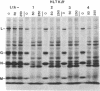
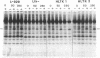
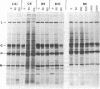
Abstract
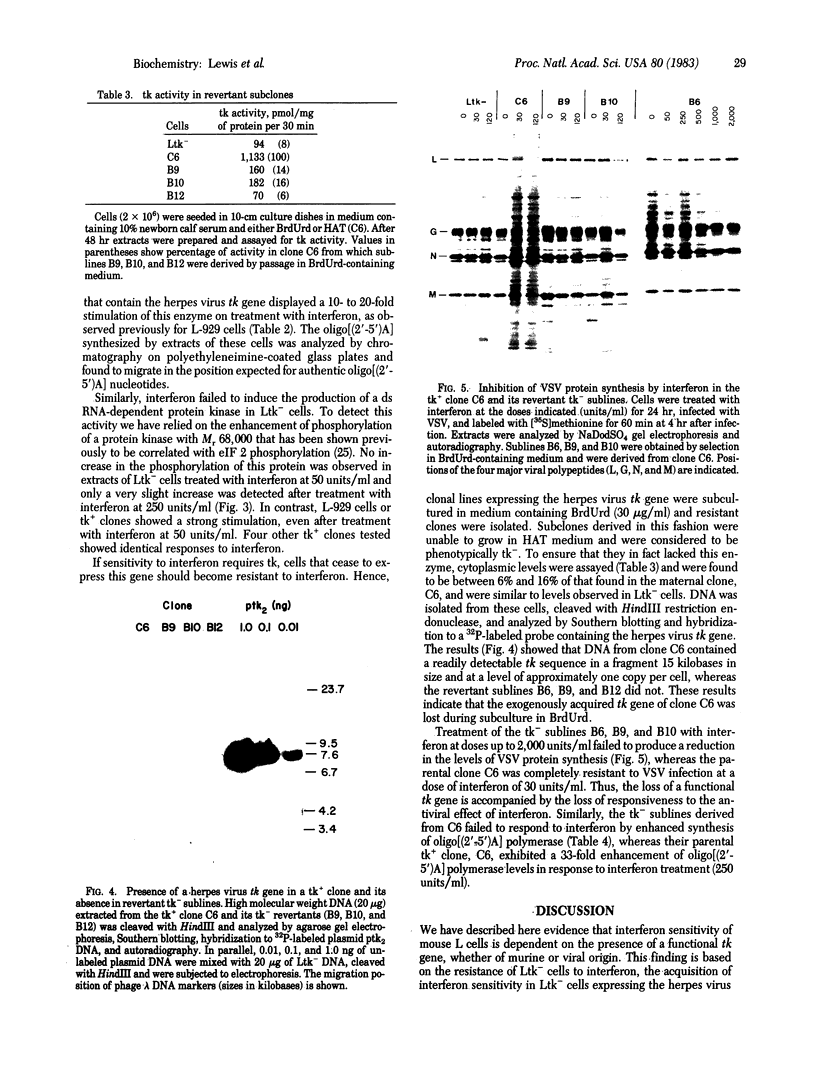
Mouse fibroblastoid cells (Ltk-) that lack thymidine kinase (tk) activity are unable to respond to murine beta-interferon by establishing antiviral activity or inducing the double-stranded RNA-dependent enzymes, oligo[(2'-5')A] polymerase and Mr 68,000 protein kinase. In contrast, the parental L-929 cell line or clonal derivatives of Ltk- cells into which the herpes virus tk gene was introduced by DNA-mediated gene transfer respond normally to interferon in developing resistance to viral infection and in inducing double-stranded RNA-dependent enzymes. Further evidence for a role of tk in the response to interferon was obtained by isolating revertants of tk+ clones that lost the herpes virus tk gene during growth in BrdUrd-containing medium. In such revertant sublines both tk enzyme activity and viral tk genes were undetectable and treatment with interferon failed to produce an antiviral effect or induce synthesis of the double-stranded RNA-dependent enzymes. Our results indicate that the ability of mouse L cells to respond to beta-interferon is dependent upon the presence of a functional tk gene. We propose that the induction of antiviral responses by interferon stringently requires a metabolite, the level of which is determined by tk activity. The system described may provide a means for elucidating the mechanisms by which responses to interferon are induced.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baglioni C. Interferon-induced enzymatic activities and their role in the antriviral state. Cell. 1979 Jun;17(2):255–264. doi: 10.1016/0092-8674(79)90151-x. [DOI] [PubMed] [Google Scholar]
- Baglioni C., Maroney P. A., West D. K. 2'5'Oligo(A) polymerase activity and inhibition of viral RNA synthesis in interferon-treated HeLa cells. Biochemistry. 1979 May 1;18(9):1765–1770. doi: 10.1021/bi00576a020. [DOI] [PubMed] [Google Scholar]
- Baglioni C., Minks M. A., Maroney P. A. Interferon action may be mediated by activation of a nuclease by pppA2'p5'A2'p5'A. Nature. 1978 Jun 22;273(5664):684–687. doi: 10.1038/273684a0. [DOI] [PubMed] [Google Scholar]
- Ball L. A. Induction of 2'5'-oligoadenylate synthetase activity and a new protein by chick interferon. Virology. 1979 Apr 30;94(2):282–296. doi: 10.1016/0042-6822(79)90462-8. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Branca A. A., Baglioni C. Evidence that types I and II interferons have different receptors. Nature. 1981 Dec 24;294(5843):768–770. doi: 10.1038/294768a0. [DOI] [PubMed] [Google Scholar]
- Clemens M. J., Williams B. R. Inhibition of cell-free protein synthesis by pppA2'p5'A2'p5'A: a novel oligonucleotide synthesized by interferon-treated L cell extracts. Cell. 1978 Mar;13(3):565–572. doi: 10.1016/0092-8674(78)90329-x. [DOI] [PubMed] [Google Scholar]
- Friedman R. M., Sonnabend J. A. Inhibition of interferon action by puromycin. J Immunol. 1965 Oct;95(4):696–703. [PubMed] [Google Scholar]
- Gupta S. L., Rubin B. Y., Holmes S. L. Interferon action: induction of specific proteins in mouse and human cells by homologous interferons. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4817–4821. doi: 10.1073/pnas.76.10.4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D., Lane D., Lipsich L., Wigler M., Botchan M. Characteristics of an SV40-plasmid recombinant and its movement into and out of the genome of a murine cell. Cell. 1980 Aug;21(1):127–139. doi: 10.1016/0092-8674(80)90120-8. [DOI] [PubMed] [Google Scholar]
- Hovanessian A. G., Meurs E., Montagnier L. Lack of systematic correlation between the interferon mediated antiviral state and the levels of 2-5A synthetase and protein kinase in three different types of murine cells. J Interferon Res. 1981 Feb;1(2):179–190. doi: 10.1089/jir.1981.1.179. [DOI] [PubMed] [Google Scholar]
- KIT S., DUBBS D. R., PIEKARSKI L. J., HSU T. C. DELETION OF THYMIDINE KINASE ACTIVITY FROM L CELLS RESISTANT TO BROMODEOXYURIDINE. Exp Cell Res. 1963 Aug;31:297–312. doi: 10.1016/0014-4827(63)90007-7. [DOI] [PubMed] [Google Scholar]
- Kerr I. M., Brown R. E., Hovanessian A. G. Nature of inhibitor of cell-free protein synthesis formed in response to interferon and double-stranded RNA. Nature. 1977 Aug 11;268(5620):540–542. doi: 10.1038/268540a0. [DOI] [PubMed] [Google Scholar]
- Knight E., Jr, Korant B. D. Fibroblast interferon induces synthesis of four proteins in human fibroblast cells. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1824–1827. doi: 10.1073/pnas.76.4.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lebleu B., Sen G. C., Shaila S., Cabrer B., Lengyel P. Interferon, double-stranded RNA, and protein phosphorylation. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3107–3111. doi: 10.1073/pnas.73.9.3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J. A., Falcoff E., Falcoff R. Dual action of double-stranded RNA inhibiting protein synthesis in extracts of interferon-treated mouse L cells. Translation is impaired at the level of initiation and by mRNA degradation. Eur J Biochem. 1978 May 16;86(2):497–509. doi: 10.1111/j.1432-1033.1978.tb12333.x. [DOI] [PubMed] [Google Scholar]
- Meurs E., Hovanessian A. G., Montagnier L. Interferon-mediated antiviral state in human MRC5 cells in the absence of detectable levels of 2-5A synthetase and protein kinase. J Interferon Res. 1981 Feb;1(2):219–232. doi: 10.1089/jir.1981.1.219. [DOI] [PubMed] [Google Scholar]
- Perucho M., Hanahan D., Lipsich L., Wigler M. Isolation of the chicken thymidine kinase gene by plasmid rescue. Nature. 1980 May 22;285(5762):207–210. doi: 10.1038/285207a0. [DOI] [PubMed] [Google Scholar]
- Perucho M., Hanahan D., Wigler M. Genetic and physical linkage of exogenous sequences in transformed cells. Cell. 1980 Nov;22(1 Pt 1):309–317. doi: 10.1016/0092-8674(80)90178-6. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R., Urlaub G., Chasin L. DNA-mediated transfer of the adenine phosphoribosyltransferase locus into mammalian cells. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1373–1376. doi: 10.1073/pnas.76.3.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]