Abstract
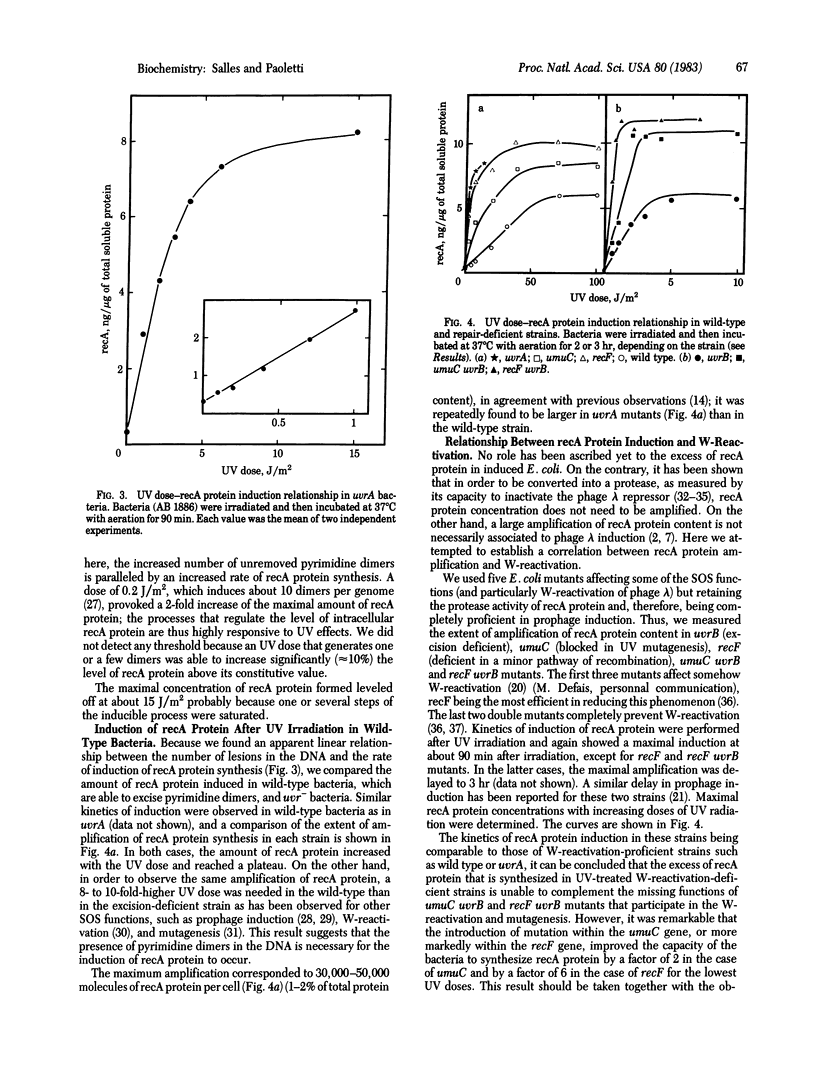
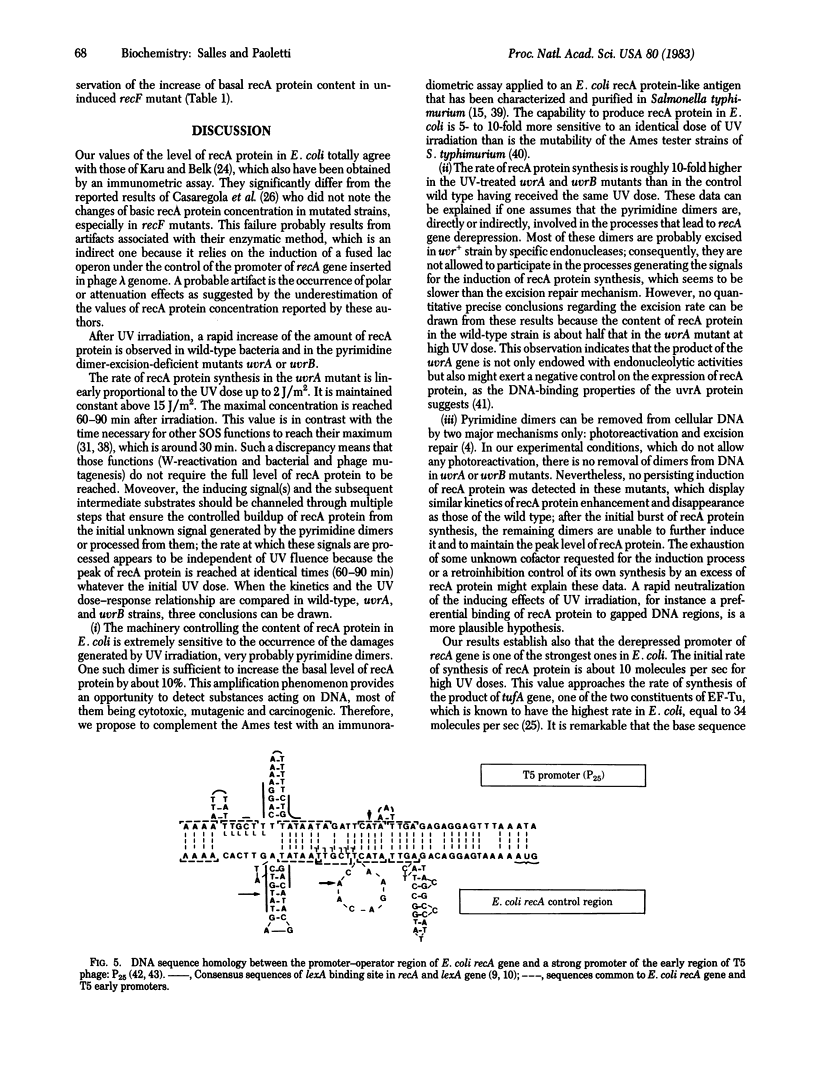
The basal level of recA protein in Escherichia coli K-12 was estimated by an immunoradiometric assay; it is approximately equal to 1,200 molecules per wild-type bacteria in midexponential phase of growth, slightly more in an excision-deficient (uvrA) strain, and markedly more in recF mutants. Kinetics of induction after UV irradiation showed a rapid increase of recA protein content, which reached a peak level after 60-90 min (20- to 55-fold amplification) and then decreased by dilution of the protein in the growing population. In order to obtain an identical extent of induction of recA protein, a 10-fold higher UV dose was necessary in a wild-type strain compared to the uvrA mutant strain. In the uvrA strain, the presence of one or only very few pyrimidine dimers on DNA was accompanied by a measurable increase of the constitutive level of recA protein; however, the unexcised dimers were unable to permanently induce the formation of recA protein. The derepressed promoter of recA gene is one of the strongest in E. coli. Its sequence displays many similarities with that of the strongest early promoters of T5 phage. Mutants (umuC uvrB and recF uvrB) unable to carry out W-reactivation produced high levels of recA protein after UV irradiation. The data suggested that the recF and umuC genes negatively control the regulation of recA protein level.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armengod M. E., Blanco M. Influence of the recF143 mutation of Escherichia coli K12 on prophage lambda induction. Mutat Res. 1978 Oct;52(1):37–47. doi: 10.1016/0027-5107(78)90093-3. [DOI] [PubMed] [Google Scholar]
- Bailone A., Levine A., Devoret R. Inactivation of prophage lambda repressor in vivo. J Mol Biol. 1979 Jul 5;131(3):553–572. doi: 10.1016/0022-2836(79)90007-x. [DOI] [PubMed] [Google Scholar]
- Baluch J., Sussman R., Resnick J. Induction of prophage lambda without amplification of recA protein. Mol Gen Genet. 1980;178(2):317–323. doi: 10.1007/BF00270478. [DOI] [PubMed] [Google Scholar]
- Casaregola S., D'Ari R., Huisman O. Quantitative evaluation of recA gene expression in Escherichia coli. Mol Gen Genet. 1982;185(3):430–439. doi: 10.1007/BF00334135. [DOI] [PubMed] [Google Scholar]
- Defais M., Caillet-Fauquet P., Fox M. S., Radman M. Induction kinetics of mutagenic DNA repair activity in E. coli following ultraviolet irradiation. Mol Gen Genet. 1976 Oct 18;148(2):125–130. doi: 10.1007/BF00268375. [DOI] [PubMed] [Google Scholar]
- Defais M., Fauquet P., Radman M., Errera M. Ultraviolet reactivation and ultraviolet mutagenesis of lambda in different genetic systems. Virology. 1971 Feb;43(2):495–503. doi: 10.1016/0042-6822(71)90321-7. [DOI] [PubMed] [Google Scholar]
- Gudas L. J., Pardee A. B. DNA synthesis inhibition and the induction of protein X in Escherichia coli. J Mol Biol. 1976 Mar 15;101(4):459–477. doi: 10.1016/0022-2836(76)90240-0. [DOI] [PubMed] [Google Scholar]
- Horii Z., Clark A. J. Genetic analysis of the recF pathway to genetic recombination in Escherichia coli K12: isolation and characterization of mutants. J Mol Biol. 1973 Oct 25;80(2):327–344. doi: 10.1016/0022-2836(73)90176-9. [DOI] [PubMed] [Google Scholar]
- Howard-Flanders P., Boyce R. P., Theriot L. Three loci in Escherichia coli K-12 that control the excision of pyrimidine dimers and certain other mutagen products from DNA. Genetics. 1966 Jun;53(6):1119–1136. doi: 10.1093/genetics/53.6.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacinski B. M., Sancar A., Rupp W. D. A general approach for purifying proteins encoded by cloned genes without using a functional assay: isolation of the uvrA gene product from radiolabeled maxicells. Nucleic Acids Res. 1981 Sep 25;9(18):4495–4508. doi: 10.1093/nar/9.18.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karu A. E., Belk E. D. Induction of E. coli recA protein via recBC and alternate pathways: quantitation by enzyme-linked immunosorbent assay (ELISA). Mol Gen Genet. 1982;185(2):275–282. doi: 10.1007/BF00330798. [DOI] [PubMed] [Google Scholar]
- Kato T., Rothman R. H., Clark A. J. Analysis of the role of recombination and repair in mutagenesis of Escherichia coli by UV irradiation. Genetics. 1977 Sep;87(1):1–18. doi: 10.1093/genetics/87.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T., Shinoura Y. Isolation and characterization of mutants of Escherichia coli deficient in induction of mutations by ultraviolet light. Mol Gen Genet. 1977 Nov 14;156(2):121–131. doi: 10.1007/BF00283484. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Little J. W., Edmiston S. H., Pacelli L. Z., Mount D. W. Cleavage of the Escherichia coli lexA protein by the recA protease. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3225–3229. doi: 10.1073/pnas.77.6.3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little J. W., Mount D. W., Yanisch-Perron C. R. Purified lexA protein is a repressor of the recA and lexA genes. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4199–4203. doi: 10.1073/pnas.78.7.4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maenhaut-Michel G., Brandenburger A., Boiteux S. Requirement of protein and RNA synthesis for lambda repressor inactivation by tif-1: effects of chloramphenicol, neomycin and rifampicin. Mol Gen Genet. 1978 Jul 25;163(3):293–299. doi: 10.1007/BF00271958. [DOI] [PubMed] [Google Scholar]
- Mattern I. E., van Winden M. P., Rörsch A. The range of action of genes controlling radiation sensitivity in Escherichia coli. Mutat Res. 1965 Apr;2(2):111–131. doi: 10.1016/0027-5107(65)90042-4. [DOI] [PubMed] [Google Scholar]
- McEntee K., Weinstock G. M., Lehman I. R. Initiation of general recombination catalyzed in vitro by the recA protein of Escherichia coli. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2615–2619. doi: 10.1073/pnas.76.6.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk M., Peacey M., Gross J. D. Repair of damage induced by ultraviolet light in DNA polymerase-defective Escherichia coli cells. J Mol Biol. 1971 Jun 14;58(2):623–630. doi: 10.1016/0022-2836(71)90376-7. [DOI] [PubMed] [Google Scholar]
- Moreau P. L., Fanica M., Devoret R. Induction of prophage lambda does not require full induction of RecA protein synthesis. Biochimie. 1980;62(10):687–694. doi: 10.1016/s0300-9084(80)80026-5. [DOI] [PubMed] [Google Scholar]
- Nakano E., Ichikawa-Ryo H., Kondo S. Comparative mutability of the Ames tester strains of Salmonella typhimurium by ultraviolet radiation and by 4-nitroquinoline 1-oxide. Mutat Res. 1982 Mar;93(1):35–44. doi: 10.1016/0027-5107(82)90123-3. [DOI] [PubMed] [Google Scholar]
- Paoletti C., Salles B., Giacomoni P. An immunoradiometric quantitative assay of Escherichia coli recA protein. Biochimie. 1982 Apr;64(4):239–246. doi: 10.1016/s0300-9084(82)80490-2. [DOI] [PubMed] [Google Scholar]
- Pedersen S., Bloch P. L., Reeh S., Neidhardt F. C. Patterns of protein synthesis in E. coli: a catalog of the amount of 140 individual proteins at different growth rates. Cell. 1978 May;14(1):179–190. doi: 10.1016/0092-8674(78)90312-4. [DOI] [PubMed] [Google Scholar]
- Pierre A., Salles B., Paoletti C. Measurement of recA protein induction in Salmonella typhimurium: a possible biochemical test for the detection of DNA damaging agents. Biochimie. 1982 Aug-Sep;64(8-9):775–781. doi: 10.1016/s0300-9084(82)80128-4. [DOI] [PubMed] [Google Scholar]
- Roberts J. W., Roberts C. W., Mount D. W. Inactivation and proteolytic cleavage of phage lambda repressor in vitro in an ATP-dependent reaction. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2283–2287. doi: 10.1073/pnas.74.6.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. W., Roberts C. W. Proteolytic cleavage of bacteriophage lambda repressor in induction. Proc Natl Acad Sci U S A. 1975 Jan;72(1):147–151. doi: 10.1073/pnas.72.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman R. H., Clark A. J. The dependence of postreplication repair on uvrB in a recF mutant of Escherichia coli K-12. Mol Gen Genet. 1977 Oct 24;155(3):279–286. doi: 10.1007/BF00272806. [DOI] [PubMed] [Google Scholar]
- Rothman R. H., Margossian L. J., Clark A. J. W-reactivation of phage lambda in recF, recL, uvrA, and uvrB mutants of E. coli K-12. Mol Gen Genet. 1979 Feb 1;169(3):279–287. doi: 10.1007/BF00382274. [DOI] [PubMed] [Google Scholar]
- Sancar A., Stachelek C., Konigsberg W., Rupp W. D. Sequences of the recA gene and protein. Proc Natl Acad Sci U S A. 1980 May;77(5):2611–2615. doi: 10.1073/pnas.77.5.2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schendel P. F., Defais M. The role of umuC gene product in mutagenesis by simple alkylating agents. Mol Gen Genet. 1980;177(4):661–665. doi: 10.1007/BF00272677. [DOI] [PubMed] [Google Scholar]
- Shibata T., DasGupta C., Cunningham R. P., Radding C. M. Purified Escherichia coli recA protein catalyzes homologous pairing of superhelical DNA and single-stranded fragments. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1638–1642. doi: 10.1073/pnas.76.4.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock G. M., McEntee K., Lehman I. R. ATP-dependent renaturation of DNA catalyzed by the recA protein of Escherichia coli. Proc Natl Acad Sci U S A. 1979 Jan;76(1):126–130. doi: 10.1073/pnas.76.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin E. M. Persistence and decay of thermoinducible error-prone repair activity in nonfilamentous derivatives of tif-1, Escherichia coli B/r: the timing of some critical events in ultraviolet mutagenesis. Mol Gen Genet. 1975 Dec 29;142(2):87–103. doi: 10.1007/BF00266092. [DOI] [PubMed] [Google Scholar]
- Witkin E. M. Ultraviolet mutagenesis and inducible DNA repair in Escherichia coli. Bacteriol Rev. 1976 Dec;40(4):869–907. doi: 10.1128/br.40.4.869-907.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Gabain A., Bujard H. Interaction of Escherichia coli RNA polymerase with promoters of several coliphage and plasmid DNAs. Proc Natl Acad Sci U S A. 1979 Jan;76(1):189–193. doi: 10.1073/pnas.76.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]


