and could therefore also identify protein-protein interaction interfaces.
An RNAPII Point Mutant Epistatic Miniarray Profile
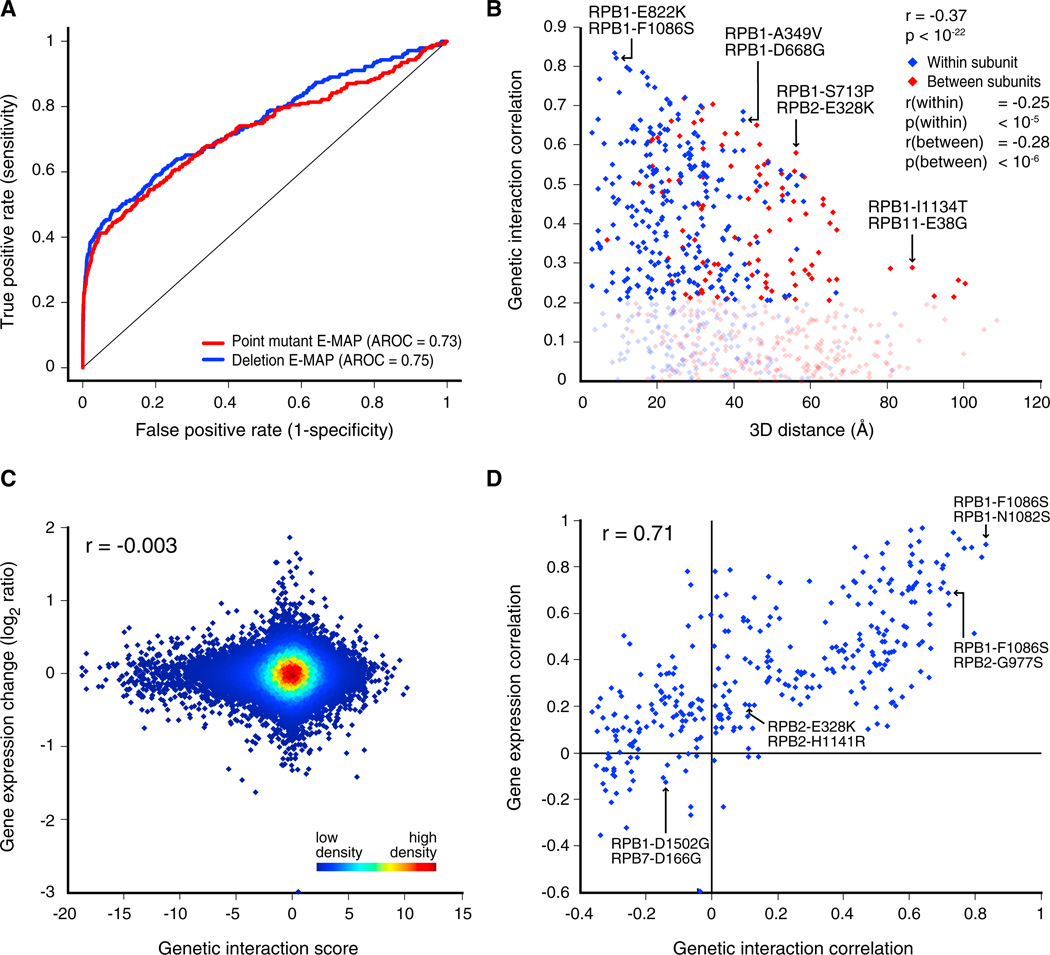
The 53 RNAPII point mutants (Figure 1B) were crossed against ~1,200 deletion and DAmP (decreased abundance by mRNA perturbation) alleles (Schuldiner et al., 2005) (Table S1), which represent all major biological processes. Thus, a quantitative pE-MAP comprising ~60,000 double mutants was created (Table S2; http://interactome-cmp.ucsf.edu). Two-dimensional hierarchical clustering of these data effectively grouped together genes from known complexes and pathways based on their interactions with the point mutants (Figure S2 and Data S1). Previous studies have demonstrated that genes encoding proteins that physically associate often have similar genetic interaction profiles (Collins et al., 2007b; Roguev et al., 2008). The data derived from the point mutants could differ in this respect, as it is based on only five subunits of a single molecular machine. A receiver operating characteristic (ROC) curve was therefore generated to measure how well the genetic profiles of the deletion and DAmP mutants in the pE-MAP predict known physical interactions between their encoded proteins (Experimental Procedures). It was found that the predictive power of the pE-MAP is similar to that of a previously published E-MAP (Collins et al., 2007b), indicating that the genetic interactions of the RNAPII point mutants report on connections among virtually all cellular processes (Figures 2A and S2 and Data S1).
Figure 2. pE-MAP Interactions Span Numerous Biological Processes, Depend on Spatial Location of Mutated Residues, and Are Not Direct Consequences of Changes in Gene Expression.
(A) ROC curves comparing the power of genetic profile correlations from the pE-MAP (red) and an E-MAP focused on chromosome biology (Collins et al., 2007b) (blue) to predict physical interactions between pairs of proteins (Collins et al., 2007a; Experimental Procedures). AROC, area under the curve.
(B) Genetic profile correlations between pairs of mutated residues compared to the three-dimensional distance between their α carbons. Blue points denote residue pairs within the same RNAPII subunit; red points represent pairs in different subunits. Negatively correlated residue pairs were excluded, as were four mutants of residues absent from the coordinate file (PDB ID: 2E2H) (Rpb1 D1502, Rpb7 V101, Rpb7 D166, and Rpb7 L168) (Wang et al., 2006). Correlations between 0 and 0.2 are dimmed to highlight trends at higher correlations.
(C) Effect of RNAPII point mutations on gene expression, compared to the corresponding genetic interaction scores between RNAPII mutants and deletion/DAmP alleles. All combinations of the 26 RNAPII mutants and 1,192 library genes/mutants that were examined via both pE-MAP and expression analyses are included. No global changes in gene expression were observed (measured by spike-in control RNA; Experimental Procedures).
(D) Comparison between pairwise RNAPII mutant correlations of genetic interaction profiles and gene expression profiles.
Next, to examine whether the spatial location of a mutated residue is a determinant of its function, we compared the similarity of pairs of RNAPII genetic profiles to the three-dimensional distance between the mutated residues (Wang et al., 2006). We observed a strong correlation (r = −0.37, p < 10−22) (Table S2), and the trend is significant both for residue pairs residing in the same subunit (r = −0.25, p < 10−5) and for those in different subunits (r = −0.28, p < 10−6) (Figure 2B). This suggests that structural proximity correlates with functional similarity and that high-resolution genetic interaction profiling could provide information for targets whose structures have not yet been determined.
Comparison of Genetic and Gene Expression Profiles Derived from the RNAPII Alleles
To determine whether any given genetic interaction might result from the point mutation affecting the expression of the corresponding gene, we subjected 26 of the RNAPII mutants to genome-wide gene expression analysis (Table S2; GEO accession number: GSE47429). We found no correlation (r = −0.003) between an RNAPII mutant’s genetic interaction score with a gene deletion or DAmP allele and the expression change of that gene due to the RNAPII mutation (Figure 2C). Therefore, connections must be due to more complex relationships between the mutated residues and the library genes. Nonetheless, these data sets allowed us to test whether the clustering of the RNAPII mutants in the pE-MAP (Figure S2 and Data S1) could be recapitulated using their gene expression profiles. We thus assessed pair-wise RNAPII mutant similarity based on genetic and gene expression profiles separately and found that these two measures are highly correlated (r = 0.71; Figure 2D). Therefore, these orthogonal data sets provide a common biological framework for functionally organizing the RNAPII mutants, allowing us to study the underlying biology behind these mutants and their phenotypes.
Functional Associations between RNAPII Residues and Protein Complexes
In an effort to link individual RNAPII mutations to specific cellular functions, data from the pE-MAP were compared to a published genetic interaction data set containing profiles from >4,000 genes (Costanzo et al., 2010). These genes were classified based on complex membership of their encoded proteins (Benschop et al., 2010), and Mann-Whitney U statistics were used to identify RNAPII mutants with high profile similarity to members of specific complexes (Experimental Procedures, Figure 3A, and Table S3). We uncovered a number of connections, including several point mutants having similar genetic profiles to mutants of components of Mediator and the Rpd3C(L) histone deacetylase complex (Carrozza et al., 2005; Keogh et al., 2005). Unexpectedly, we also observed that several RNAPII mutants are significantly correlated genetically to kinetochore mutants (Figure 3A). We carried out chromosome transmission fidelity (CTF) assays on 19 of our RNAPII mutants, including those linked specifically to the kinetochore (Experimental Procedures and Table S3) (Spencer et al., 1990). Only four of the tested mutants exhibited chromosome loss in more than 15% of their colonies, and these were genetically linked to the kinetochore in our analysis and had similar genetic profiles to each other in our pE-MAP (Figures 3A and 3B). Recent studies indicate that a certain level of transcription by RNAPII at the centromere is required for centromere function and high-fidelity chromosome segregation in budding yeast (Ohkuni and Kitagawa, 2011). Using specific constructs designed to ascertain centromere sensitivity to transcriptional readthrough, we did not observe any defects in kinetochore integrity in these RNAPII mutants (data not shown). Ultimately, further work will be required to understand the connection between these RNAPII point mutants and chromosome segregation. A full point mutant module map from alleles of all subunits is presented in Figure S3.
Figure 3. Comparison of the pE-MAP with Previously Collected Genetic Interaction Data Reveals Functional Associations between RNAPII Residues and Protein Complexes.
(A) Module map depicting genetic similarity of RNAPII mutants with genes encoding the indicated protein complex subunits (Experimental Procedures). Edge widths correspond to statistical significance of connections. Only RPB1 edges with a false discovery rate <0.1 are displayed. Four mutated residues linked to the kinetochore are highlighted in blue, and the blow-up indicates their structural locations.
(B) Nineteen mutants were examined using a chromosome transmission fidelity (CTF) assay. The four kinetochore-linked mutants highlighted in (A) exhibit chromosome loss in >15% of their colonies (blue bars), whereas unlinked mutants display no or weak phenotype (red bars, representative set).
pE-MAP Identifies Alleles Involved in Start Site Selection and Can Finely Distinguish between Different Phenotypic Categories
What other transcription defects might underlie differences in the genetic profiles of specific RNAPII alleles? Recent work has shown that mutations in the Rpb1 trigger loop (TL), a dynamic element in the active site that couples correct NTP substrate recognition with catalysis, can alter transcription start site selection in vivo. For example, rpb1 E1103G shifts transcription start site selection upstream at ADH1, whereas rpb1 H1085Y shifts distribution of start sites downstream (Kaplan et al., 2012). The pE-MAP subcluster containing E1103G includes an additional ten mutants in RPB1, RPB2, and RPB7 (Figure 4A and Data S1). We examined eight of these for defects in start site selection at ADH1 by primer extension and found that, like E1103G, all had a preference for upstream start site selection (Figures 4B, S4A, and Table S4), consistent with their clustering with E1103G (Figure 4A). Four of these mutants are also in the TL (rpb1 F1084I, M1079R, A1076T, and M1079V); however, two are in other regions of Rpb1 (rpb1 I1327V and S713P). Further inspection reveals that these are in close proximity to the TL (Figure 4C) (Kettenberger et al., 2004; Wang et al., 2006), suggesting that they too may directly regulate the active site. Interestingly, the other two mutants tested are not close to the TL (rpb2 E328K and rpb7 D166G); these mutations may allosterically impact the active site or may function independently of the TL by recruiting other factors to the transcription apparatus. Importantly, rpb7 D166G is the first identified RPB7 mutation with a start site defect. We additionally examined start site selection in rpb4Δ and rpb6 Q100R, as both are expected to reduce the association of Rpb4/Rpb7 with RNAPII (Edwards et al., 1991; Tan et al., 2003). Neither altered start site selection at ADH1 (Figure S4B), indicating that the rpb7 D166G mutant exerts a unique effect on RNAPII function (see Discussion). These data provide an example of how mechanistic information on structure-function relationships can be extracted from the pE-MAP.
Figure 4. pE-MAP Profiles Differentiate between Subtle Changes in Transcription-Related Phenotypes and Identify RNAPII Mutations that Affect Start Site Selection.
(A) pE-MAP clustering in relation to MPA and Spt− phenotypes of alleles. The RNAPII alleles are clustered by pE-MAP profiles, and their colors indicate degrees of MPA and Spt− phenotypes (determined from the spot tests).
(B) Effect of RNAPII mutations on start site selection at ADH1 determined by primer extension analysis. The heatmap describes the fractional change of start site in each bin of the ADH1 schematic (bottom).
(C) Rpb1 I1327 and Rpb1 S713 connect to the TL (magenta). Mutations in I1327 could affect the structural region of the TL (E1103) via a network of loops and helices in Rpb1 (gray), and S713 is close to the TL catalytic site in its open conformation, via a Rpb9 loop (orange). In particular, the proline substitution, S713P, could result in structural changes affecting the TL. Coordinates for TL, Rpb9, and the S713 loop are from PDB ID 1Y1V (Kettenberger et al., 2004), and all others are from 2E2H (Wang et al., 2006). The bridge helix is shown in cyan; template DNA, blue; nontemplate DNA, green; and RNA, red. The incoming GTP base is colored by atom.
In our screening process, we had also identified an rpb2 allele mutated at two residues in close proximity (E437G/F442S) within the tip of the Rpb2 protrusion domain, whose genetic profile also clusters with the upstream start site mutants (Figure S4C and Table S2). The Rpb2 lobe and protrusion domains physically contact TFIIF (Chen et al., 2007), and consistent with this, we observed that, similar to what has been reported for TFIIF alleles (Eichner et al., 2010; Ghazy et al., 2004) (Figure S6B), rpb2 E437G/F442S is sensitive to MPA (Figure S4D) and has a preferential upstream start site selection (Figure 4B) (see Discussion). Additional rpb2 alleles that confer MPA sensitivity also map near the protrusion (R120C) or to the lobe (D399H); however, these did not alter ADH1 start site selection and are genetically distinct from rpb1 E1103G or other rpb2 alleles, illustrating the fine resolving power of the pE-MAP (Figures 4A and 4B).
Despite its unbiased nature, the pE-MAP precisely grouped the mutants within the upstream start site cluster based on their Spt− and MPA phenotypes in the plate assays (Figure 4A). However, there are other mutants with MPA or Spt− phenotypes that did not exhibit upstream start preference and, notably, cluster apart from the ones that do (Figures 4A, 4B, and S4A and Table S4). These data collectively suggest that the pE-MAP has the resolving power to categorize the mutations causing upstream start site selection and, within this group, can further separate them into specific Spt− and MPA phenotypic categories.
In Vitro Biochemical Activity Correlates with pE-MAP Profiles and Gene Expression
We next focused on the genetic profiles of a series of active-site mutants whose in vitro elongation rates range from <0.1 to >2-fold that of WT RNAPII (Kaplan et al., 2008, 2012; Malagon et al., 2006). This series allowed for addressing questions regarding the in vivo consequences of altered elongation rates. Clustering these mutants based on their pE-MAP profiles yielded two distinct subsets that differ by transcription rate: fast mutants (rpb1 E1103G, L1101S, and F1084I) and slow mutants (rpb1 F1086S, N1082S, N479S, and H1085Q) (Figure 5A dendrogram). The gene expression profiles of these mutants also group them into the same two subsets. Thus, pairs of mutants with similar in vitro transcription rates show highly similar genetic and expression profiles (Figure 5B), indicating that altered catalytic activity may likely be a defining feature in vivo.
Figure 5. pE-MAP and Expression Profiles Are Indicative of Biochemical Activity.
(A) In vitro transcription rates from (Kaplan et al., 2012) and in vivo growth rates relative to WT for RNAPII active-site mutants. The dendrogram was generated via hierarchical clustering of the genetic profiles. Error bars represent 95% confidence intervals for transcription rates and SD for growth rates. Means and SD of growth rates were derived from three technical replicates. Note that rpb1 G1097D was too sick for reproducible E-MAP analysis.
(B) In vitro transcription rate difference between pairs of active-site mutants in relation to their genetic and expression profile correlations.
(C) Residues H1085 and F1086 reside in the catalytic site of the TL, whereas E1103 is part of the distal flanking α helix that structurally constrains the TL in open conformations. TL is shown in green (closed) and magenta (open); template DNA, blue; and RNA, red. The incoming GTP base is colored by atom. Coordinates for open TL are from PDB ID 1Y1V, and all others are from 2E2H.
(D) Counts of high-scoring interactions (pE-MAP score >3.3 [97.5 percentile] or <−5.1 [2.5 percentile]) in the complete genetic profiles or changes >1.7-fold in the genome-wide expression profiles of the indicated RNAPII mutants. In vitro transcription rates are indicated on the scale on the right.
See also Figure S5.
We reasoned that, because combining two TL mutations that individually increase and decrease elongation rate results in RNAPII with near-WT elongation rate in vitro and growth rate in vivo (Kaplan et al., 2012) (Figure S5A), these double mutants would also exhibit near-WT pE-MAP and gene expression profiles. Indeed, when we combine rpb1 E1103G (fast) and F1086S (slow), we see very few high-scoring genetic interactions compared to the single mutants and few genes whose expression changes by more than 1.7-fold (Figures 5C and 5D and Table S2). Similar trends were observed in the genetic and expression profiles of a rpb1 E1103G/N1082S double mutant (data not shown). However, the pE-MAP and gene expression assays had the resolving power to also identify a double mutant (rpb1 E1103G/H1085Q) that deviated from this general rule (Figures 5C and 5D and Table S2). Despite exhibiting partially suppressive genetic and expression patterns, there are still significant effects that correlate well with E1103G (Figure S5B), suggesting a more complex genetic relationship with this double mutant. Taken together, our double-mutant analyses are consistent with the notion that the genetic interactions and gene expression changes in fast and slow mutants are, for the most part, defined by the catalytic activity of RNAPII.
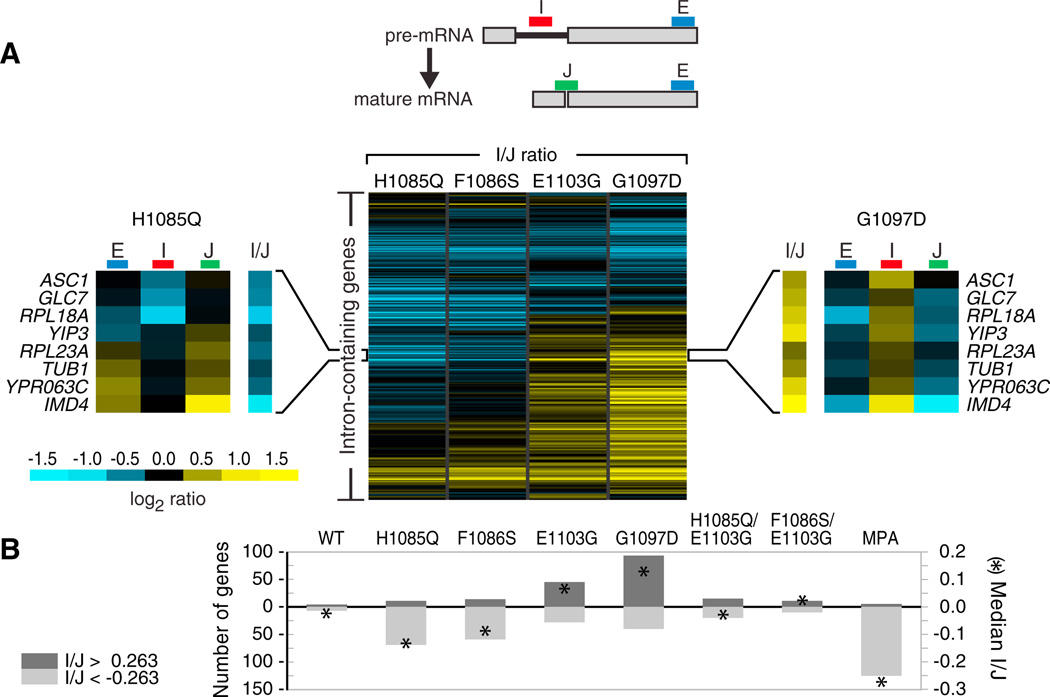
Genome-wide and Gene-Specific Effects of Altering Polymerase Speed on In Vivo Splicing Efficiency
Several steps in mRNA processing are coupled to the process of transcription (Bentley, 2005). Based primarily on experiments from metazoa, a kinetic coupling model has been invoked to explain how decreasing polymerase speed affects the usage of alternative splice sites, primarily favoring the recognition of otherwise weak splice sites (de la Mata et al., 2011). We used the RNAPII mutants that differ in their in vitro elongation rates to determine whether this type of coupling exists in S. cerevisiae, in which splice sites are of consensus or near-consensus sequence and the sole readout is splicing efficiency. We used splicing-sensitive microarrays to measure the change in total, pre-mRNA, and mature mRNA for each intron-containing gene (Figure 6A, top) in response to mutations in the RNAPII TL. Many genes exhibit the reciprocal pre- and mature mRNA values indicative of changes in splicing (Figure 6A, side panels), and to allow facile comparison across multiple mutants, we calculated pre-mRNA/mature mRNA ratios (Clark et al., 2002) for each gene (Figure 6 and Table S5). Both gene-by-gene (Figure 6A) and global (Figure 6B) measures show that rpb1 mutants characterized as fast in vitro (E1103G and G1097D) lead to an increase in this ratio for many genes, which indicates a defect in the splicing of those transcripts. Conversely, mutants with slow in vitro elongation (H1085Q and F1086S) decrease this ratio for many genes, a phenotype that is consistent with more efficient splicing. Thus, the global trend that we observe is an anticorrelation between polymerase rate and splicing efficiency (p < 10−5 for each mutant in Figure 6A compared to WT; Table S5). Using qPCR, we confirmed this trend at several genes (Figure S6A) and found that these genes exhibit between 1% and 40% unspliced mRNA in WT but up to 70% in G1097D (data not shown).
Figure 6. Effects of Altering RNAPII Transcription Rate on In Vivo Splicing Efficiency.
(A) (Top) Microarray schematic for each intron-containing gene: probe I (intron) hybridizes to premRNA, J (junction) to mature mRNA, and E (exon) to both. (Center) Heatmap of I/J log2 ratios for the slow (rpb1 H1085Q and F1086S) and fast (rpb1 E1103G and G1097D) mutants, corresponding to the enrichment of pre-mRNA over mature mRNA. The side panels highlight a subset of genes that behave reciprocally in fast and slow RNAPII backgrounds.
(B) Number of genes exhibiting >20% change in pre-mRNA-to-mature mRNA ratio (bars, scale on left) and median I/J log2 ratio (asterisks, scale on right) across entire array. MPA treatment was 10 min; WT denotes competitive hybridization between two WT cultures. I/J denotes I/J log2 ratio.
Given that polymerase rate and splicing efficiency are anticor-related, it follows that the TL double mutants (rpb1 E1103G/F1086S and E1103G/H1085Q), which transcribe at near-WT rate in vitro, should also exhibit near-WT splicing. Consistent with this prediction, these strains had very few genes with splicing defects (Figure 6B and Table S5). Thus, suppressive relationships are observed in the double mutants with respect to growth, genetic and expression profiling, in vitro transcription rate, and also mRNA splicing. Furthermore, slowing polymerase by chemical means should phenocopy a genetically slow polymerase mutant. We therefore evaluated splicing in WT cells treated with mycophenolic acid (MPA), which is known to impede transcriptional elongation (Mason and Struhl, 2005). A 10 min MPA treatment resulted in even more genes with improved splicing when compared to a slow polymerase mutant (Figure 6B, p < 10−15 compared to WT; Table S5), possibly because MPA elicits an acute stress. Taken together, these splicing phenotypes are consistent with a direct kinetic coupling between elongation rate and splicing in vivo.
Genetic Interactions between RNAPII Alleles and Other Mutants Reveal Relationships between Transcription Factors and RNAPII Activity
The observation of growth suppression in the TL double mutants (Kaplan et al., 2012) (Figure S5A) suggests that some of the deletion/DAmP genetic interactors that were detected in the pE-MAP might directly regulate, or collaborate with, RNAPII. We reasoned that disruption of a positively acting transcription factor would result in positive genetic interactions (suppression) with fast RNAPII mutants but negative interactions (synthetic sickness) with slow mutants. Conversely, a negatively acting factor would show opposite genetic trends. To identify these factors, the deletion/DAmP mutants were sorted based on the difference in their average genetic interaction score with fast and slow RNAPII mutants (Experimental Procedures, Figure 7A, and Table S6). We focused on genes that behaved as positively acting factors and observed that sub1Δ had the strongest pattern in this regard. Interestingly, previous evidence has implicated Sub1 as a positive factor in in vitro transcription assays (reviewed in Conesa and Acker, 2010), as well as in vivo (García et al., 2012). The genetic relationships from the pE-MAP were confirmed using standard growth assays in which sub1Δ exacerbated slow RNAPII alleles (Figure 7B) and partially suppressed fast RNAPII alleles (Figure 7C). Deletion of SUB1 also exacerbated and suppressed the relevant RNAPII mutant phenotypes (MPA, Spt−, and GalR) (Figure S7A) (as did other mutants [Figure S7B]). Furthermore, gene expression analysis of sub1Δ, rpb1 E1103G, and sub1Δ/E1103G showed an epistatic relationship between the E1103G mutant and sub1Δ (Figure 7D), consistent with their positive interaction and suggesting that a fast RNAPII mutant can bypass the requirement for Sub1.
Figure 7. Genetic Interaction Patterns with Fast and Slow RNAPII Mutants Reveal Sub1 as a Transcription Factor that Regulates Start Site Selection and Influences mRNA Splicing.
(A) Genetic profiles of library mutants, sorted on the difference between their average interaction with fast and slow RNAPII mutants (Table S6).
(B) Patch tests examining the sensitivity of slow TL mutants to sub1Δ. WT RPB1 plasmid covering rpb1 mutants in left panel is lost in right panel.
(C) Spot tests examining the effect of sub1Δ on fast mutants in absence (left) and presence (right) of MPA.
(D) Comparison of sub1Δ effect on gene expression in rpb1 E1103G (difference between red and blue) and WT (difference between green and y = 0). Included are all array transcripts exhibiting a >1.5-fold expression change in at least one of the three mutants. Transcripts are sorted by expression change in E1103G.
(E) Primer extension at ADH1 to map transcription start sites for rpb1 F1086S, E1103G, and sub1Δ (Figure S7C). Bar colors correspond to sequence windows in the ADH1 schematic (top), and heights specify the mean fraction change of transcription start in mutant compared to WT. Error bars represent SD.
(F) Splicing microarray analysis of sub1Δ, as in Figure 6 (Table S5). Number of genes exhibiting >20% change in pre-mRNA-to-mature mRNA ratio (bars, scale on left) and median I/J log2 ratio (asterisks, scale on right).
(G) Model for the effect of Sub1 and RNAPII activity on start site selection and splicing. Fast RNAPII mutations (class I) result in upstream transcription start and diminished splicing efficiency, whereas sub1Δ or slow RNAPII mutations (class II) shift transcription start downstream and enhance splicing.
See also Figures S6 and S7 and Tables S4, S5, and S6.
Our recent work has implicated changes in RNAPII activity with the alteration of start site selection in vivo (Kaplan et al., 2012) (Figure 4). Furthermore, Sub1 genetically interacts with TFIIB (sua7) (Knaus et al., 1996; Wu et al., 1999), is broadly recruited to RNAPII/III promoters in vivo (Rosonina et al., 2009; Tavenet et al., 2009), and was implicated as a member of the RNAPII preinitiation complex (Sikorski et al., 2011). We therefore sought to determine whether Sub1 might modulate RNAPII start site choice. Notably, primer extension analysis at ADH1 revealed that deleting SUB1 led to a significant downstream shift in start site (Figures 7E and S7C). Slow RNAPII TL mutant rpb1 F1086S (Figure 4B) and sua7 alleles (Pinto et al., 1992) (Figure S6B) also initiate downstream and are synthetic sick with sub1Δ. These data are consistent with the notion that Sub1 promotes transcription initiation. Double-mutant analysis revealed that sub1Δ also exacerbated the downstream start site shift of the slow RNAPII TL allele rpb1 F1086S and slightly suppressed the rpb1 E1103G allele (Figure 7E). Because sub1Δ has also been linked to another RNA processing step, namely 3′ end processing (reviewed in Conesa and Acker, 2010), we examined the effect of sub1Δ on splicing and observed a statistically significant increase in splicing efficiency (p < 10−8; Table S5), again phenocopying the slow RNAPII mutants (Figure 7F and Table S5).
Based on these data, we propose a model in which transcription start and splicing are intimately coupled with RNAPII elongation: fast RNAPII mutations result in upstream transcription start and diminished splicing, whereas slow mutations or sub1Δ give rise to downstream transcription start and enhanced splicing (Figure 7G). Given the possibility of direct coupling between start site selection and downstream mRNA processing, we globally measured splicing defects in sua7-3 (TFIIB) (Pinto et al., 1994; Wu et al., 1999) and tgf2Δ261-273 (TFIIF) (Eichner et al., 2010), mutants that exhibit downstream and upstream start site selection, respectively (Figure S6B). If splicing were strictly coupled to start site choice, one would expect these mutants to have similar splicing defects to the slow and fast RNAPII mutants. However, such a correlation was not observed (Figure S6C), suggesting that these processes are, in fact, genetically separable. Taken together, these data support a model in which the catalytic rate of RNAPII has multiple, separable effects on start site selection and mRNA processing and highlight the importance of WT elongation rate for multiple steps in gene expression.
DISCUSSION
In this study, we have described an important extension of our E-MAP genetic interaction mapping strategy, the functional interrogation of individual protein residues. We have first used this approach to genetically dissect RNAPII and demonstrate how the pE-MAP successfully characterized functionally distinct, individual amino acids within this multifunctional complex. This analysis provided not only insight into global structure-function relationships within RNAPII, but also specific details about how RNAPII regulates and is regulated by different factors and processes.
Insights into RNAPII Function Derived from the pE-MAP
Multiple aspects of the genetic interaction map provided insights into transcriptional regulation. First, by comparing the RNAPII genetic interaction profiles with those from previous deletion/knockdown studies, we could assign function to individual residues, which allowed us to generate a point mutant-protein complex connectivity map (Figures 3 and S3). Furthermore, analyzing double mutants, both within RNAPII itself (Figures 5C and 5D) and between RNAPII and other genes (Figure 7), allowed for a better understanding of TL function, as well as the identification of other factors that directly or indirectly impinge on RNAPII activity. Indeed, the pE-MAP allowed us to identify putative transcription factors (negative and positive), such as Sub1. Although Sub1 was previously implicated at the promoter (Sikorski et al., 2011), we here demonstrated that it positively regulates RNAPII by influencing start site usage (Figure 7). The epistatic relationships between fast RNAPII alleles and sub1Δ suggest that Sub1 activity may be bypassed when RNAPII catalytic activity is increased. Collectively, our data indicate that Sub1 plays a direct role in transcriptional initiation and influences mRNA splicing, possibly via its effect on elongation (García et al., 2012).
Finally, structural analysis revealed that mutations close in three-dimensional (3D) space have very similar genetic profiles, including those in different subunits (Figure 2B), suggesting that structural information is ultimately contained within the pE-MAP and can be used to identify specific protein-protein interaction interfaces. For example, the Rpb2 mutation E437G/F442S, which shifts start site selection upstream, is in a domain that contacts TFIIF at a region in which mutations also result in upstream start site shifts (Chen et al., 2007; Eichner et al., 2010). Furthermore, the identification of an Rpb7 mutant (D166G) that alters start site selection is intriguing, as this region interacts with TFIIH in a cryo-EM structure of the RNAPII preinitiation complex (He et al., 2013) and TFIIH also alters start site selection (Goel et al., 2012). Therefore, the pE-MAP technique could supplement other methods, such as crosslinking and electron microscopy, to identify physically interacting protein regions.
Coordination of Transcriptional Rate with Start Site Selection and mRNA Splicing
The importance of maintaining WT rates of transcription is evidenced by the phenotypic defects observed in the fast and slow mutants (Kaplan et al., 2012) (Figures 5 and 6), as well as the striking mutual suppression seen when combining two mutations that individually make RNAPII slow or fast (Figures 5 and 6). Interestingly, the pE-MAP identified two groups of RNAPII mutants: one that preferentially initiates upstream and exhibits an increased rate of transcription and one that initiates downstream and transcribes slowly (Figure 7) (Kaplan et al., 2012). We propose that both phenotypes are direct consequences of the efficiency of nucleotide selection and incorporation, as the addition of the first nucleotides at initiation is biochemically similar to adding nucleotides during elongation. These data support the model that RNAPII engages in “scanning” during initiation in S. cerevisiae (Giardina and Lis, 1993; Kaplan, 2013; Kaplan et al., 2012; Kuehner and Brow, 2006). Whether RNAPII catalysis drives this scanning or whether scanning occurs in the absence of nucleotide incorporation, perhaps driven by TFIIH, is unknown. We also note that, because changes in start site selection alter the 5′ UTR length and composition, initial transcription decisions may have downstream effects on gene expression such as changes in RNA stability or translational efficiency of the mRNA (Arribere and Gilbert, 2013; Rojas-Duran and Gilbert, 2012).
The pE-MAP has allowed insights into the cotranscriptional process of mRNA splicing. It is now clear that most introns are removed while RNA polymerase is still associated with the DNA template. In metazoans, alternative splicing decisions can be influenced by factors impinging on transcription, including promoter identity and polymerase speed (reviewed in Perales and Bentley, 2009). Slowing the rate of elongation by mutation of RNAPII or chemical means can improve the recognition of splice sites that deviate from consensus signals (de la Mata et al., 2003; Howe et al., 2003; Ip et al., 2011). Because the spliceosome undergoes stepwise assembly on each intron, slowing transcription can afford more time for formation of the catalytically active machine before transcription of a downstream, stronger site. Although budding yeast lack alternative splicing, it nonetheless follows that the efficiency of cotranscriptional splicing would be favored by allowing sufficient time for spliceosome assembly. Indeed, recent work suggests that RNA polymerase may slow down to favor co-versus posttranscriptional splicing (Aitken et al., 2011; Alexander et al., 2010; Carrillo Oesterreich et al., 2010). Our microarray analyses that directly compare faster and slower RNAPII show a clear trend in which splicing efficiency is anticorrelated with transcription rate (Figure 6); thus, these results satisfy the predictions of kinetic coupling in S. cerevisiae.
Interestingly, we observed correlation among start site selection, elongation rate, and splicing efficiency. In fact, promoter-proximal events are known to be able to influence downstream RNA transactions: promoter identity can influence alternative splicing or mRNA stability in other systems (Cramer et al., 1997; Harel-Sharvit et al., 2010; Trcek et al., 2011), and 5′ UTR length, determined by start site selection, can strongly alter translation efficiency in budding yeast (Rojas-Duran and Gilbert, 2012). However, when we measured splicing efficiency using mutants in the general transcription factors TFIIF and TFIIB that alter start site selection, we found that not all initiation phenotypes are predictive of splicing efficiency (Figure S6C). This suggests that RNAPII catalytic rate has several separable effects on gene expression, a claim supported by recent evidence showing kinetic coupling between RNAPII transcription and Sen1-dependent termination (Hazelbaker et al., 2013).
Taken together, our data highlight the important impact of transcription speed determined by the genetic status of RNAPII and trans-acting factors (e.g., Sub1) on start site selection and mRNA splicing. We propose that RNA polymerase may have been evolutionarily tuned to coordinate between multiple steps in gene expression, and we predict that polymerase rate may influence multiple additional cotranscriptional steps in gene expression, including mRNP assembly, 3′ end processing, and export.
Future Studies Using the pE-MAP Approach
S. cerevisiae RNAPII provided the groundwork for validating the pE-MAP approach. Application to other molecular machines, including the ribosome, the proteasome, HSP70, HSP90, histones, and DNA polymerases, should prove informative. This analysis can also be carried out in other organisms that are genetically tractable and are amenable to high-throughput genetic interaction mapping, including S. pombe (Roguev et al., 2008; Roguev et al., 2007; Ryan et al., 2012) and E. coli (Butland et al., 2008; Typas et al., 2008). Furthermore, pE-MAPs could be used to gain structural insight into proteins and complexes that have unknown structures. Finally, as genetic interaction mapping strategies become more prevalent in mammalian cells (Bassik et al., 2013; Laufer et al., 2013; Lin et al., 2012; Roguev et al., 2013) and with the development of genome editing (Gaj et al., 2013), similar work characterizing the function of individual amino acids will have great impact on understanding how point mutations in specific genes result in different disease states.
EXPERIMENTAL PROCEDURES
E-MAP-compatible MATα RNAPII mutant strains carrying a marked rpb deletion and mutant rpb on a CEN plasmid were mated with 1,200 MATa DAmP/deletion strains by pinning on solid media. Sporulation was induced, and double-mutant MATa spores were isolated on selective media. Genetic interactions were scored based on double-mutant colony sizes, which were extracted using automated imaging software.
For gene expression and splicing arrays, total RNA was extracted from mutant and WT log-phase cells. Competitive hybridizations were performed between mutant and WT complementary DNA (cDNA) (splicing) or complementary RNA (cRNA) (gene expression).
CTF assays were carried out by plating strains carrying ade2-101 and a chromosome VII fragment containing SUP11 on SC medium with 20% adenine and measuring the fraction of red colonies. The red color caused by ade2-101 is counteracted by SUP11; chromosome fragment loss results in red colonies.
For transcription start site analysis, we performed primer extension from a 32P end-labeled oligo annealed to total RNA. cDNAs were separated by PAGE and bands quantified.
Detailed descriptions of experiments and computational analyses are provided in the Extended Experimental Procedures.
Supplementary Material
ACKNOWLEDGMENTS
The authors wish to thank members of the Krogan, Kaplan, and Guthrie labs; David Agard, Brad Cairns, Ada Cheng, C. James Ingles, Paivand Jalalian, Tanja Kortemme, Andrej Sali, and Keith Yamamoto for helpful discussion; Steve Buratowski, Olga Calvo, and Eva Nogales for sharing results prior to publication; Steve Hahn and Mike Hampsey for providing tfg2 and sua7 mutant plasmids, respectively; Colm Ryan for help with statistical analysis; and Ricardo Almeida and Jiewei Xu for assistance with growth assays. This work was supported by grants from QB3 at UCSF and the NIH (R01GM084448, R01GM084279, P50GM081879, and R01GM098101 to N.J.K.; R01GM036659 to Roger D. Kornberg for support of C.D.K. for a portion of this work; R01GM097260 to C.D.K.; R01GM021119 to C.G.; and DP5OD009180 to J.S.F.). C.G. is an ACS Research Professor of Molecular Genetics. E.A.M. was supported by an NSF graduate research fellowship. C.D.K. acknowledges a Helen Hay Whitney Fellowship for the early stages of this work. H.B. was supported by the UCSF Biophysics Graduate Program. N.J.K. is a Searle Scholar and a Keck Young Investigator. The content is solely the responsibility of the authors and does not necessarily represent the official views of any of the acknowledged funding agencies.
H.B., E.A.M., C.G., C.D.K., and N.J.K. designed the research; H.B., H.J., E.A.M., Y.A.C., S.W., J.J.B., C.Q., F.H., and L.K.T. carried out experimental assays; H.B. analyzed data; M.S., J.H.M., and J.S.F. provided computational support; F.C.P.H., P.H., C.G., C.D.K., and N.J.K. supervised the research; H.B., E.A.M., C.G., C.D.K., and N.J.K. wrote the paper.
Footnotes
ACCESSION NUMBERS
The GEO accession number for the gene expression profiles reported in this paper is GSE47429.
SUPPLEMENTAL INFORMATION
Supplemental Information includes Extended Experimental Procedures, seven figures, and six tables and can be found with this article online at http://dx.doi.org/10.1016/j.cell.2013.07.033.
REFERENCES
- Aguilar PS, Fröhlich F, Rehman M, Shales M, Ulitsky I, Olivera-Couto A, Braberg H, Shamir R, Walter P, Mann M, et al. A plasma-membrane E-MAP reveals links of the eisosome with sphingolipid metabolism and endosomal trafficking. Nat. Struct. Mol. Biol. 2010;17:901–908. doi: 10.1038/nsmb.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitken S, Alexander RD, Beggs JD. Modelling reveals kinetic advantages of co-transcriptional splicing. PLoS Comput. Biol. 2011;7:e1002215. doi: 10.1371/journal.pcbi.1002215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander RD, Innocente SA, Barrass JD, Beggs JD. Splicing-dependent RNA polymerase pausing in yeast. Mol. Cell. 2010;40:582–593. doi: 10.1016/j.molcel.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archambault J, Friesen JD. Genetics of eukaryotic RNA polymerases I, II, and III. Microbiol. Rev. 1993;57:703–724. doi: 10.1128/mr.57.3.703-724.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arribere JA, Gilbert WV. Roles for transcript leaders in translation and mRNA decay revealed by transcript leader sequencing. Genome Res. 2013;23:977–987. doi: 10.1101/gr.150342.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassik MC, Kampmann M, Lebbink RJ, Wang S, Hein MY, Poser I, Weibezahn J, Horlbeck MA, Chen S, Mann M, et al. A systematic mammalian genetic interaction map reveals pathways underlying ricin susceptibility. Cell. 2013;152:909–922. doi: 10.1016/j.cell.2013.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltrao P, Cagney G, Krogan NJ. Quantitative genetic interactions reveal biological modularity. Cell. 2010;141:739–745. doi: 10.1016/j.cell.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benschop JJ, Brabers N, van Leenen D, Bakker LV, van Deutekom HW, van Berkum NL, Apweiler E, Lijnzaad P, Holstege FC, Kemmeren P. A consensus of core protein complex compositions for Saccharomyces cerevisiae. Mol. Cell. 2010;38:916–928. doi: 10.1016/j.molcel.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Bentley DL. Rules of engagement: co-transcriptional recruitment of pre-mRNA processing factors. Curr. Opin. Cell Biol. 2005;17:251–256. doi: 10.1016/j.ceb.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Butland G, Babu M, Díaz-Mejía JJ, Bohdana F, Phanse S, Gold B, Yang W, Li J, Gagarinova AG, Pogoutse O, et al. eSGA: E. coli synthetic genetic array analysis. Nat. Methods. 2008;5:789–795. doi: 10.1038/nmeth.1239. [DOI] [PubMed] [Google Scholar]
- Carrillo Oesterreich F, Preibisch S, Neugebauer KM. Global analysis of nascent RNA reveals transcriptional pausing in terminal exons. Mol. Cell. 2010;40:571–581. doi: 10.1016/j.molcel.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Carrozza MJ, Li B, Florens L, Suganuma T, Swanson SK, Lee KK, Shia WJ, Anderson S, Yates J, Washburn MP, Workman JL. Histone H3 methylation by Set2 directs deacetylation of coding regions by Rpd3S to suppress spurious intragenic transcription. Cell. 2005;123:581–592. doi: 10.1016/j.cell.2005.10.023. [DOI] [PubMed] [Google Scholar]
- Chen HT, Warfield L, Hahn S. The positions of TFIIF and TFIIE in the RNA polymerase II transcription preinitiation complex. Nat. Struct. Mol. Biol. 2007;14:696–703. doi: 10.1038/nsmb1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark TA, Sugnet CW, Ares M., Jr Genomewide analysis of mRNA processing in yeast using splicing-specific microarrays. Science. 2002;296:907–910. doi: 10.1126/science.1069415. [DOI] [PubMed] [Google Scholar]
- Collins SR, Kemmeren P, Zhao XC, Greenblatt JF, Spencer F, Holstege FC, Weissman JS, Krogan NJ. Toward a comprehensive atlas of the physical interactome of Saccharomyces cerevisiae. Mol. Cell. Proteomics. 2007a;6:439–450. doi: 10.1074/mcp.M600381-MCP200. [DOI] [PubMed] [Google Scholar]
- Collins SR, Miller KM, Maas NL, Roguev A, Fillingham J, Chu CS, Schuldiner M, Gebbia M, Recht J, Shales M, et al. Functional dissection of protein complexes involved in yeast chromosome biology using a genetic interaction map. Nature. 2007b;446:806–810. doi: 10.1038/nature05649. [DOI] [PubMed] [Google Scholar]
- Collins SR, Roguev A, Krogan NJ. Quantitative genetic interaction mapping using the E-MAP approach. Methods Enzymol. 2010;470:205–231. doi: 10.1016/S0076-6879(10)70009-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conesa C, Acker J. Sub1/PC4 a chromatin associated protein with multiple functions in transcription. RNA Biol. 2010;7:287–290. doi: 10.4161/rna.7.3.11491. [DOI] [PubMed] [Google Scholar]
- Costanzo M, Baryshnikova A, Bellay J, Kim Y, Spear ED, Sevier CS, Ding H, Koh JL, Toufighi K, Mostafavi S, et al. The genetic landscape of a cell. Science. 2010;327:425–431. doi: 10.1126/science.1180823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer P, Bushnell DA, Kornberg RD. Structural basis of transcription: RNA polymerase II at 2.8 angstrom resolution. Science. 2001;292:1863–1876. doi: 10.1126/science.1059493. [DOI] [PubMed] [Google Scholar]
- Cramer P, Pesce CG, Baralle FE, Kornblihtt AR. Functional association between promoter structure and transcript alternative splicing. Proc. Natl. Acad. Sci. USA. 1997;94:11456–11460. doi: 10.1073/pnas.94.21.11456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Mata M, Alonso CR, Kadener S, Fededa JP, Blaustein M, Pelisch F, Cramer P, Bentley D, Kornblihtt AR. A slow RNA polymerase II affects alternative splicing in vivo. Mol. Cell. 2003;12:525–532. doi: 10.1016/j.molcel.2003.08.001. [DOI] [PubMed] [Google Scholar]
- de la Mata M, Muñoz MJ, Alló M, Fededa JP, Schor IE, Kornblihtt AR. RNA Polymerase II Elongation at the Crossroads of Transcription and Alternative Splicing. Genet. Res. Int. 2011;2011:309865. doi: 10.4061/2011/309865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards AM, Kane CM, Young RA, Kornberg RD. Two dissociable subunits of yeast RNA polymerase II stimulate the initiation of transcription at a promoter in vitro. J. Biol. Chem. 1991;266:71–75. [PubMed] [Google Scholar]
- Eichner J, Chen HT, Warfield L, Hahn S. Position of the general transcription factor TFIIF within the RNA polymerase II transcription preinitiation complex. EMBO J. 2010;29:706–716. doi: 10.1038/emboj.2009.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler D, Braberg H, Mehta M, Chechik G, Cagney G, Mukherjee P, Silva AC, Shales M, Collins SR, van Wageningen S, et al. Functional organization of the S. cerevisiae phosphorylation network. Cell. 2009;136:952–963. doi: 10.1016/j.cell.2008.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaj T, Gersbach CA, Barbas CF., 3rd ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol. 2013;31:397–405. doi: 10.1016/j.tibtech.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García A, Collin A, Calvo O. Sub1 associates with Spt5 and influences RNA polymerase II transcription elongation rate. Mol. Biol. Cell. 2012;23:4297–4312. doi: 10.1091/mbc.E12-04-0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazy MA, Brodie SA, Ammerman ML, Ziegler LM, Ponticelli AS. Amino acid substitutions in yeast TFIIF confer upstream shifts in transcription initiation and altered interaction with RNA polymerase II. Mol. Cell. Biol. 2004;24:10975–10985. doi: 10.1128/MCB.24.24.10975-10985.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardina C, Lis JT. DNA melting on yeast RNA polymerase II promoters. Science. 1993;261:759–762. doi: 10.1126/science.8342041. [DOI] [PubMed] [Google Scholar]
- Gnatt AL, Cramer P, Fu J, Bushnell DA, Kornberg RD. Structural basis of transcription: an RNA polymerase II elongation complex at 3.3 A resolution. Science. 2001;292:1876–1882. doi: 10.1126/science.1059495. [DOI] [PubMed] [Google Scholar]
- Goel S, Krishnamurthy S, Hampsey M. Mechanism of start site selection by RNA polymerase II: interplay between TFIIB and Ssl2/XPB helicase subunit of TFIIH. J. Biol. Chem. 2012;287:557–567. doi: 10.1074/jbc.M111.281576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greger IH, Proudfoot NJ. Poly(A) signals control both transcriptional termination and initiation between the tandem GAL10 and GAL7 genes of Saccharomyces cerevisiae. EMBO J. 1998;17:4771–4779. doi: 10.1093/emboj/17.16.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harel-Sharvit L, Eldad N, Haimovich G, Barkai O, Duek L, Choder M. RNA polymerase II subunits link transcription and mRNA decay to translation. Cell. 2010;143:552–563. doi: 10.1016/j.cell.2010.10.033. [DOI] [PubMed] [Google Scholar]
- Hazelbaker DZ, Marquardt S, Wlotzka W, Buratowski S. Kinetic competition between RNA Polymerase II and Sen1-dependent transcription termination. Mol. Cell. 2013;49:55–66. doi: 10.1016/j.molcel.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Fang J, Taatjes DJ, Nogales E. Structural visualization of key steps in human transcription initiation. Nature. 2013;495:481–486. doi: 10.1038/nature11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe KJ, Kane CM, Ares M., Jr Perturbation of transcription elongation influences the fidelity of internal exon inclusion in Saccharomyces cerevisiae. RNA. 2003;9:993–1006. doi: 10.1261/rna.5390803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip JY, Schmidt D, Pan Q, Ramani AK, Fraser AG, Odom DT, Blencowe BJ. Global impact of RNA polymerase II elongation inhibition on alternative splicing regulation. Genome Res. 2011;21:390–401. doi: 10.1101/gr.111070.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan CD. Basic mechanisms of RNA polymerase II activity and alteration of gene expression in Saccharomyces cerevisiae. Biochim. Biophys. Acta. 2013;1829:39–54. doi: 10.1016/j.bbagrm.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan CD, Holland MJ, Winston F. Interaction between transcription elongation factors and mRNA 3′-end formation at the Saccharomyces cerevisiae GAL10-GAL7 locus. J. Biol. Chem. 2005;280:913–922. doi: 10.1074/jbc.M411108200. [DOI] [PubMed] [Google Scholar]
- Kaplan CD, Jin H, Zhang IL, Belyanin A. Dissection of Pol II trigger loop function and Pol II activity-dependent control of start site selection in vivo. PLoS Genet. 2012;8:e1002627. doi: 10.1371/journal.pgen.1002627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan CD, Larsson KM, Kornberg RD. The RNA polymerase II trigger loop functions in substrate selection and is directly targeted by alpha-amanitin. Mol. Cell. 2008;30:547–556. doi: 10.1016/j.molcel.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keogh MC, Kurdistani SK, Morris SA, Ahn SH, Podolny V, Collins SR, Schuldiner M, Chin K, Punna T, Thompson NJ, et al. Cotranscriptional set2 methylation of histone H3 lysine 36 recruits a repressive Rpd3 complex. Cell. 2005;123:593–605. doi: 10.1016/j.cell.2005.10.025. [DOI] [PubMed] [Google Scholar]
- Kettenberger H, Armache KJ, Cramer P. Complete RNA polymerase II elongation complex structure and its interactions with NTP and TFIIS. Mol. Cell. 2004;16:955–965. doi: 10.1016/j.molcel.2004.11.040. [DOI] [PubMed] [Google Scholar]
- Knaus R, Pollock R, Guarente L. Yeast SUB1 is a suppressor of TFIIB mutations and has homology to the human co-activator PC4. EMBO J. 1996;15:1933–1940. [PMC free article] [PubMed] [Google Scholar]
- Kuehner JN, Brow DA. Quantitative analysis of in vivo initiator selection by yeast RNA polymerase II supports a scanning model. J. Biol. Chem. 2006;281:14119–14128. doi: 10.1074/jbc.M601937200. [DOI] [PubMed] [Google Scholar]
- Laufer C, Fischer B, Billmann M, Huber W, Boutros M. Mapping genetic interactions in human cancer cells with RNAi and multiparametric phenotyping. Nat. Methods. 2013;10:427–431. doi: 10.1038/nmeth.2436. [DOI] [PubMed] [Google Scholar]
- Lin YY, Kiihl S, Suhail Y, Liu SY, Chou YH, Kuang Z, Lu JY, Khor CN, Lin CL, Bader JS, et al. Functional dissection of lysine deacetylases reveals that HDAC1 and p300 regulate AMPK. Nature. 2012;482:251–255. doi: 10.1038/nature10804. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Malagon F, Kireeva ML, Shafer BK, Lubkowska L, Kashlev M, Strathern JN. Mutations in the Saccharomyces cerevisiae RPB1 gene conferring hypersensitivity to 6-azauracil. Genetics. 2006;172:2201–2209. doi: 10.1534/genetics.105.052415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason PB, Struhl K. Distinction and relationship between elongation rate and processivity of RNA polymerase II in vivo. Mol. Cell. 2005;17:831–840. doi: 10.1016/j.molcel.2005.02.017. [DOI] [PubMed] [Google Scholar]
- Ohkuni K, Kitagawa K. Endogenous transcription at the centromere facilitates centromere activity in budding yeast. Curr. Biol. 2011;21:1695–1703. doi: 10.1016/j.cub.2011.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X, Yuan DS, Xiang D, Wang X, Sookhai-Mahadeo S, Bader JS, Hieter P, Spencer F, Boeke JD. A robust toolkit for functional profiling of the yeast genome. Mol. Cell. 2004;16:487–496. doi: 10.1016/j.molcel.2004.09.035. [DOI] [PubMed] [Google Scholar]
- Perales R, Bentley D. “Cotranscriptionality”: the transcription elongation complex as a nexus for nuclear transactions. Mol. Cell. 2009;36:178–191. doi: 10.1016/j.molcel.2009.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto I, Ware DE, Hampsey M. The yeast SUA7 gene encodes a homolog of human transcription factor TFIIB and is required for normal start site selection in vivo. Cell. 1992;68:977–988. doi: 10.1016/0092-8674(92)90040-j. [DOI] [PubMed] [Google Scholar]
- Pinto I, Wu WH, Na JG, Hampsey M. Characterization of sua7 mutations defines a domain of TFIIB involved in transcription start site selection in yeast. J. Biol. Chem. 1994;269:30569–30573. [PubMed] [Google Scholar]
- Roguev A, Bandyopadhyay S, Zofall M, Zhang K, Fischer T, Collins SR, Qu H, Shales M, Park HO, Hayles J, et al. Conservation and rewiring of functional modules revealed by an epistasis map in fission yeast. Science. 2008;322:405–410. doi: 10.1126/science.1162609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roguev A, Talbot D, Negri GL, Shales M, Cagney G, Bandyopadhyay S, Panning B, Krogan NJ. Quantitative genetic-interaction mapping in mammalian cells. Nat. Methods. 2013;10:432–437. doi: 10.1038/nmeth.2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roguev A, Wiren M, Weissman JS, Krogan NJ. High-throughput genetic interaction mapping in the fission yeast Schizosaccharomyces pombe. Nat. Methods. 2007;4:861–866. doi: 10.1038/nmeth1098. [DOI] [PubMed] [Google Scholar]
- Rojas-Duran MF, Gilbert WV. Alternative transcription start site selection leads to large differences in translation activity in yeast. RNA. 2012;18:2299–2305. doi: 10.1261/rna.035865.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosonina E, Willis IM, Manley JL. Sub1 functions in osmoregulation and in transcription by both RNA polymerases II and III. Mol. Cell. Biol. 2009;29:2308–2321. doi: 10.1128/MCB.01841-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan CJ, Roguev A, Patrick K, Xu J, Jahari H, Tong Z, Beltrao P, Shales M, Qu H, Collins SR, et al. Hierarchical modularity and the evolution of genetic interactomes across species. Mol. Cell. 2012;46:691–704. doi: 10.1016/j.molcel.2012.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuldiner M, Collins SR, Thompson NJ, Denic V, Bhamidipati A, Punna T, Ihmels J, Andrews B, Boone C, Greenblatt JF, et al. Exploration of the function and organization of the yeast early secretory pathway through an epistatic miniarray profile. Cell. 2005;123:507–519. doi: 10.1016/j.cell.2005.08.031. [DOI] [PubMed] [Google Scholar]
- Schuldiner M, Collins SR, Weissman JS, Krogan NJ. Quantitative genetic analysis in Saccharomyces cerevisiae using epistatic miniarray profiles (E-MAPs) and its application to chromatin functions. Methods. 2006;40:344–352. doi: 10.1016/j.ymeth.2006.07.034. [DOI] [PubMed] [Google Scholar]
- Shaw RJ, Reines D. Saccharomyces cerevisiae transcription elongation mutants are defective in PUR5 induction in response to nucleotide depletion. Mol. Cell. Biol. 2000;20:7427–7437. doi: 10.1128/mcb.20.20.7427-7437.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski TW, Ficarro SB, Holik J, Kim T, Rando OJ, Marto JA, Buratowski S. Sub1 and RPA associate with RNA polymerase II at different stages of transcription. Mol. Cell. 2011;44:397–409. doi: 10.1016/j.molcel.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer F, Gerring SL, Connelly C, Hieter P. Mitotic chromosome transmission fidelity mutants in Saccharomyces cerevisiae. Genetics. 1990;124:237–249. doi: 10.1093/genetics/124.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Q, Prysak MH, Woychik NA. Loss of the Rpb4/Rpb7 subcomplex in a mutant form of the Rpb6 subunit shared by RNA polymerases I, II, and III. Mol. Cell. Biol. 2003;23:3329–3338. doi: 10.1128/MCB.23.9.3329-3338.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavenet A, Suleau A, Dubreuil G, Ferrari R, Ducrot C, Michaut M, Aude JC, Dieci G, Lefebvre O, Conesa C, Acker J. Genome-wide location analysis reveals a role for Sub1 in RNA polymerase III transcription. Proc. Natl. Acad. Sci. USA. 2009;106:14265–14270. doi: 10.1073/pnas.0900162106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong AH, Lesage G, Bader GD, Ding H, Xu H, Xin X, Young J, Berriz GF, Brost RL, Chang M, et al. Global mapping of the yeast genetic interaction network. Science. 2004;303:808–813. doi: 10.1126/science.1091317. [DOI] [PubMed] [Google Scholar]
- Trcek T, Larson DR, Moldón A, Query CC, Singer RH. Single-molecule mRNA decay measurements reveal promoter-regulated mRNA stability in yeast. Cell. 2011;147:1484–1497. doi: 10.1016/j.cell.2011.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Typas A, Nichols RJ, Siegele DA, Shales M, Collins SR, Lim B, Braberg H, Yamamoto N, Takeuchi R, Wanner BL, et al. High-throughput, quantitative analyses of genetic interactions in E. coli. Nat. Methods. 2008;5:781–787. doi: 10.1038/nmeth.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Bushnell DA, Westover KD, Kaplan CD, Kornberg RD. Structural basis of transcription: role of the trigger loop in substrate specificity and catalysis. Cell. 2006;127:941–954. doi: 10.1016/j.cell.2006.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmes GM, Bergkessel M, Bandyopadhyay S, Shales M, Braberg H, Cagney G, Collins SR, Whitworth GB, Kress TL, Weissman JS, et al. A genetic interaction map of RNA-processing factors reveals links between Sem1/Dss1-containing complexes and mRNA export and splicing. Mol. Cell. 2008;32:735–746. doi: 10.1016/j.molcel.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston F, Sudarsanam P. The SAGA of Spt proteins and transcriptional analysis in yeast: past, present, and future. Cold Spring Harb. Symp. Quant. Biol. 1998;63:553–561. doi: 10.1101/sqb.1998.63.553. [DOI] [PubMed] [Google Scholar]
- Wu WH, Pinto I, Chen BS, Hampsey M. Mutational analysis of yeast TFIIB. A functional relationship between Ssu72 and Sub1/Tsp1 defined by allele-specific interactions with TFIIB. Genetics. 1999;153:643–652. doi: 10.1093/genetics/153.2.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.